Process Kinetics of the Carbonation of Fly Ashes: A Research Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
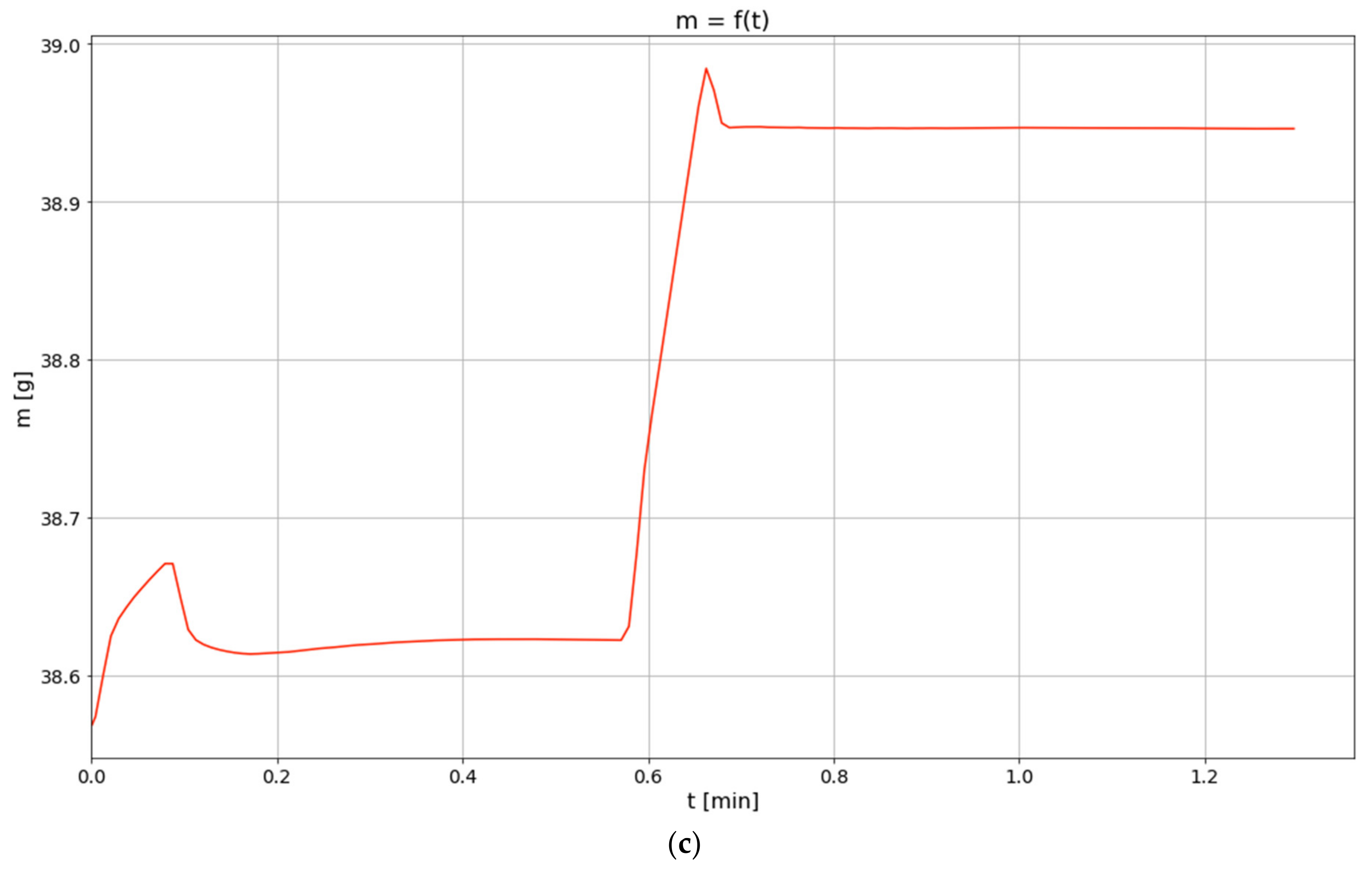
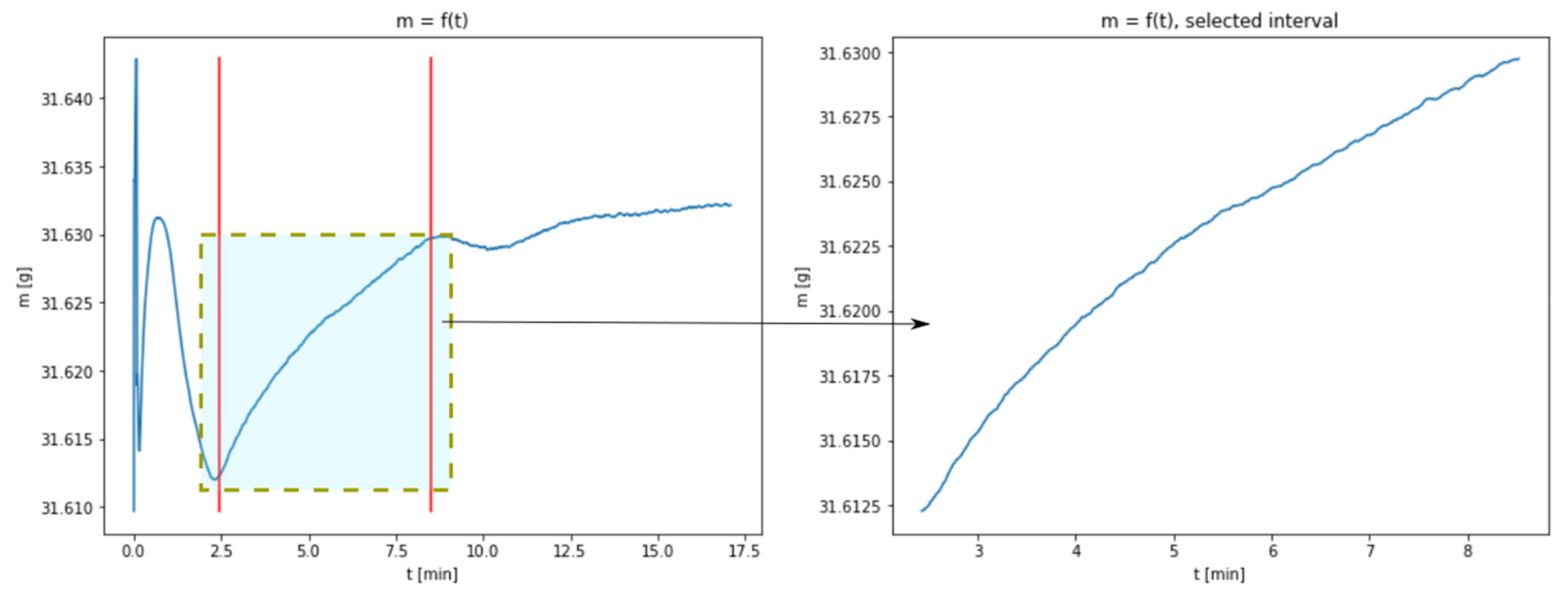
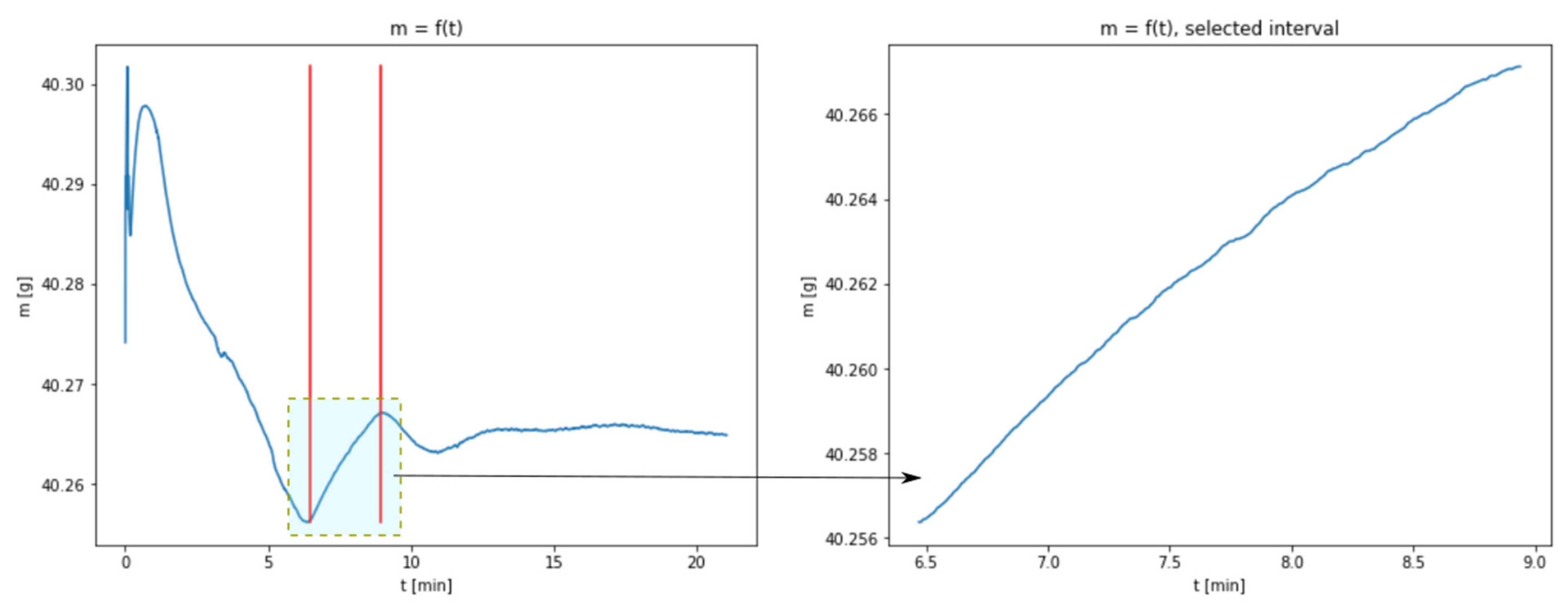
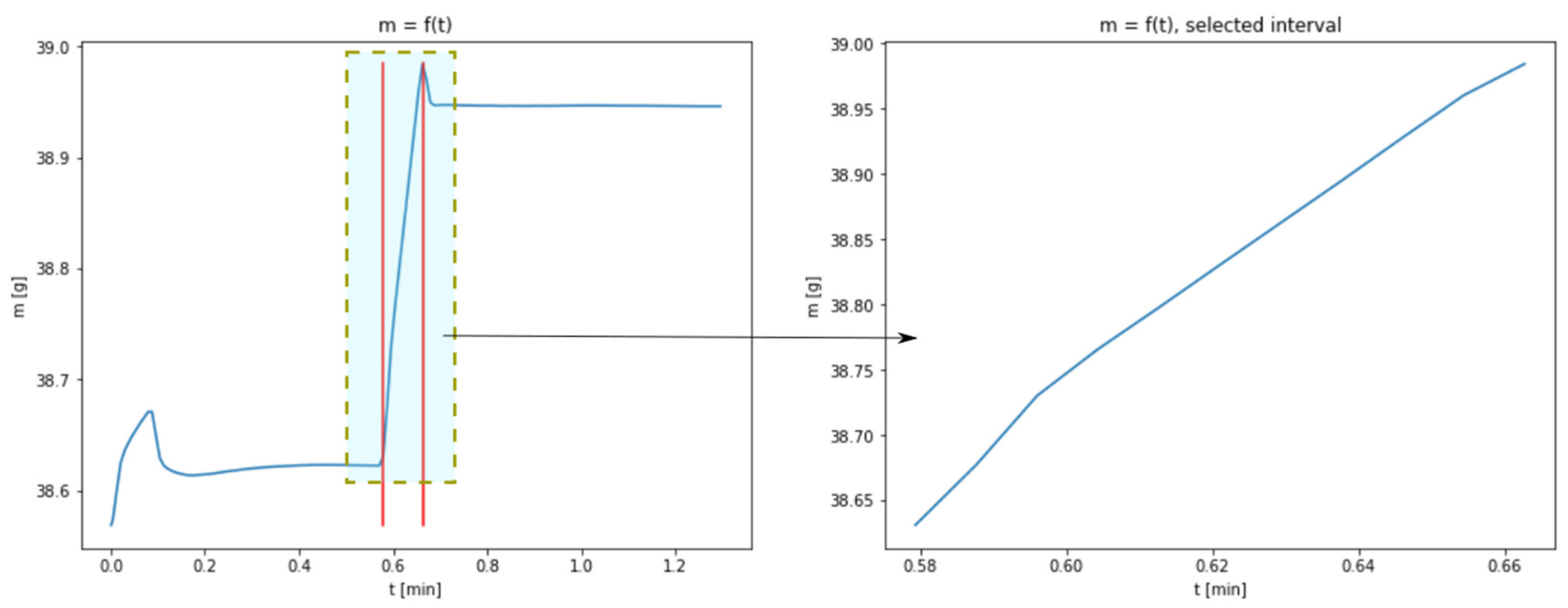
3.1. Results of Thermogravimetric Analyses
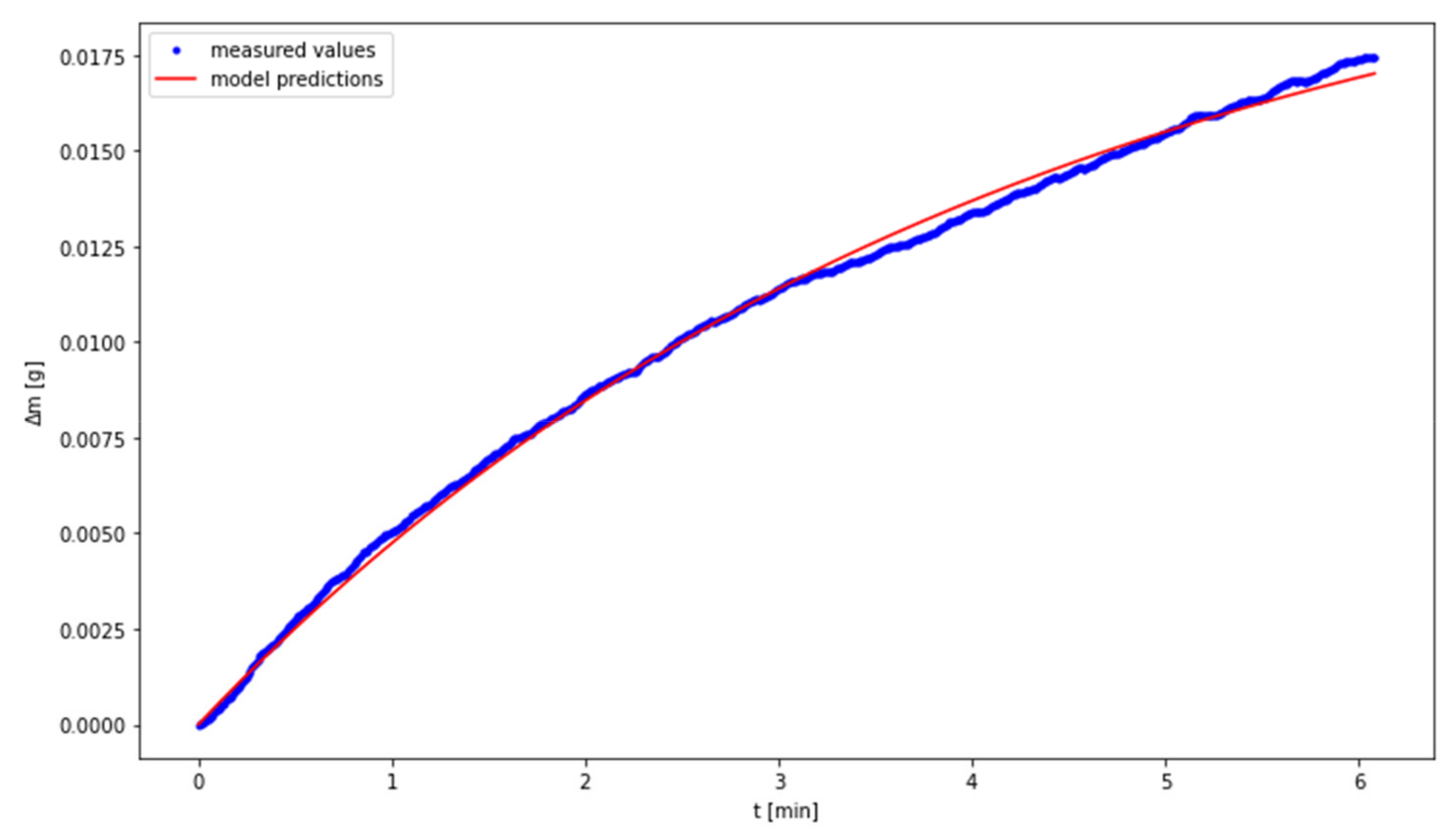
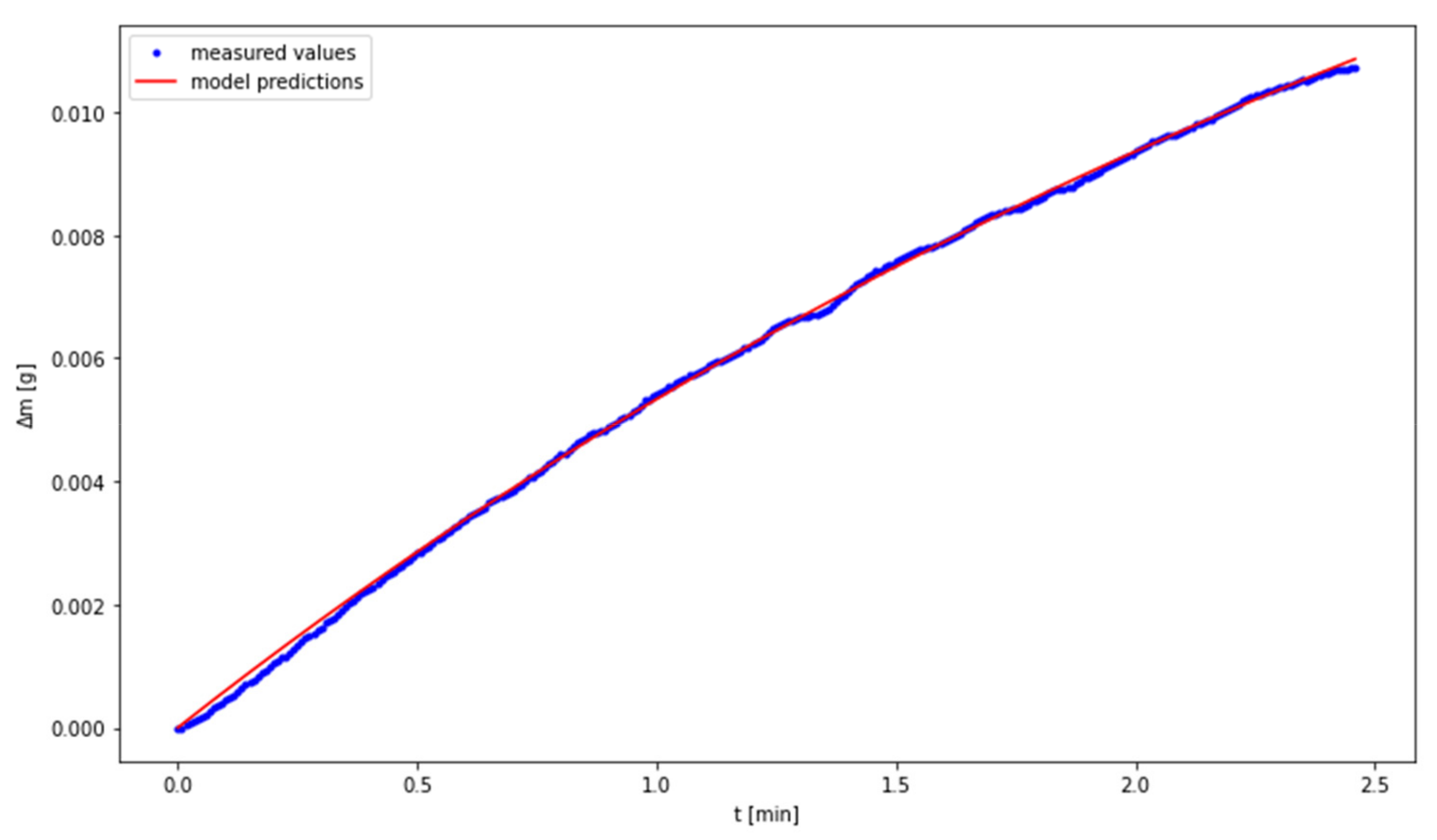
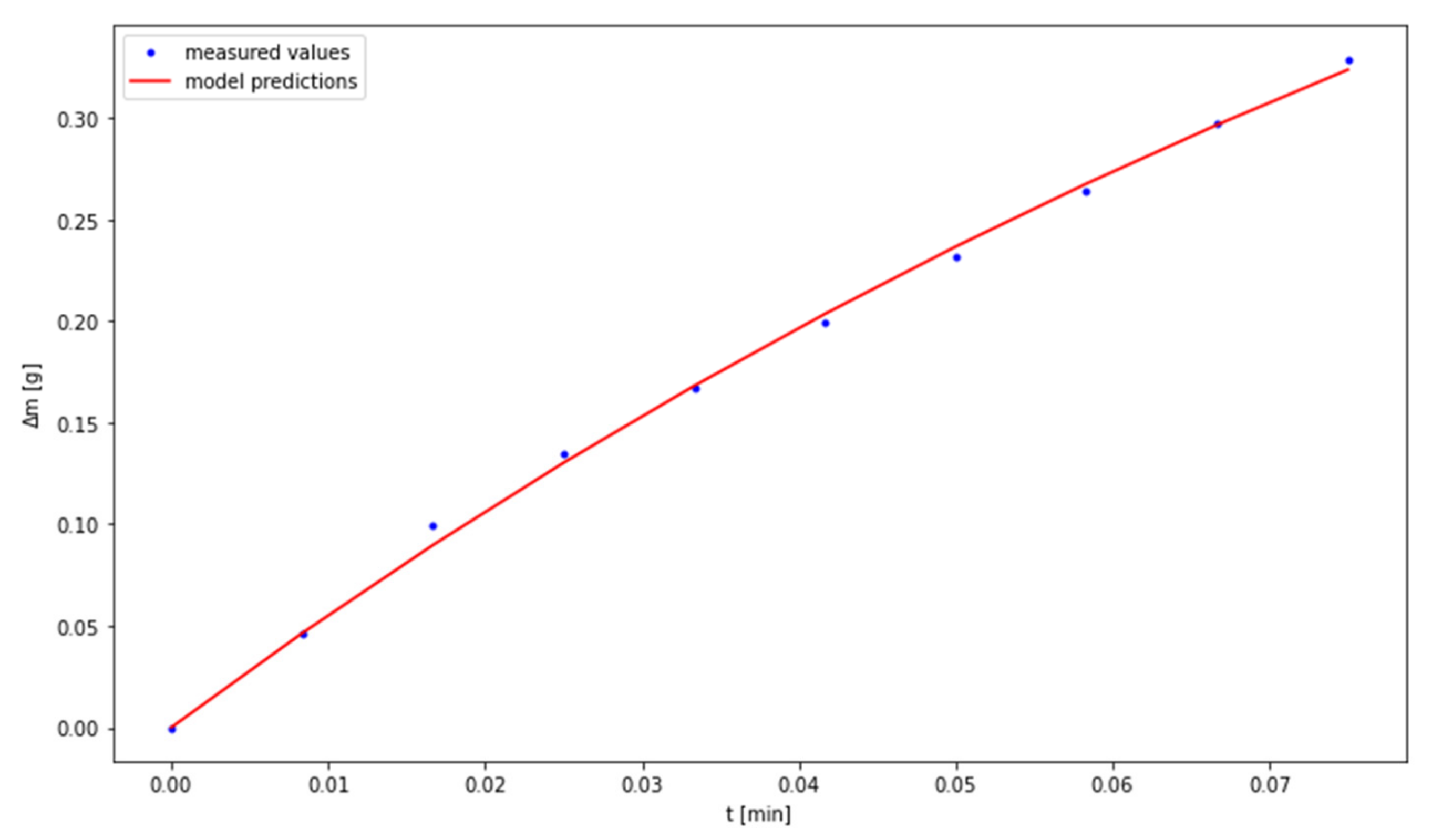
3.2. The Use of a Dynamic Model Describing the Carbonation Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EPRI. Evaluation of Processes for Post Combustion Control of Emissions from Circulating Fluidized-Bed Power Plants; EPRI: Palo Alto, CA, USA, 2003; p. 1004883. [Google Scholar]
- EPRI. Developments in Circulating Fluidized-Bed Combustion Technology; EPRI: Palo Alto, CA, USA, 2009; p. 1015695. [Google Scholar]
- Romanov, V.; Soong, Y.; Carney, C.; Rush, G.; Nielsen, B.; O’Connor, W. Mineralization of Carbon Dioxide: Literature Review. ChemBioEng Rev. 2015, 2, 231–256. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andrés, J.M.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicová, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- EPRI. Coal Ash: Characteristics, Management, and Environmental Issues. Technical Results, Program: Coal Combustion Products; EPRI: Palo Alto, CA, USA, 2009; p. 1019022. [Google Scholar]
- Norm EN 450-1:2005+A1 Fly Ash for Concrete—Part 1.
- Smoliński, A.; Stempin, M.; Howaniec, N. Determination of Rare Earth Elements in Combustion Ashes from Selected Polish Coal Mines by Wavelength Dispersive X-ray Fluorescence Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2016, 116, 63–74. [Google Scholar] [CrossRef]
- Regulations of the European Parliament and the Council of Europe (WE) No. 1272/2008.
- Anthony, E.J.; Jia, L.; Caris, M.; Preto, F.; Burwell, S. An examination of the exothermic nature of fluidized bed combustion (FBC) residues. Waste Manag. 1999, 19, 293–305. [Google Scholar] [CrossRef]
- Gogola, K.; Rogala, T.; Magdziarczyk, M.; Smoliński, A. The mechanisms of endogenous fires occurring in extractive waste dumping facilities. Sustainability 2020, 12, 2856. [Google Scholar] [CrossRef] [Green Version]
- Chećko, J.; Urych, T.; Magdziarczyk, M.; Smoliński, A. Resource Assessment and Numerical Modeling of CBM Extraction in the Upper Silesian Coal Basin, Poland. Energies 2020, 13, 2153. [Google Scholar] [CrossRef]
- Smoliński, A.; Stańczyk, K.; Kapusta, K.; Howaniec, N. Analysis of the organic contaminants in the condensate produced in the in-situ underground coal gasification process. Water Sci. Technol. 2013, 67, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Bapat, J.D. Mineral Admixtures in Cement and Concrete; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- EN 197–1: 2011, IDT.; Cement—Part 1: Composition, specifications and conformity for common cements 2011.
- Krzemień, A.; Więckol-Ryk, A.; Smolinski, A.; Koteras, A.; Więcław-Solny, L. Assessing the risk of corrosion in amine-based CO2 Capture Process. J. Loss Prev. Process Ind. 2016, 43, 189–197. [Google Scholar] [CrossRef]
- Anthony, E.J.; Jia, L.; Wu, Y.; Caris, M. CFBC Ash Hydratation Studies. Fuel 2005, 84, 1393–1397. [Google Scholar] [CrossRef]
- Gunning, P.; Hills, C.; Carey, P. Accelerated carbonation treatment of industrial wastes. Waste Manag. 2010, 30, 1081–1090. [Google Scholar] [CrossRef]
- Bauer, M.; Gassen, N.; Stanjek, H.; Peiffer, S. Carbonation of lignite fly ash at ambient T and P in a semi-dry reaction system for CO2 sequestration. Appl. Geochem. 2011, 26, 1502–1512. [Google Scholar] [CrossRef]
- Uliasz-Bochenczyk, A.; Mokrzycki, E.; Piotrowski, Z.; Pomykala, R. Estimation of CO2 sequestration potential via mineral carbonation in fly ash from lignite combustion in Poland. Energy Procedia 2009, 1, 4873–4879. [Google Scholar] [CrossRef] [Green Version]
- Montes-Hernandez, G.; Pérez-López, R.; Renard, F.; Nieto, J.; Charlet, L. Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J. Hazard. Mater. 2009, 161, 1347–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howaniec, N.; Smolinski, A.; Cempa-Balewicz, M. Experimental study of nuclear high temperaturę reactor excess heat use in the coal and energy crops co-gasification process to hydrogen-rich gas. Energy 2015, 84, 455–461. [Google Scholar] [CrossRef]
- Uliasz-Bochenczyk, A. Mineral Sequestration of CO2 Using Water Suspensions of Selected Fly Ashes from the Combustion of Lignite Coal. Miner. Resour. Manag. 2011, 27, 145–153. [Google Scholar]
- Ukwattage, N.; Ranjith, P.; Wang, S. Investigation of the potential of coal combustion fly ash for mineral sequestration of CO2 by accelerated carbonation. Energy 2013, 52, 230–236. [Google Scholar] [CrossRef]
- Rao, A.; Anthony, E.J.; Jia, L.; Macchi, A. Carbonation of FBC ash by sonochemical treatment. Fuel 2007, 86, 2603–2615. [Google Scholar] [CrossRef]
- Baron, J.; Douvare, C. Technical and economic aspects of the use of limestone filler additions in cement. World Cem. 1987, 18, 100–104. [Google Scholar]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydratation. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland Cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Stefanoni, M.; Maltese, C.; Pistolesi, C.; Bravo, A. Effect of calcium-carbonate-based aggregates on alkali-free accelerators. Adv. Cem. Res. 2014, 26, 194–204. [Google Scholar] [CrossRef]
- Łączny, M.J.; Iwaszenko, S.; Gogola, K.; Bajerski, A.; Janoszek, T.; Klupa, A.; Cempa-Balewicz, M. Study on the possibilities of treatment of combustion by-products from fluidized bed boilers into a product devoid of free calcium oxide. J. Sustain. Min. 2015, 14, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Łączny, M.J.; Majka, G.; Cempa-Balewicz, M. Study on the impact of fluidized bed ash processed by carbonation on mechanical properties of cement mortar. Cem. Wapno Beton. 2016, 21, 265–273. [Google Scholar]
- Łączny, M.J.; Bzowski, Z. Transformations of calcium sulphates in solidified carbonated volatile fluidized ashes. J. Sustain. Min. 2017, 16, 151–155. [Google Scholar] [CrossRef]
- Scott, F. Elements of Chemical Reaction Engineering; Prentice Hall Profesional: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Anthony, E.J.; Granatstein, D.L. Sulfation phenomena in fluidized bed combustion systems. Prog. Energy Combust. Sci. 2001, 27, 215–236. [Google Scholar] [CrossRef]
- Romano, M. Coal-fired power plant with calcium oxide carbonation for postcombustion CO2 capture. Energy Procedia 2009, 1, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Grasaa, G.; Martineza, I.; Diegob, M.E.; Abanades, J.C. Determination of CaO carbonation kinetics under re-carbonation. Energy Fuels 2014, 28, 4033–4042. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, M.; Tremain, P.; Doroodchi, E.; Moghtaderi, B. Determination of carbonation/calcination reaction kinetics of a limestone sorbent in low CO2 partial pressures using TGA Experiments. Energy Procedia 2017, 114, 259–270. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Chiriac, R.; Toche, F.; Renardl, F. Gas-solid carbonation of Ca(OH)2 and CaO particles under non-isothermal conditions by using a thermogravimetric analyzer: Implications for CO2 capture. Int. J. Greenh. Gas Control. 2012, 11, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.Y.; Kim, J.H.; Lee, Y.J.; Lee, M.; Choh, S.J. Evaluation of factors affecting mineral carbonation of CO2 using coal fly ash in aqueous solutions under ambient conditions. Chem. Eng. J. 2012, 183, 77–87. [Google Scholar] [CrossRef]
- Bertos, M.F.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, B112, 193–205. [Google Scholar]

| No | Ash Component | Content (wt.%) |
|---|---|---|
| 1 | SiO2 | 36.02 |
| 2 | Al2O3 | 21.02 |
| 3 | Fe2O3 | 6.41 |
| 4 | CaO (including free CaO) | 18.02 (4.18) |
| 5 | MgO | 2.10 |
| 6 | BaO | 0.07 |
| 7 | P2O5 | 0.33 |
| 8 | Na2O | 0.91 |
| 9 | K2O | 2.15 |
| 10 | SO3 | 9.12 |
| 11 | TiO2 | 0.63 |
| 12 | LOI | 2.55 |
| Examined Samples | CaOw (wt.%) |
|---|---|
| Fly ash—on the day of delivery | 4.18 |
| Fly ash after 18 days of storage | 4.03 |
| Fly ash after 36 days of storage | 3.68 |
| Bottom ash—on the day of delivery | 2.76 |
| Parameter | Unit | Value |
|---|---|---|
| Characteristic grain d | d10 * (µm) | 1.23 |
| d50 * (µm) | 2.86 | |
| d90 * (µm) | 7.60 | |
| Averaged diameter | (µm) | 4.15 |
| Standard deviation | (µm) | 4.93 |
| Constant | 40 °C | 60 °C | 80 °C |
|---|---|---|---|
| m0 [g] | 0.02815867 | 0.02773818 | 0.91305838 |
| k [min−1] | 0.24125613 | 0.28122913 | 8.02458336 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łączny, M.J.; Iwaszenko, S.; Smoliński, A. Process Kinetics of the Carbonation of Fly Ashes: A Research Study. Materials 2021, 14, 253. https://doi.org/10.3390/ma14020253
Łączny MJ, Iwaszenko S, Smoliński A. Process Kinetics of the Carbonation of Fly Ashes: A Research Study. Materials. 2021; 14(2):253. https://doi.org/10.3390/ma14020253
Chicago/Turabian StyleŁączny, Marian Jacek, Sebastian Iwaszenko, and Adam Smoliński. 2021. "Process Kinetics of the Carbonation of Fly Ashes: A Research Study" Materials 14, no. 2: 253. https://doi.org/10.3390/ma14020253