Ultrashort Sintering and Near Net Shaping of Zr-Based AMZ4 Bulk Metallic Glass
Abstract
:1. Introduction
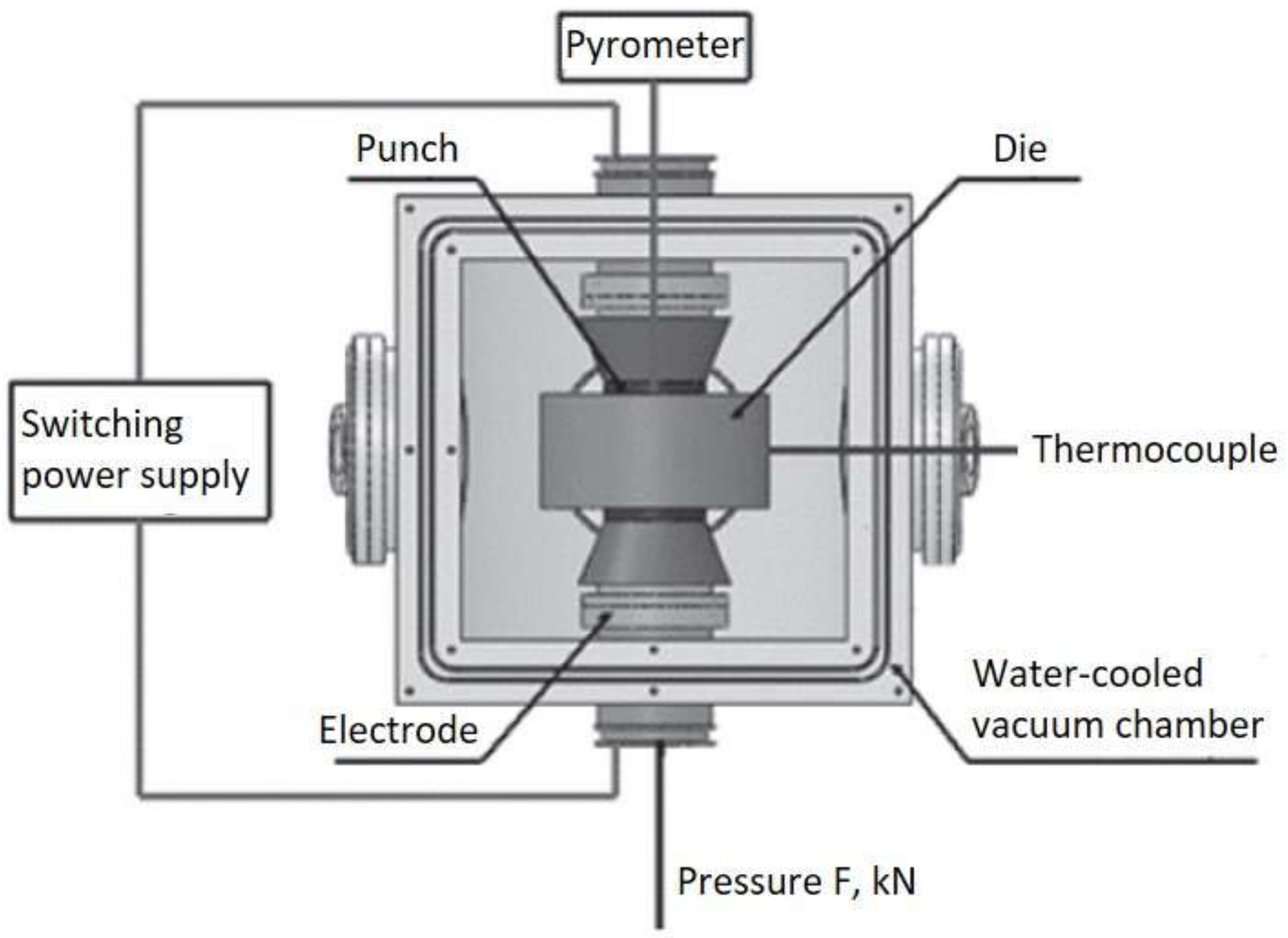
2. Materials and Methods
3. Results and Discussion
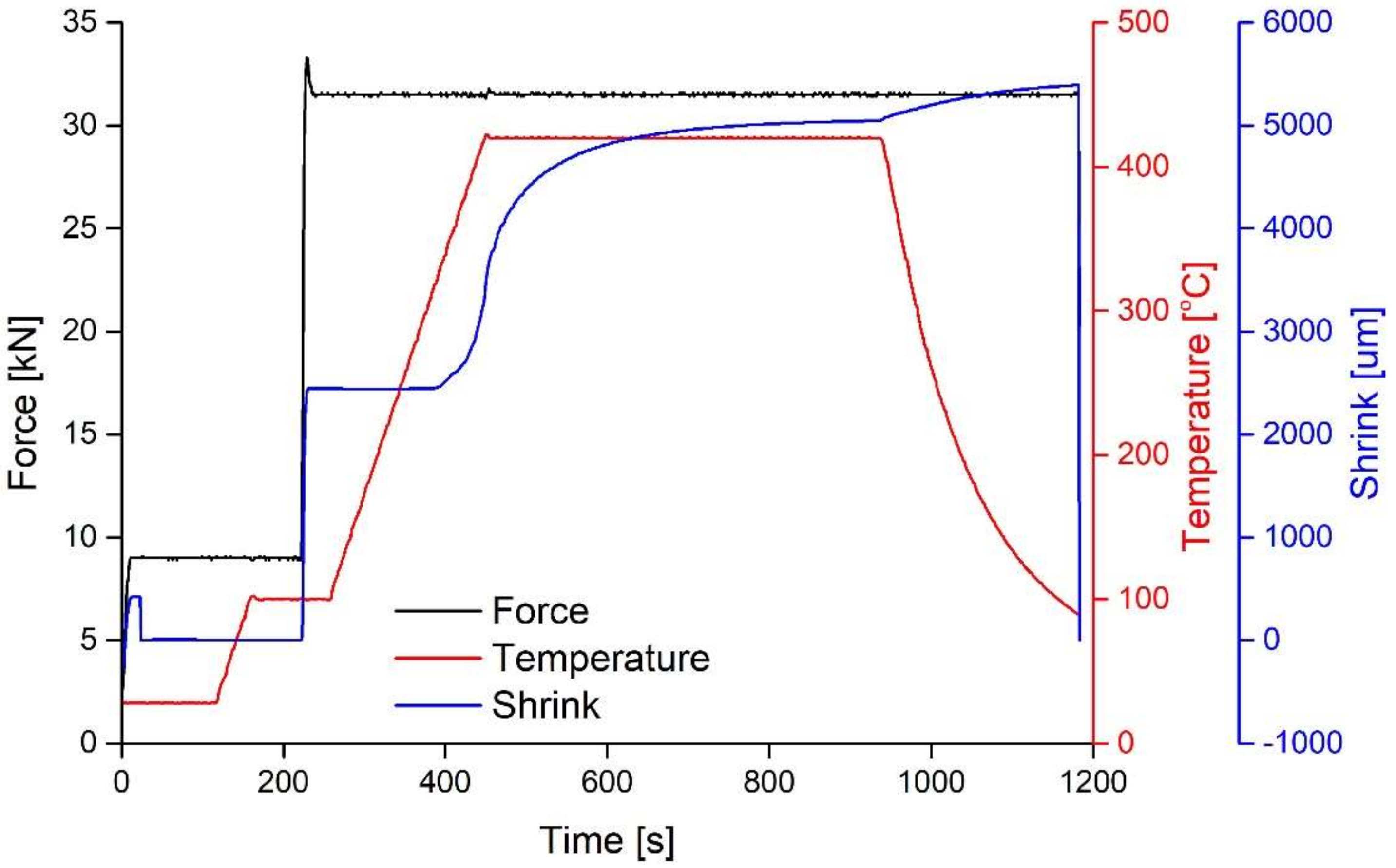
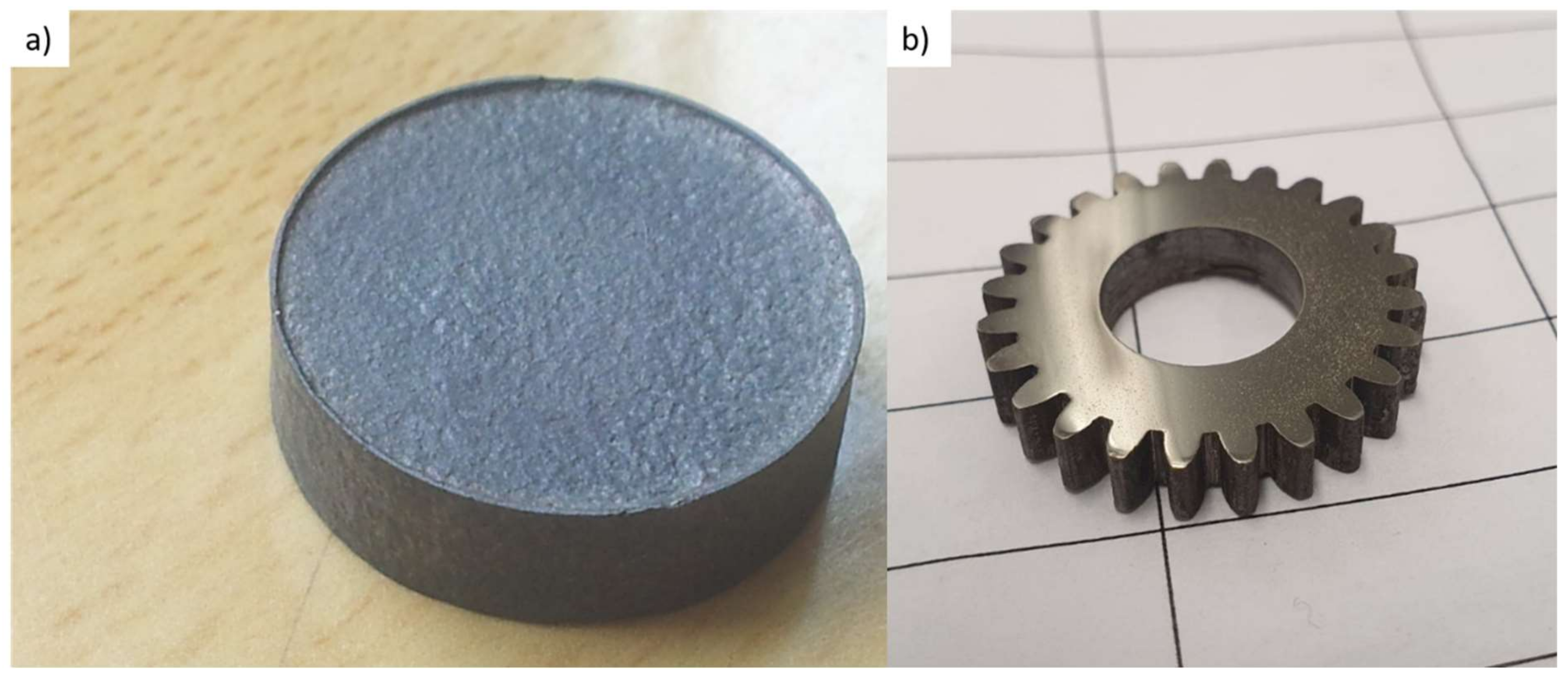
3.1. Manufacturing Process
3.2. Sample Characterization
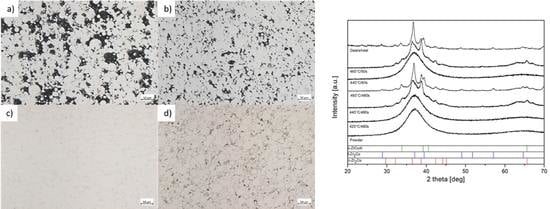
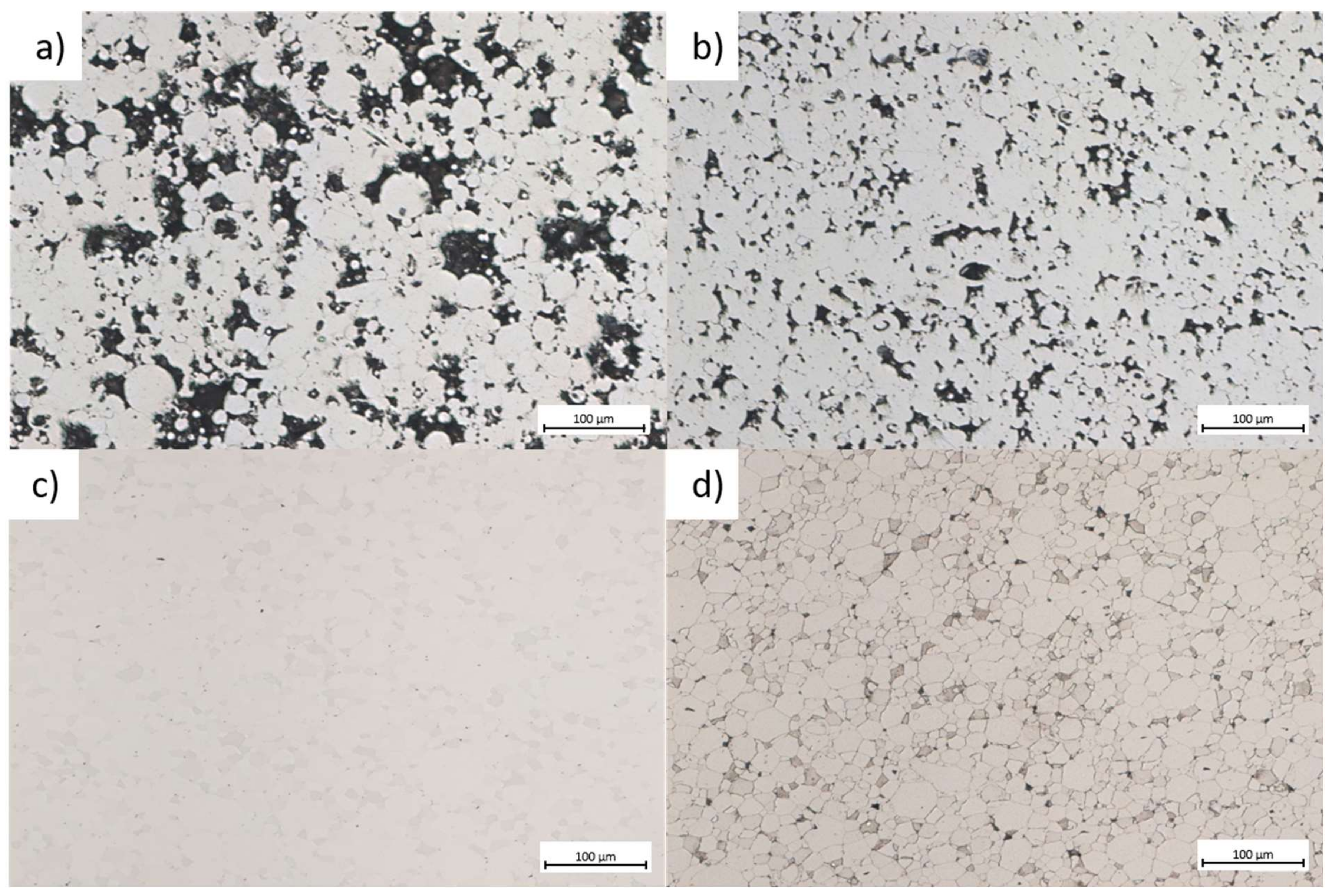
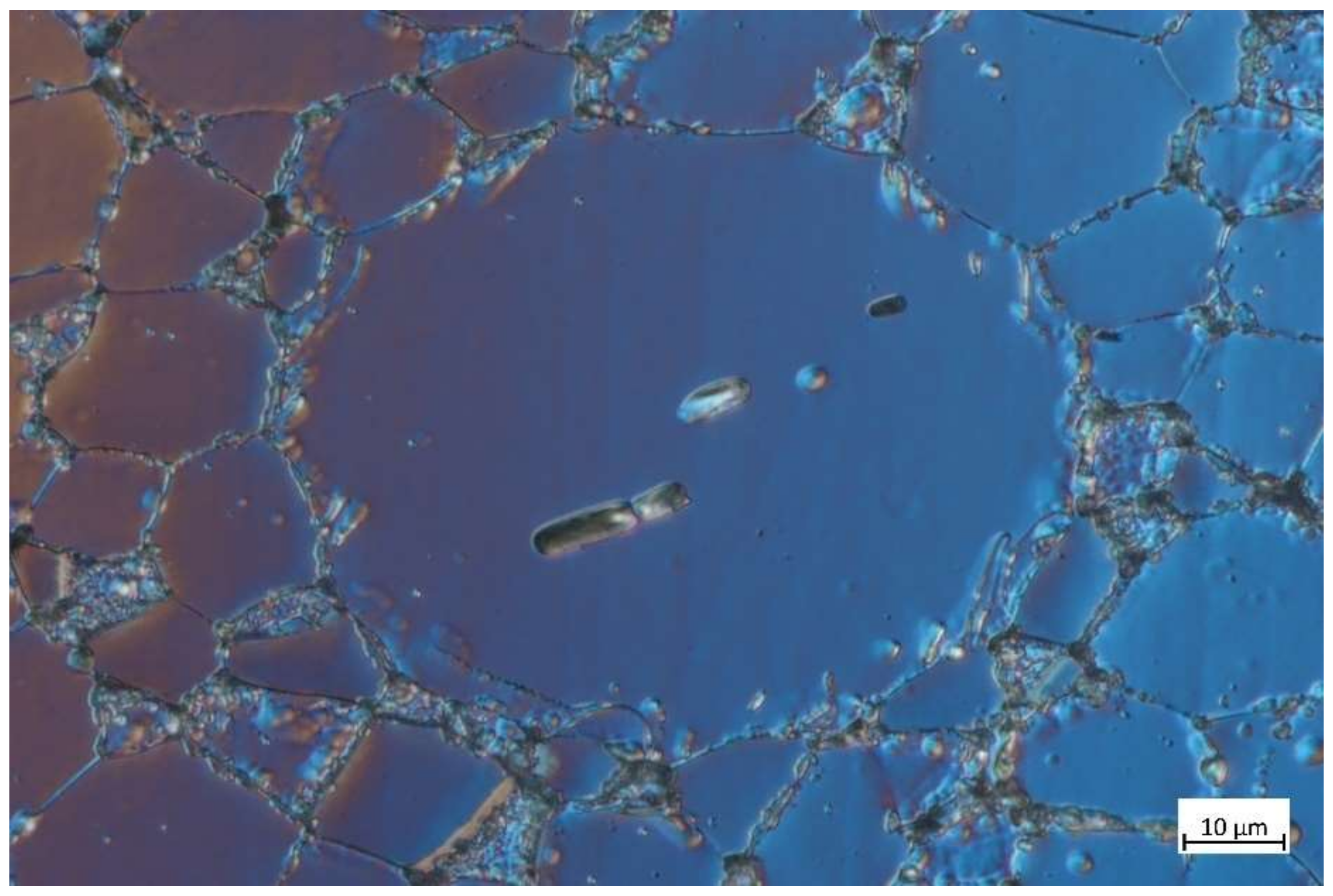
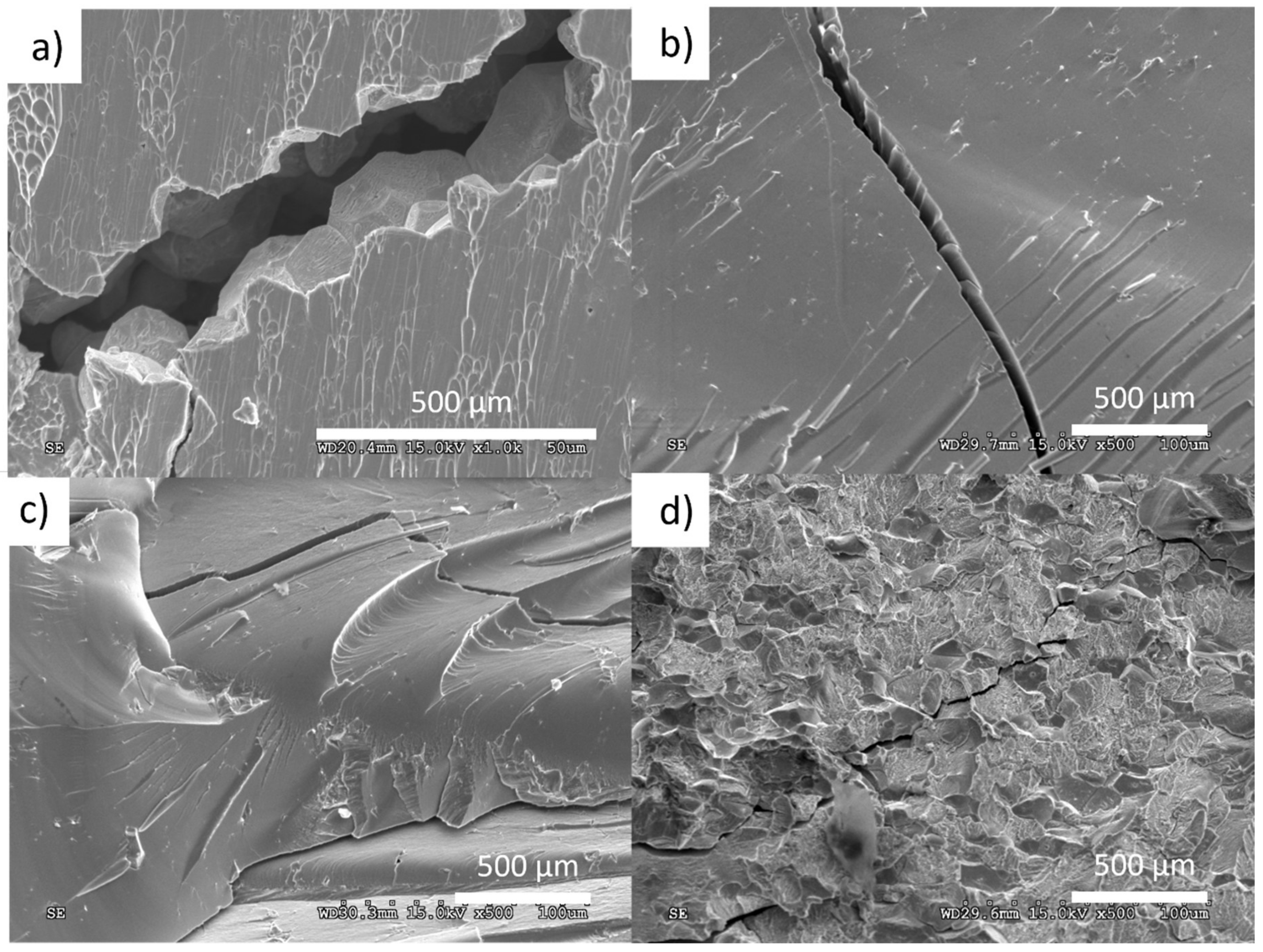
3.2.1. Microstructural Characterization
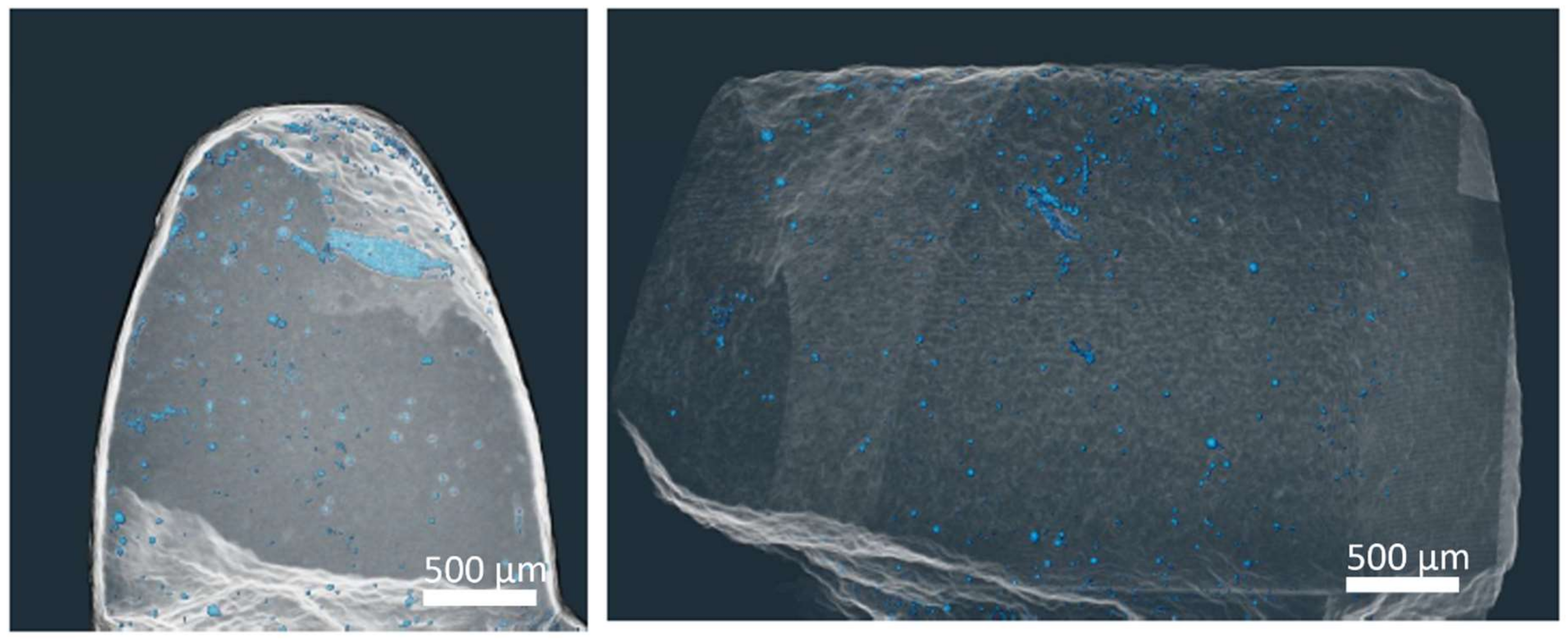
3.2.2. Computer Tomography
3.2.3. XRD Phase Analysis
3.2.4. DSC Thermal Analyses
- (1)
- The processing temperature is in fact higher than that actually measured by the thermocouple, even if it is really close to the sample. However, the electrical current generated during processing, both in graphite mould and powder, may induce a difference between the temperature values inside and outside the mould. In addition, the temperature can be strongly inhomogenous inside the compact as the local electrical resistance depends on the particles’ contact surface area. Therefore, a weak contact results in high local temperature and vice-versa.
- (2)
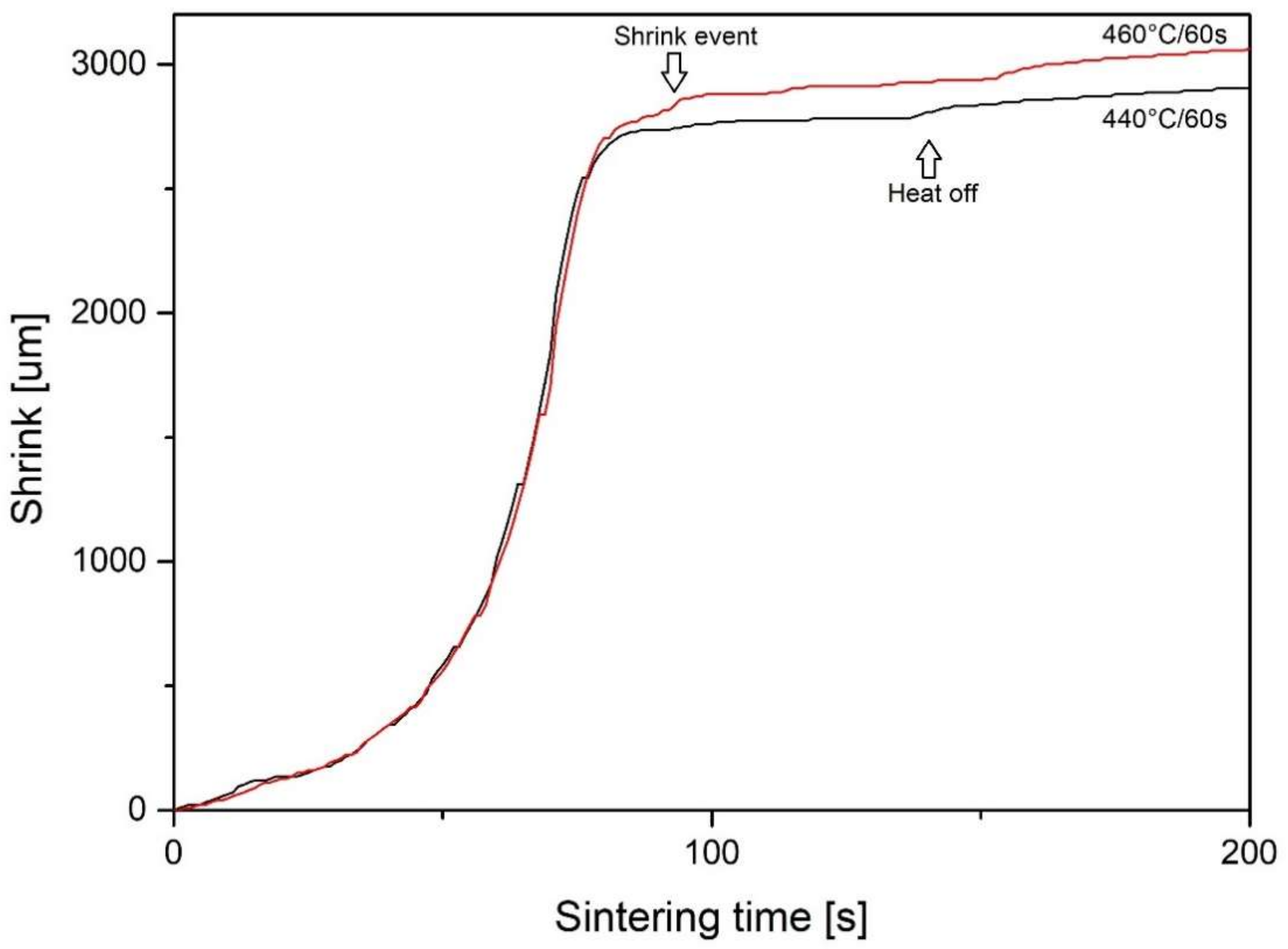
- Sintering is an isothermal process performed for 5 min in Ts, while DSC experiments are performed during continuous heating. Since crystallization is a time and temperature-dependent kinetic phenomenon, it can occur at temperatures lower than Tx due to the influence of time.
- (3)
- During the whole sintering cycle, a pressure of 60 MPa is used. On one hand, the crystalline phase is slightly denser than its glassy counterpart, thus higher pressure should promote crystallization. On the other hand, the pressure tends to limit the diffusion of atoms, usually necessary for crystallization in BMGs. For this reason, the formation of crystalline particles should be delayed. Therefore, this influence is difficult to unveil, yet it still might have occurred.
3.2.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hennicke, J.; Kessel, H.U. Field assisted sintering technology (“FAST”), for the consolidation of innovative materials. In Cfi-Ceramic Forum International; Göller Verlag GmbH: Baden, Germany, 2004; Volume 81, pp. E14–E16. [Google Scholar]
- Kessel, H.U.; Hennicke, J.; Schmidt, J.; Weißgärber, T.; Kieback, B.F.; Herrmann, M.; Räthel, J. “FAST” field assisted sintering technology—A new process for the production of metallic and ceramic sintering materials. In Pulvermetallurgie in Wissenschaft und Praxis 22—Pulvermetallurgie—Kompetenz und Perspektive; Heimdall Verlag: Witten, Germay, 2008. [Google Scholar]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Inoue, A.; Kato, A.; Zhang TG, K.S.; Masumoto, T. Mg–Cu–Y amorphous alloys with high mechanical strengths produced by a metallic mold casting method. Mater. Trans. JIM 1991, 32, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Zhang, T.; Masumoto, T. Al–La–Ni amorphous alloys with a wide supercooled liquid region. Mater. Trans. JIM 1989, 30, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Wang, Y.; Qiang, J.; Dong, C. Bulk metallic glasses in the Zr-Al-Ni-Cu system. Acta Mater. 2003, 51, 1899–1907. [Google Scholar] [CrossRef]
- Waniuk, T.A.; Schroers, J.; Johnson, W.L. Critical cooling rate and thermal stability of Zr–Ti–Cu–Ni–Be alloys. Appl. Phys. Lett. 2001, 78, 1213–1215. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Inoue, A. Thermal and mechanical properties of Ti–Ni–Cu–Sn amorphous alloys with a wide supercooled liquid region before crystallization. Mater. Trans. JIM 1998, 39, 1001–1006. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, J.; Dey, G.K.; Murty, B.S. Thermodynamic and Topological Modeling and Synthesis of Cu-Zr-Ti-Ni–Based Bulk Metallic Glasses by Mechanical Alloying. Metall. Mater. Trans. A 2008, 39, 1543–1551. [Google Scholar] [CrossRef]
- Wei, B.C.; Löser, W.; Xia, L.; Roth, S.; Pan, M.X.; Wang, W.H.; Eckert, J. Anomalous thermal stability of Nd–Fe–Co–Al bulk metallic glass. Acta Mater. 2002, 50, 4357–4367. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. B R Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Xie, G.; Louzguine-Luzgin, D.V.; Kimura, H.; Inoue, A. Nearly full density Ni 52.5 Nb 10 Zr 15 Ti 15 Pt 7.5 bulk metallic glass obtained by spark plasma sintering of gas atomized powders. Appl. Phys. Lett. 2007, 90, 241902. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Drescher, P.; Witte, K.; Yang, B.; Steuer, R.; Kessler, O.; Burkel, E.; Schick, C.; Seitz, H. Composites of amorphous and nanocrystalline Zr–Cu–Al–Nb bulk materials synthesized by spark plasma sintering. J. Alloy. Compd. 2016, 667, 109–114. [Google Scholar] [CrossRef]
- Perrière, L.; Champion, Y.; Bernard, F. Spark plasma sintering of metallic glasses. In Spark Plasma Sintering of Materials; Springer: Cham, Switzerland, 2019; pp. 291–335. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V. Early stage crystallization kinetics in metallic glass-forming alloys. J. Alloy. Compd. 2014, 586, 216–219. [Google Scholar] [CrossRef]
- Paul, T.; Harimkar, S.P. Viscous flow activation energy adaptation by isochronal spark plasma sintering. Scr. Mater. 2017, 126, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Zhang, W.; Louzguine-Luzgin, D.V.; Kimura, H.; Inoue, A. Fabrication of porous Zr–Cu–Al–Ni bulk metallic glass by spark plasma sintering process. Scriptamaterialia 2006, 55, 687–690. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, W.; Louzguine-Luzgin, D.V.; Kimura, H.; Inoue, A. Microstructure and mechanical properties of porous Zr55Cu30Al10Ni5 bulk metallic glass fabricated by spark plasma sintering process. Mater. Trans. 2007, 0706180023. [Google Scholar] [CrossRef]
- Xie, G.; Fukuhara, M.; Louzguine-Luzgin, D.V.; Inoue, A. Ultrasonic characteristics of porous Zr55Cu30Al10Ni5 bulk metallic glass fabricated by spark plasma sintering. Intermetallics 2010, 18, 2014–2018. [Google Scholar] [CrossRef]
- Nowak, S.; Perrière, L.; Dembinski, L.; Tusseau-Nenez, S.; Champion, Y. Approach of the spark plasma sintering mechanism in Zr57Cu20Al10Ni8Ti5 metallic glass. J. Alloy. Compd. 2011, 509, 1011–1019. [Google Scholar] [CrossRef]
- Szlezynger, M.; Maj, Ł.; Pomorska, M.; Morgiel, J.; Jach, K.; Rosinski, M. Effect of upgraded field assisted sintering technology on microstructure of NiAl/CrB2 composites. Compos. Theory Pract. 2020, 20, 7–10. [Google Scholar]
- Heraeus. Amorphous Alloy AMLOY-ZR01. Available online: https://www.heraeus.com/media/media/group/media_group/products/amorphous_metals/datasheets_1/Datasheet_AMLOY-ZR01~2.pdf (accessed on 20 March 2020).
- Perrière, L.; Thai, M.T.; Tusseau-Nenez, S.; Blétry, M.; Champion, Y. Spark Plasma Sintering of a Zr-Based Metallic Glass. Adv. Eng. Mater. 2011, 13, 581–586. [Google Scholar] [CrossRef]
- Hao, T.; Tanimoto, H.; Mizubayashi, H. Transformation to nanocrystallites in amorphous alloys induced by resonant electropulsing. Mater. Trans. 2005, 46, 2898–2907. [Google Scholar] [CrossRef] [Green Version]




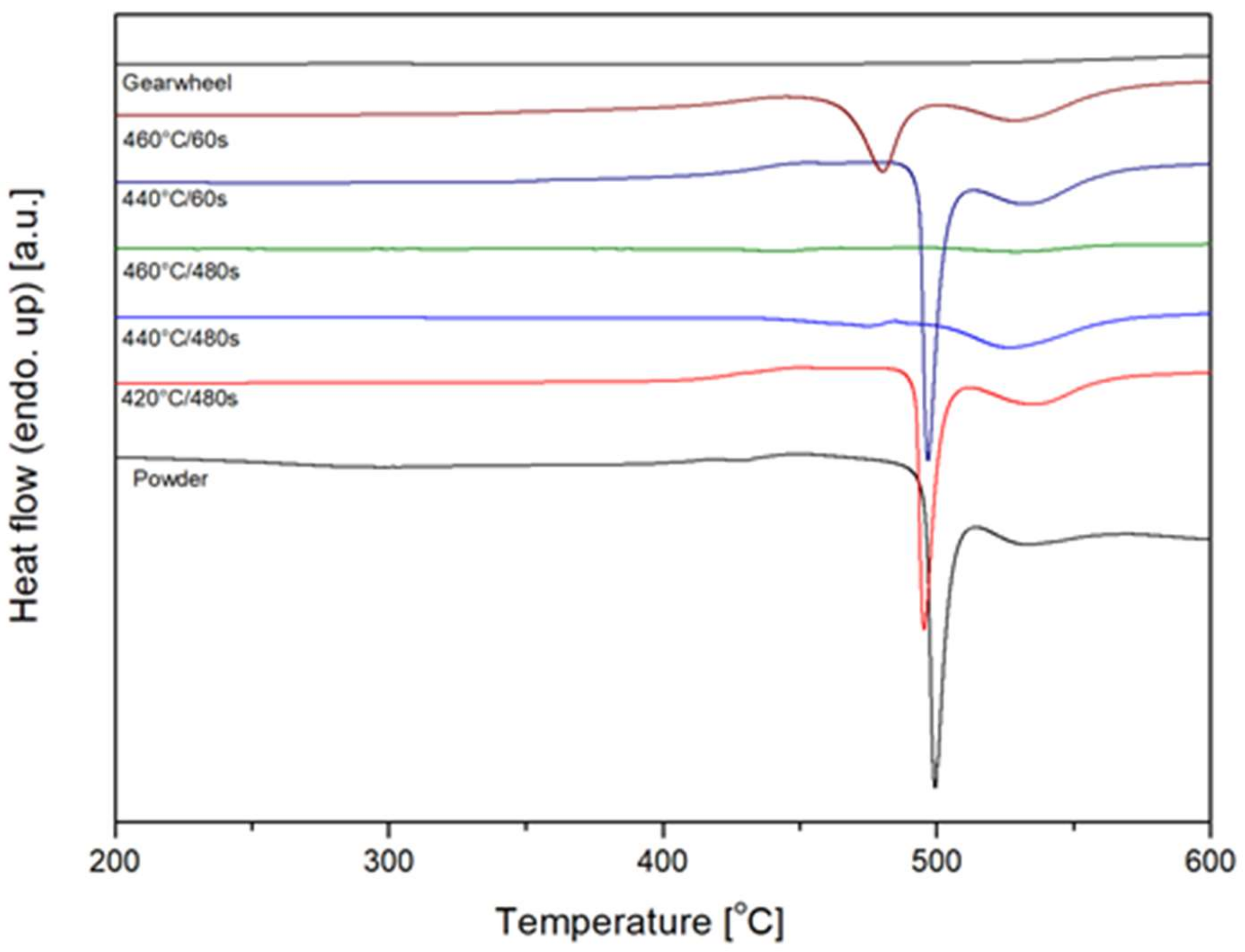
| Sample | Crystallization Enthalpy (J/g) | Crystallization Temperature (Peak) (°C) |
|---|---|---|
| Powder | 57.6 | 500 |
| 420 °C/480 s | 56.4 | 496 |
| 440 °C/480 s | 13.7 | x |
| 460 °C/480 s | 4.7 | x |
| 440 °C/60 s | 56.4 | 496 |
| 460 °C/60 s | 41.7 | 480 |
| Gear wheel | 0 | x |
| Sample | Density (g/cm3) | Relative Density (%) | Amorphous Content (%) | Hardness (HV1) | Compressive Strength (MPa) |
|---|---|---|---|---|---|
| 380 °C/480 s | 4.563 | 68.2% | ~100% | x | x |
| 400 °C/480 s | 5.747 | 85.9% | ~100% | x | x |
| 420 °C/480 s | 6.658 | 99.5% | ~100% | 519 ± 13 | 1551 ± 39 |
| 440 °C/480 s | 6.766 | 101.1%* | 24 | 610 ± 3 | 1097 ± 54 |
| 460 °C/480 s | 6.779 | 101.35%* | 8 | 633 ± 8 | 1052 ± 85 |
| 440 °C/60 s | 6.691 | ~100% | ~100% | 519 ± 5 | 1625 ± 31 |
| 460 °C/60 s | 6.712 | 100.3%* | 72 | 554 ± 13 | 1409 ± 46 |
| Heraeus Cast sample | 6.68 | 100% | 100% | 480 | 1700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żrodowski, Ł.; Wróblewski, R.; Choma, T.; Rygier, T.; Rosiński, M.; Morończyk, B.; Kasonde, M.; Leonowicz, M.; Jaroszewicz, J.; Ostrysz, M.; et al. Ultrashort Sintering and Near Net Shaping of Zr-Based AMZ4 Bulk Metallic Glass. Materials 2021, 14, 5862. https://doi.org/10.3390/ma14195862
Żrodowski Ł, Wróblewski R, Choma T, Rygier T, Rosiński M, Morończyk B, Kasonde M, Leonowicz M, Jaroszewicz J, Ostrysz M, et al. Ultrashort Sintering and Near Net Shaping of Zr-Based AMZ4 Bulk Metallic Glass. Materials. 2021; 14(19):5862. https://doi.org/10.3390/ma14195862
Chicago/Turabian StyleŻrodowski, Łukasz, Rafał Wróblewski, Tomasz Choma, Tomasz Rygier, Marcin Rosiński, Bartosz Morończyk, Maweja Kasonde, Marcin Leonowicz, Jakub Jaroszewicz, Mateusz Ostrysz, and et al. 2021. "Ultrashort Sintering and Near Net Shaping of Zr-Based AMZ4 Bulk Metallic Glass" Materials 14, no. 19: 5862. https://doi.org/10.3390/ma14195862