One-Pot Synthesis of Al-P-O Catalysts and Their Catalytic Properties for O-Methylation of Catechol and Methanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Performance Evaluation
3. Results and Discussion
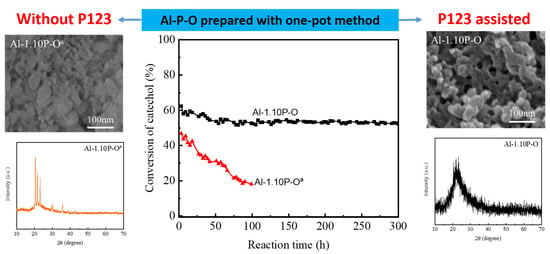
3.1. Catalyst Characterization
3.2. Catalytic Performance Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.; Zhou, W.; Seshan, K.; Li, Y. Green and efficient synthesis route of catechol from guaiacol. J. Mol. Catal. A: Chem. 2013, 368, 61–65. [Google Scholar] [CrossRef]
- Tran, N.T.; Uemura, Y.; Chowdhury, S.; Ramli, A. Vapor-phase hydrodeoxygenation of guaiacol on Al-MCM-41 supported Ni and Co catalysts. Appl. Catal. A: Gen. 2016, 512, 93–100. [Google Scholar] [CrossRef]
- Mao, J.B.; Zhou, J.X.; Xia, Z.; Wang, Z.G.; Xu, Z.W.; Xu, W.J.; Yan, P.F.; Liu, K.R.; Guo, X.W.; Zhang, Z.C. Anatase TiO2 activated by gold nanoparticles for selective hydrodeoxygenation of guaiacol to phenolics. ACS Catal. 2017, 7, 695–705. [Google Scholar] [CrossRef]
- Nair, V.; Vinu, R. Production of guaiacols via catalytic fast pyrolysis of alkali lignin using titania, zirconia and ceria. J. Anal. Appl. Pyrolysis 2016, 119, 31–39. [Google Scholar] [CrossRef]
- Kakhlon, O.; Ferreira, I.; Solmesky, L.J.; Khazanov, N.; Lossos, A.; Alvarez, R.; Yetil, D.; Pampou, S.; Weil, M.; Senderowitz, H.; et al. Guaiacol as a drug candidate for treating adult polyglucosan body disease. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Torregrosa, L.; Zalacain, A.; Ojeda, H.; Bouckenooghe, V.; Schneider, R.; Alonso, G.L.; Salinas, M.R. Behavior of glycosylated aroma precursors in Microvine fruits after guaiacol foliar application. Sci. Hortic. 2019, 246, e1–e8. [Google Scholar] [CrossRef]
- Pardo-Garcia, A.I.; Wilkinson, K.L.; Culbert, J.A.; Lloyd, N.D.; Alonso, G.L.; Salinas, M.R. Accumulation of guaiacol glycoconjugates in fruit, leaves and shoots of Vitis vinifera cv. Monastrell following foliar applications of guaiacol or oak extract to grapevines. Food Chem. 2017, 217, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From lignin-derived aromatic compounds to novel biobased polymers. Macromol. Rapid Commun. 2015, 37, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Zhang, C.; Meng, T.; Wang, H.; Hu, X.; Xiao, Z.; Wang, C.; Liu, J. Effect and mechanism of aluminum(III) For guaiacol–glyoxylic acid condensation reaction in vanillin production. ACS Omega 2020, 5, 24526–24536. [Google Scholar] [CrossRef]
- García-Bofill, M.; Sutton, P.W.; Guillén, M.; Álvaro, G. Enzymatic synthesis of vanillin catalysed by an eugenol oxidase. Appl. Catal. A: Gen. 2019, 582, 117117. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Cheng, Y.; Sun, H.; Bai, S.; Li, C. Identifying environmental hotspots and improvement strategies of vanillin production with life cycle assessment. Sci. Total. Environ. 2021, 769, 144771. [Google Scholar] [CrossRef]
- Navaruckiene, A.; Bridziuviene, D.; Raudoniene, V.; Rainosalo, E.; Ostrauskaite, J. Influence of vanillin acrylate-based resin composition on resin photocuring kinetics and antimicrobial properties of the resulting polymers. Materials 2021, 14, 653. [Google Scholar] [CrossRef]
- Bal, R.; Tope, B.B.; Sivasanker, S. Vapour phase O-methylation of dihydroxy benzenes with methanol over cesium-loaded silica, a solid base. J. Mol. Catal. A Chem. 2002, 181, 161–171. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Balakrishna, G.; Rajesh, B.; Jayasri, V.; Sikhwivhilu, L.M.; Coville, N.J. Alkylation of catechol with methanol to give guaiacol over sulphate-modified zirconia solid acid catalysts: The influence of structural modification of zirconia on catalytic performance. Catal. Commun. 2008, 9, 2422–2427. [Google Scholar] [CrossRef]
- Fu, Y.; Baba, T.; Ono, Y. Vapor-phase reactions of catechol with dimethyl carbonate. Part II. Selective synthesis of guaiacol over alumina loaded with alkali hydroxide. Appl. Catal. A: Gen. 1998, 166, 425–430. [Google Scholar] [CrossRef]
- Jafari, A.A.; Khodadadi, A.A.; Mortazavi, Y. Vapor-phase selective O-alkylation of catechol with methanol over lanthanum phosphate and its modified catalysts with Ti and Cs. J. Mol. Catal. A: Chem. 2013, 372, 79–83. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Zou, X.; Wang, Y.; Jia, M.; Zhang, W. Supported ammonium metatungstate as highly efficient catalysts for the vapour-phase O-methylation of catechol with methanol. Catal. Commun. 2006, 7, 579–582. [Google Scholar] [CrossRef]
- Jyothi, T.; Raja, T.; Talawar, M.; Rao, B. Selective O-methylation of catechol using dimethyl carbonate over calcined Mg Al hydrotalcites. Appl. Catal. A: Gen. 2001, 211, 41–46. [Google Scholar] [CrossRef]
- Lui, M.Y.; Lokare, K.S.; Hemming, E.; Stanley, J.N.G.; Perosa, A.; Selva, M.; Masters, A.F.; Maschmeyer, T. Microwave-assisted methylation of dihydroxybenzene derivatives with dimethyl carbonate. RSC Adv. 2016, 6, 58443–58451. [Google Scholar] [CrossRef]
- Kabra, S.K.; Huuhtanen, M.; Keiski, R.L.; Yadav, G.D. Selectivity engineering of O-methylation of hydroxybenzenes with dimethyl carbonate using ionic liquid as catalyst. React. Chem. Eng. 2016, 1, 330–339. [Google Scholar] [CrossRef]
- Vijayaraj, M.; Gopinath, C. Selective production of methoxyphenols from dihydroxybenzenes on alkali metal ion-loaded MgO. J. Catal. 2006, 243, 376–388. [Google Scholar] [CrossRef]
- Liao, X.Z.; Zhou, Z.; Wang, Z.L.; Zou, X.J.; Liu, G.; Jia, M.J.; Zhang, W.X. Preformed precursor of microporous alumino-phosphate coating on mesoporous SBA-15: Synthesis, characterization, and catalytic property for selective O-methylation of catechol. J. Colliod Interface Sci. 2007, 308, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.; Campelo, J.M.; Conesa, T.D.; Luna, D.; Marinas, J.M.; Romero, A.A. Catechol O-methylation with dimethyl car-bonate over different acid-base catalysts. N. J. Chem. 2006, 30, 1228–1234. [Google Scholar] [CrossRef]
- Mao, H.F.; Li, X.L.; Xu, F.; Xiao, Z.B.; Zhang, W.X.; Meng, T. Vapour-phase selective O-methylation of catechol with methanol over metal phosphate catalysts. Catalysts 2021, 11, 531. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, W.X.; Liu, G.; Jiang, L.; Zhu, X.M.; Pan, C.L.; Jiang, D.Z.; Tang, A.Q. Effect of the P/Al ratio of Al-P-O on the catalytic activity of O-methylation of catechol with methanol. React. Kinet. Catal. Lett. 2003, 79, 365–371. [Google Scholar] [CrossRef]
- Zhu, X.M.; Li, X.M.; Jia, M.J.; Liu, G.; Zhang, W.X.; Jiang, D.Z. Vapour-phase selective O-methylation of catechol with methanol over Ti-containing aluminium phosphate catalysts. Appl. Catal. A: Gen. 2005, 282, 155–161. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.L.; Jia, M.J.; Zou, X.J.; Zhu, X.M.; Zhang, W.X.; Jiang, D.Z. Thermally stable amorphous mesoporous alumi-nophosphates with controllable P/Al ratio: Synthesis, characterization, and catalytic performance for selective O-methylation of catechol. J. Phys. Chem. B 2006, 110, 16953–16960. [Google Scholar] [CrossRef]
- Yang, L.; Seshan, K.; Li, Y. Transetherification of guaiacol to o-ethoxyphenol with gamma Al2O3 as a catalyst in supercritical ethanol. Catal. Commun. 2012, 30, 36–39. [Google Scholar] [CrossRef]
- Fu, Z.H.; Yu, Y.; Yin, D.L.; Xu, Y.Z.; Liu, H.P.; Liao, H.Y.; Xu, Q.; Tan, F.Q.; Wang, J. Vapor-phase highly selective O-methylation of catechol with methanol over ZnCl2 modified gamma-Al2O3 catalysts. J. Mol. Catal. A Chem. 2005, 232, 69–75. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Raghavan, K.V.; Ndou, S.; Sikhwivhilu, L.; Plint, N.; Coville, N.J. Evidence for weak base site participation in the vapour phase methylation of catechol over solid base catalysts. Chem. Commun. 2001, 893–894. [Google Scholar] [CrossRef]
- Harish, N.; Kathyayini, N.; Nagaraju, N. Studies on the catalytic activity of mesoporous alumina-aluminophosphate (Al2O3-AlPO4) materials in the synthesis of NN’-diphenyl urea. React. Kinet. Mech. Cat. 2018, 125, 937–949. [Google Scholar] [CrossRef]
- Campelo, J.M.; Jaraba, M.; Luna, D.; Luque, R.; Marinas, A.J.M.; Romero, A.A.; Navío, J.A.; Macias, M. Effect of phosphate precursor and organic additives on the structural and catalytic properties of amorphous mesoporous alpo4materials. Chem. Mater. 2003, 15, 3352–3364. [Google Scholar] [CrossRef]
- Liao, Y.; Li, F.; Dai, X.; Zhao, N.; Xiao, F. Solid base catalysts derived from Ca-M-Al (M = Mg, La, Ce, Y) layered double hydroxides for dimethyl carbonate synthesis by transesterification of methanol with propylene carbonate. Chin. J. Catal. 2017, 38, 1860–1869. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, M.; Shangguan, L. Synthesis of a novel and salt sensitive superabsorbent hydrogel using soybean dregs by UV-irradiation. Materials 2018, 11, 2198. [Google Scholar] [CrossRef] [Green Version]
- Seesanong, S.; Boonchom, B.; Chaiseeda, K.; Boonmee, W.; Laohavisuti, N. Conversion of bivalve shells to monocalcium and tricalcium phosphates: An approach to recycle seafoodwastes. Materials 2021, 14, 4395. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, Y.Y.; Liu, W.R.; Cai, H.H.; Lv, J.H.; Liu, J.D. Amorphous magnesium substituted mesoporous alumino-phosphate: An acid-base sites synergistic catalysis for transesterification of diethyl carbonate and dimethyl carbonate in fixed-bed reactor. Microporous Mesoporous Mater. 2020, 292, 109757. [Google Scholar] [CrossRef]
- Chada, R.R.; Ketike, T.; Boilla, A.R.; Gangadharam, S.D.; Kamaraju, S.R.R.; Burri, D.R. Preparation and characterization of lanthanum phosphate catalysts for O-methylation of phenol to anisole in gas phase. Mol. Catal. 2017, 438, 224–229. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Ruaux, V.; Massin, L.; Lorentz, C.; Afanasiev, P.; Maugé, F.; Bellière-Baca, V.; Rey, P.; Millet, J. Synthesis, characterization and study of lanthanum phosphates as light alcohols dehydration catalysts. Appl. Catal. B: Environ. 2014, 166, 432–444. [Google Scholar] [CrossRef]






| Catalysts | AlPO4 Particle Size (nm) by XRD | SBET (m2·g−1) | VP (cm3·g−1) | Da (nm) | Total Acidity (μmol·g−1) | Total Basicity (μmol·g−1) |
|---|---|---|---|---|---|---|
| Al-0P-O | ─ | 249 | 1.19 | 14.9 | 27.14 | 34.06 |
| Al-0.25P-O | ─ | 319 | 2.03 | 21.6 | 40.45 | 32.99 |
| Al-0.50P-O | ─ | 273 | 1.72 | 23.7 | 56.16 | 28.75 |
| Al-0.75P-O | ─ | 220 | 1.40 | 22.6 | 82.63 | 46.63 |
| Al-1.00P-O | ─ | 176 | 1.25 | 22.9 | 56.65 | 35.70 |
| Al-1.10P-O | ─ | 147 | 1.08 | 23.9 | 53.44 | 21.61 |
| Al-1.10P-Oa | 37.8 | 99 | 0.47 | 15.2 | 21.96 | 13.17 |
| Al-1.15P-O | 30.9 | 30 | 0.10 | 14.2 | 5.76 | 4.15 |
| Al-1.20P-O | 34.3 | 23 | 0.05 | 10.7 | 4.20 | 3.79 |
| Catalysts | Conversion of Catechol (%) | Selectivity (%) | Yield (%) | ||
|---|---|---|---|---|---|
| Guaiacol | 1,2-Dimethoxybenzene | Others | |||
| Al-0P-O | 45.1 | 77.4 | 2.1 | 20.5 | 34.9 |
| Al-0.25P-O | 64.1 | 83.6 | 4.4 | 12.0 | 53.6 |
| Al-0.50P-O | 66.5 | 83.1 | 5.0 | 11.9 | 55.3 |
| Al-0.75P-O | 76.8 | 89.5 | 3.1 | 7.4 | 68.7 |
| Al-1.00P-O | 63.1 | 92.4 | 2.1 | 5.5 | 58.3 |
| Al-1.10P-O | 58.0 | 97.6 | 2.3 | 0.1 | 56.6 |
| Al-1.10P-Oa | 43.6 | 97.7 | 2.2 | 0.1 | 42.6 |
| Al-1.15P-O | 25.2 | 99.3 | 0.7 | 0 | 25.0 |
| Al-1.20P-O | 22.9 | 99.4 | 0.6 | 0 | 22.8 |
| Catalysts | Reaction Time (h) | Carbon Amount by TG (mg·gcat−1) | Average Carbon Depositions per Hour (mg·gcat−1·h−1) |
|---|---|---|---|
| Al-1.1P-O-S300 | 300 | 38 | 0.13 |
| Al-1.1P-Oa-S100 | 100 | 45 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Ren, J.; Yue, S.; Zou, X.; Shang, X.; Wang, X. One-Pot Synthesis of Al-P-O Catalysts and Their Catalytic Properties for O-Methylation of Catechol and Methanol. Materials 2021, 14, 5942. https://doi.org/10.3390/ma14205942
Xu D, Ren J, Yue S, Zou X, Shang X, Wang X. One-Pot Synthesis of Al-P-O Catalysts and Their Catalytic Properties for O-Methylation of Catechol and Methanol. Materials. 2021; 14(20):5942. https://doi.org/10.3390/ma14205942
Chicago/Turabian StyleXu, Dongfei, Jiaan Ren, Shengnan Yue, Xiujing Zou, Xingfu Shang, and Xueguang Wang. 2021. "One-Pot Synthesis of Al-P-O Catalysts and Their Catalytic Properties for O-Methylation of Catechol and Methanol" Materials 14, no. 20: 5942. https://doi.org/10.3390/ma14205942