Biomass Porous Carbons Derived from Banana Peel Waste as Sustainable Anodes for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
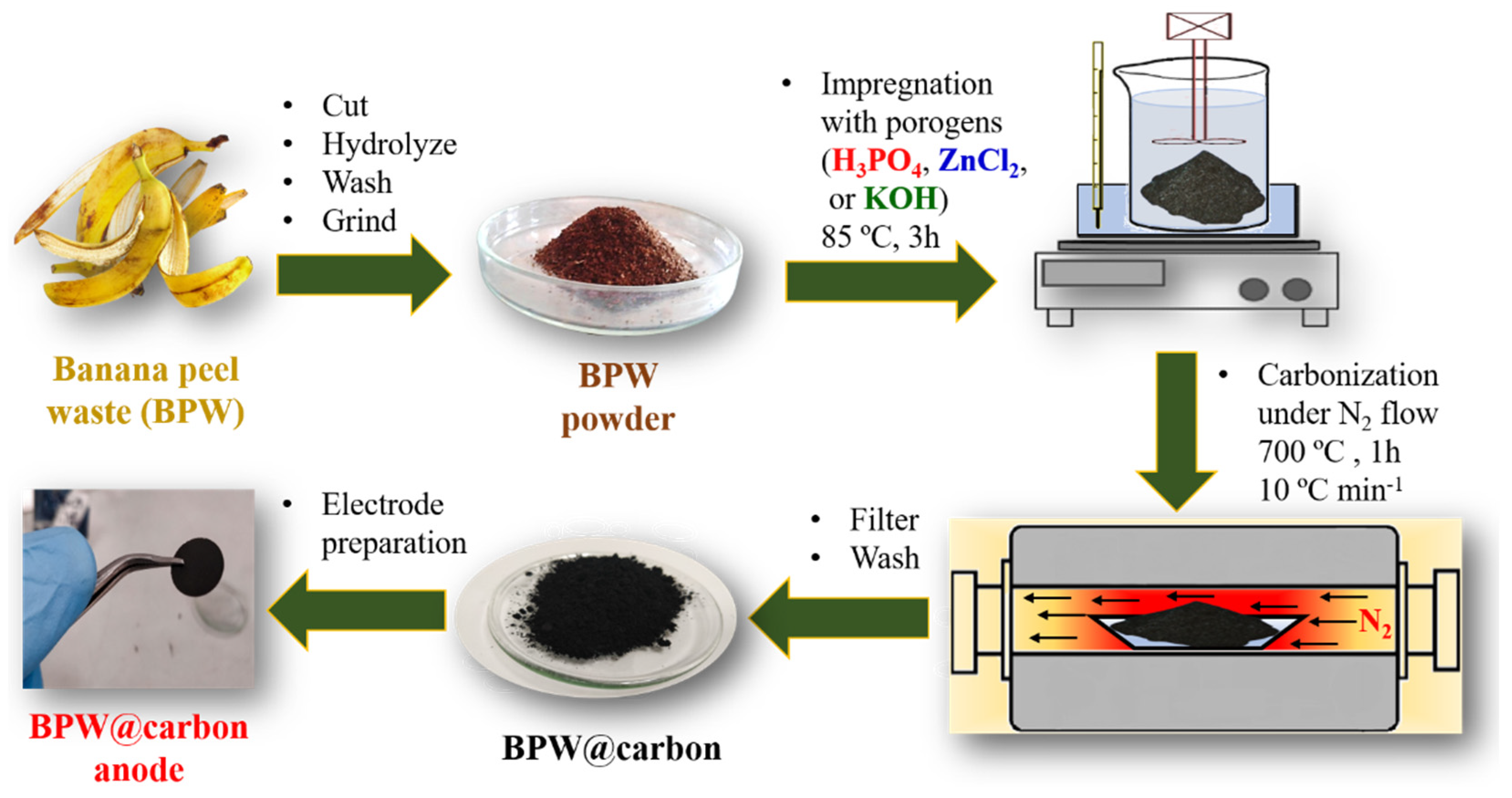
2.2. Activated Carbon Synthesis
2.3. Characterization
2.4. Preparation of Electrodes
2.5. Cell Assembly and Electrochemical Measurements
3. Results and Discussion
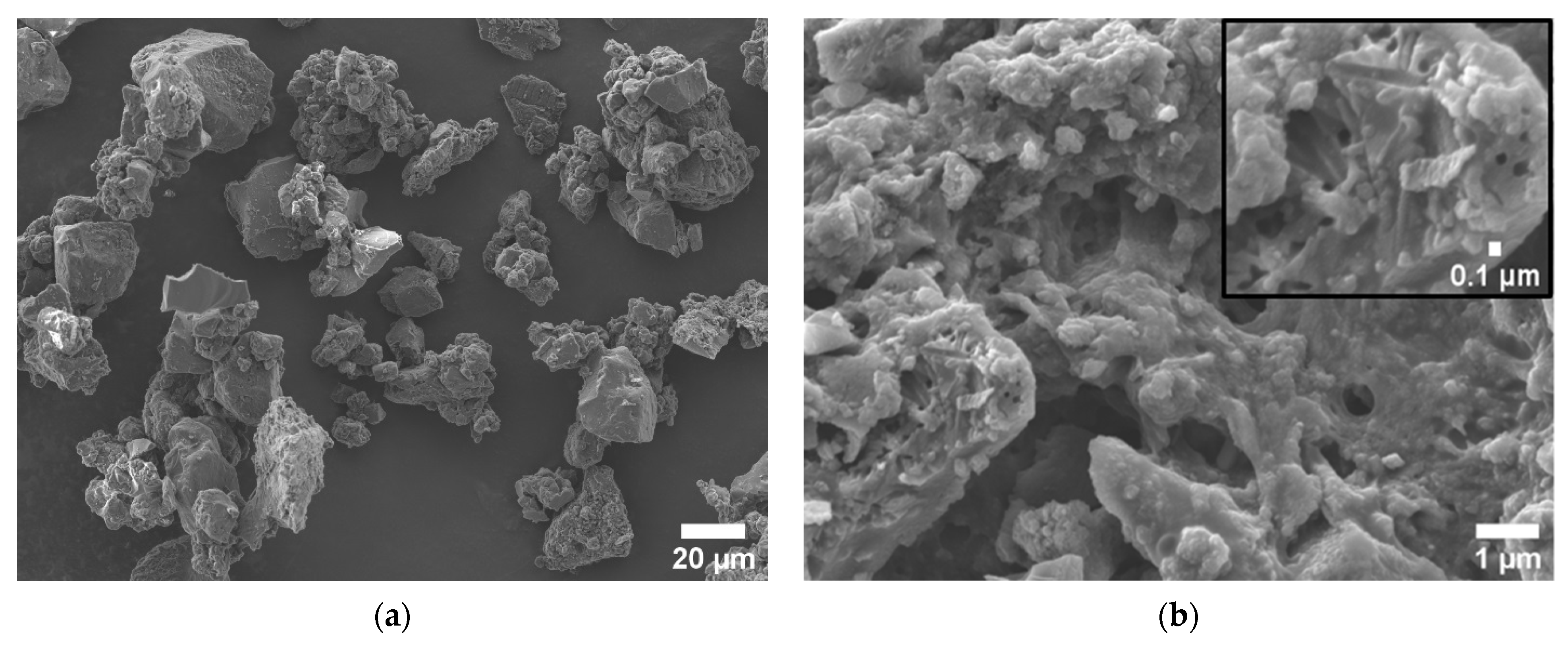
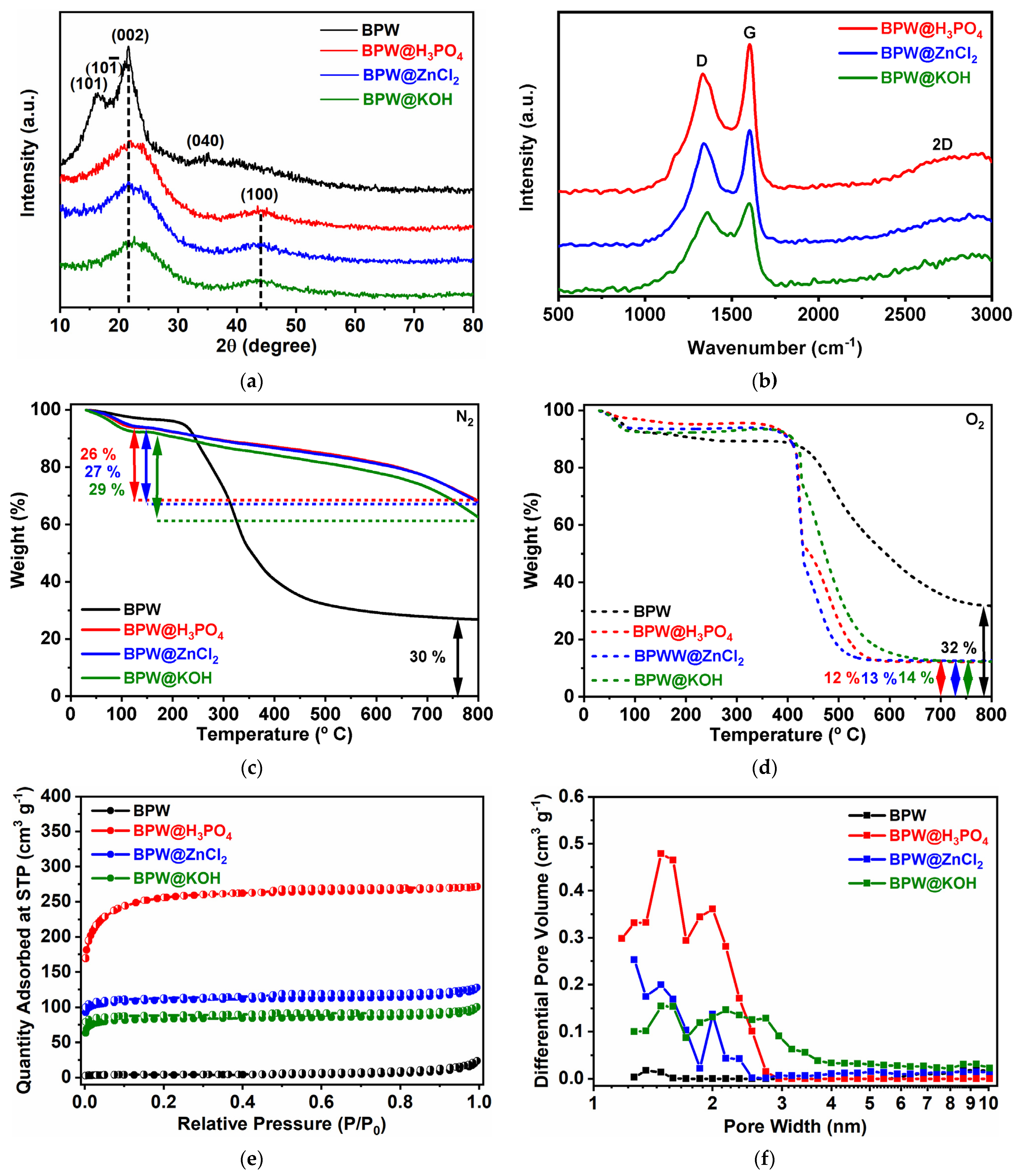
3.1. Structural, Chemical, and Textural Characterization
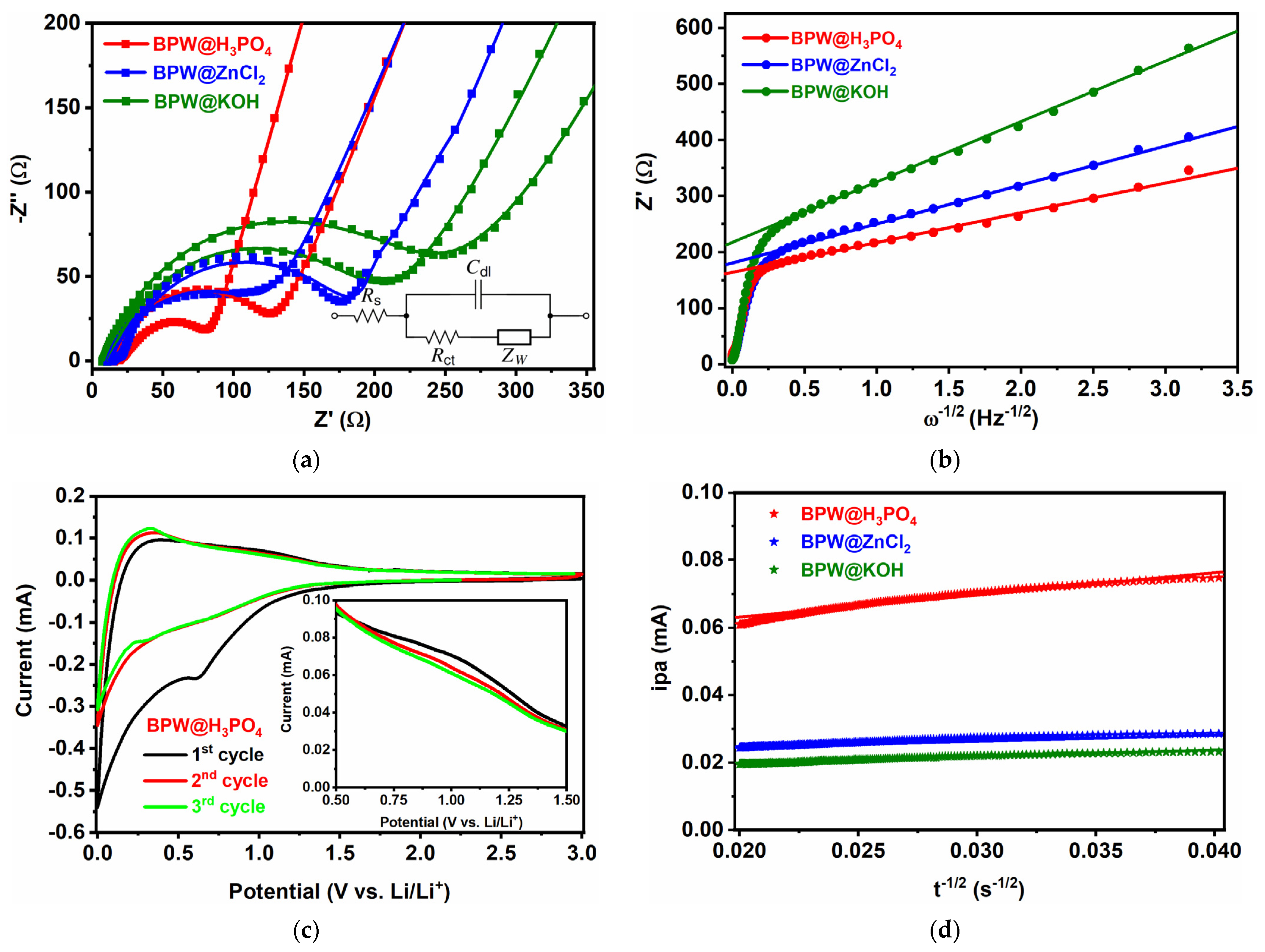
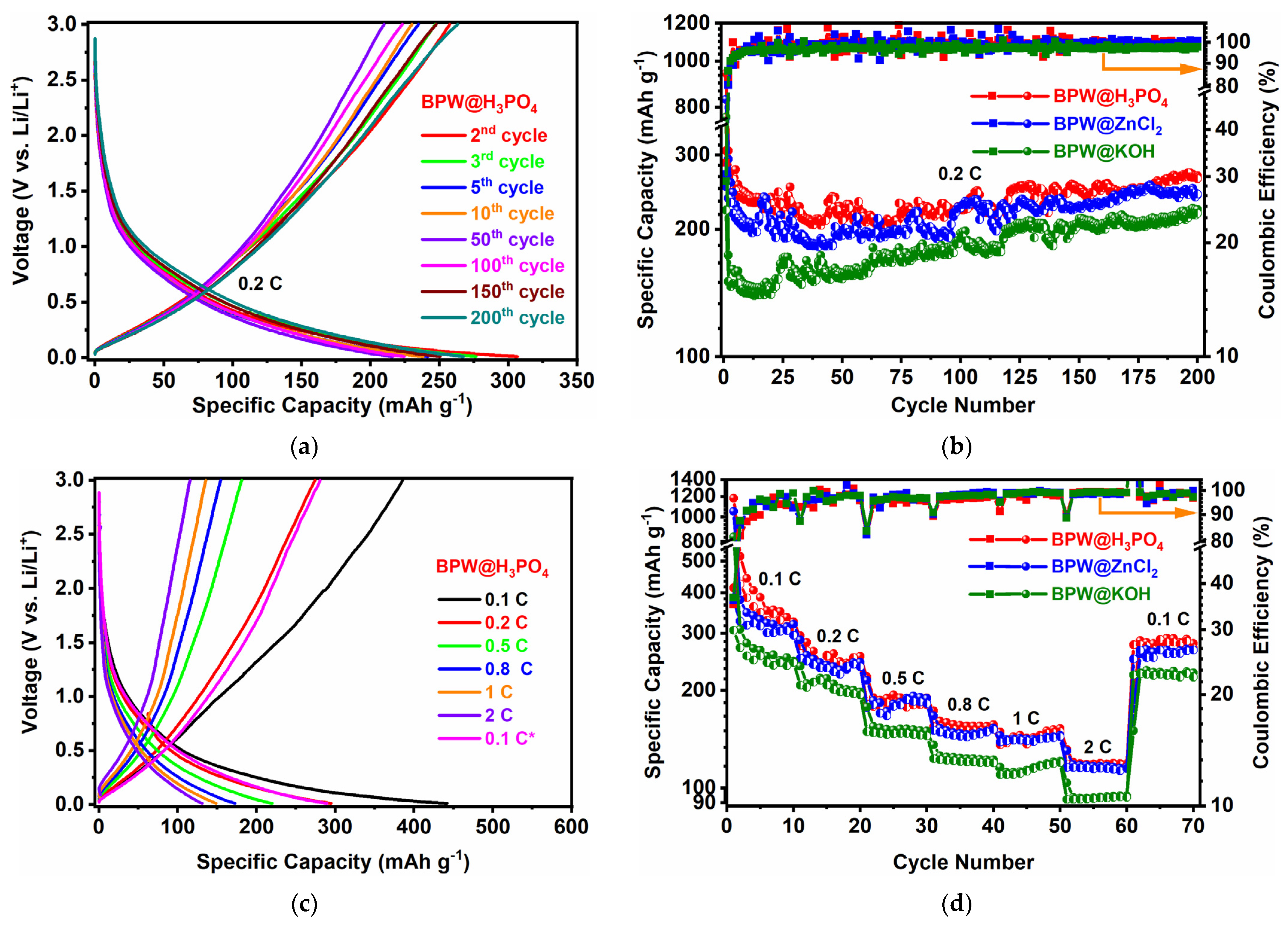
3.2. Electrochemical Properties in LIBs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akorede, M.F.; Hizam, H.; Pouresmaeil, E. Distributed energy resources and benefits to the environment. Renew. Sustain. Energy Rev. 2010, 14, 724–734. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Mater. Sustain. Energy A Collect. Peer-Rev. Res. Rev. Artic. Nat. Publ. Gr. 2010, 414, 171–179. [Google Scholar] [CrossRef]
- Mao, C.; Wood, M.; David, L.; An, S.J.; Sheng, Y.; Du, Z.; Meyer, H.M.; Ruther, R.E.; Wood, D.L. Selecting the Best Graphite for Long-Life, High-Energy Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A1837–A1845. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Barbosa, L.; Luna-Lama, F.; González Peña, Y.; Caballero, A. Simple and Eco-Friendly Fabrication of Electrode Materials and Their Performance in High-Voltage Lithium-Ion Batteries. ChemSusChem 2020, 13, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Roselin, L.S.; Juang, R.-S.; Hsieh, C.-T.; Sagadevan, S.; Umar, A.; Selvin, R.; Hegazy, H.H. Recent Advances and Perspectives of Carbon-Based Nanostructures as Anode Materials for Li-ion Batteries. Materials 2019, 12, 1229. [Google Scholar] [CrossRef] [Green Version]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, W.; Ren, J.; Weng, W.; Lin, H.; Zhang, Z.; Peng, H. Super-stretchy lithium-ion battery based on carbon nanotube fiber. J. Mater. Chem. A 2014, 2, 11054–11059. [Google Scholar] [CrossRef]
- Hernández-Rentero, C.; Vargas, O.; Caballero, A.; Morales, J.; Martín, F. Solvothermal-induced 3D graphene networks: Role played by the structural and textural properties on lithium storage. Electrochim. Acta 2016, 222, 914–920. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220. [Google Scholar] [CrossRef]
- Kalyani, P.; Anitha, A. Biomass carbon & its prospects in electrochemical energy systems. Int. J. Hydrogen Energy 2013, 38, 4034–4045. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Li, R.; Huang, J.; Li, J.; Cao, L.; Zhong, X.; Yu, A.; Lu, G. Nitrogen-doped porous hard carbons derived from shaddock peel for high-capacity lithium-ion battery anodes. J. Electroanal. Chem. 2020, 862, 114044. [Google Scholar] [CrossRef]
- Fromm, O.; Heckmann, A.; Rodehorst, U.C.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from biomass precursors as anode materials for lithium ion batteries: New insights into carbonization and graphitization behavior and into their correlation to electrochemical performance. Carbon 2018, 128, 147–163. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Jaybhaye, S.V.; Sharon, M.; Sathiyamoorthy, D.; Dasgupta, K.; Jagadale, P.; Gupta, A.; Patil, B.; Ozha, G.; Pandey, S.; et al. Carbon Nanomaterial from Tea leaves as an Anode in Lithium Secondary Batteries. Asian J. Exp. Sci. 2008, 22, 89–93. [Google Scholar]
- Luna-Lama, F.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Muñoz-Batista, M.J.; Caballero, A.; Balu, A.M.; Romero, A.A.; Luque, R. Non-porous carbonaceous materials derived from coffee waste grounds as highly sustainable anodes for lithium-ion batteries. J. Clean. Prod. 2019, 207, 411–417. [Google Scholar] [CrossRef]
- Ryu, D.J.; Oh, R.G.; Seo, Y.D.; Oh, S.Y.; Ryu, K.S. Recovery and electrochemical performance in lithium secondary batteries of biochar derived from rice straw. Environ. Sci. Pollut. Res. 2015, 22, 10405–10412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Qi, H.; Yu, K.; Liang, C. Mesoporous activated carbon from corn stalk core for lithium ion batteries. Chem. Phys. 2018, 506, 10–16. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Caballero, A.; Hernán, L.; Morales, J.; Olivares-Marín, M.; Gómez-Serrano, V. Improving the Performance of Biomass-Derived Carbons in Li-Ion Batteries by Controlling the Lithium Insertion Process. J. Electrochem. Soc. 2010, 157, A791. [Google Scholar] [CrossRef]
- Caballero, A.; Hernán, L.; Morales, J. Limitations of disordered carbons obtained from biomass as anodes for real lithium-ion batteries. ChemSusChem 2011, 4, 658–663. [Google Scholar] [CrossRef]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.; Liu, H.; Fan, H.; Wang, G. Naturally nitrogen doped porous carbon derived from waste shrimp shells for high-performance lithium ion batteries and supercapacitors. Microporous Mesoporous Mater. 2017, 246, 72–80. [Google Scholar] [CrossRef]
- Campbell, B.; Ionescu, R.; Favors, Z.; Ozkan, C.S.; Ozkan, M. Bio-Derived, Binderless, Hierarchically Porous Carbon Anodes for Li-ion Batteries. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Nie, P.; Ding, B.; Dong, S.; Hao, X.; Dou, H.; Zhang, X. Biomass derived carbon for energy storage devices. J. Mater. Chem. A 2017, 5, 2411–2428. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, L.; Fan, Z. Biomass-derived carbon materials with structural diversities and their applications in energy storage. Sci. China Mater. 2018, 61, 133–158. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 9 January 2021).
- Zhang, Y.; Gao, Z.; Song, N.; Li, X. High-performance supercapacitors and batteries derived from activated banana-peel with porous structures. Electrochim. Acta 2016, 222, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Correa, C.; Stollovsky, M.; Hehr, T.; Rauscher, Y.; Rolli, B.; Kruse, A. Influence of the Carbonization Process on Activated Carbon Properties from Lignin and Lignin-Rich Biomasses. ACS Sustain. Chem. Eng. 2017, 5, 8222–8233. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A review of chemicals to produce activated carbon from agricultural waste biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef] [Green Version]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Xiong, J.; Ou, X.; Su, W.; Zhong, G.; Yang, C. Activated Amorphous Carbon With High-Porosity Derived From Camellia Pollen Grains as Anode Materials for Lithium/Sodium Ion Batteries. Front. Chem. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Z.; Yin, S.; Guo, Z.; Wang, S.; Feng, C. Biomass carbon micro/nano-structures derived from ramie fibers and corncobs as anode materials for lithium-ion and sodium-ion batteries. Appl. Surf. Sci. 2016, 379, 73–82. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.Y.; Li, J.; Zou, Y.L.; Tang, J.J. Study of nano-porous hard carbons as anode materials for lithium ion batteries. Mater. Chem. Phys. 2012, 135, 445–450. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, H.; Wang, H.; Zhao, X.; Sun, S.; Shi, J.; Huang, M.; Liu, W.; Zheng, Y.; Li, P. Dual-doped hierarchical porous carbon derived from biomass for advanced supercapacitors and lithium ion batteries. RSC Adv. 2019, 9, 32382–32394. [Google Scholar] [CrossRef] [Green Version]
- Fey, G.T.K.; Cho, Y.D.; Chen, C.L.; Lin, Y.Y.; Kumar, T.P.; Chan, S.H. Pyrolytic carbons from acid/base-treated rice husk as lithium-insertion anode materials. Pure Appl. Chem. 2010, 82, 2157–2165. [Google Scholar] [CrossRef]
- Tang, S.; Baker, G.A.; Ravula, S.; Jones, J.E.; Zhao, H. PEG-functionalized ionic liquids for cellulose dissolution and saccharification. Green Chem. 2012, 14, 2922–2932. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Carvalho Benini, K.C.C.; Watashi, C.Y.; Voorwald, H.J.C.; Cioffi, M.O.H. Characterization of high density polyethylene (HDPE) reinforced with banana peel fibers. BioResources 2013, 8, 2351–2365. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.; Hu, X. Nitrogen doped biomass-derived porous carbon as anode materials of lithium ion batteries. Solid State Ionics 2019, 341, 115030. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-density sodium and lithium ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, J.S.; Zheng, T.; Dahn, J.R. Mechanism of lithium insertion in hard carbons prepared by pyrolysis of epoxy resins. Carbon 1996, 34, 193–200. [Google Scholar] [CrossRef]
- Sun, F.; Wang, L.; Peng, Y.; Gao, J.; Pi, X.; Qu, Z.; Zhao, G.; Qin, Y. Converting biomass waste into microporous carbon with simultaneously high surface area and carbon purity as advanced electrochemical energy storage materials. Appl. Surf. Sci. 2018, 436, 486–494. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, Z.; Fan, W.; Peng, G.; Qu, M. Effects of functional groups of graphene oxide on the electrochemical performance of lithium-ion batteries. RSC Adv. 2015, 5, 90041–90048. [Google Scholar] [CrossRef]
- Xiang, X.; Huang, Z.; Liu, E.; Shen, H.; Tian, Y.; Xie, H.; Wu, Y.; Wu, Z. Lithium storage performance of carbon nanotubes prepared from polyaniline for lithium-ion batteries. Electrochim. Acta 2011, 56, 9350–9356. [Google Scholar] [CrossRef]
- Wu, Y.P.; Rahm, E.; Holze, R. Effects of heteroatoms on electrochemical performance of electrode materials for lithium ion batteries. Electrochim. Acta 2002, 47, 3491–3507. [Google Scholar] [CrossRef]
- Fey, G.T.K.; Chen, C.L. High-capacity carbons for lithium-ion batteries prepared from rice husk. J. Power Sources 2001, 97–98, 47–51. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Huang, J.; Xing, L.; Hu, H.; Xiao, Y.; Dong, H.; Liu, Y.; Zheng, M. Mixed-Biomass Wastes Derived Hierarchically Porous Carbons for High-Performance Electrochemical Energy Storage. ACS Sustain. Chem. Eng. 2019, 7, 10393–10402. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Bai, W.; Ke, J. The preparation of biomass carbon materials and its energy storage research. Ionics 2019, 25, 2543–2548. [Google Scholar] [CrossRef]
- Machnikowski, J.; Grzyb, B.; Weber, J.V.; Frackowiak, E.; Rouzaud, J.N.; Béguin, F. Structural and electrochemical characterisation of nitrogen enriched carbons produced by the co-pyrolysis of coal-tar pitch with polyacrylonitrile. Electrochim. Acta 2004, 49, 423–432. [Google Scholar] [CrossRef]
- Agarkar, S.; Yadav, P.; Fernandes, R.; Kothari, D.; Suryawanshi, A.; Ogale, S. Minute-made activated porous carbon from agro-waste for Li-ion battery anode using a low power microwave oven. Electrochim. Acta 2016, 212, 535–544. [Google Scholar] [CrossRef]
- Zhang, D.; Popov, B.N.; White, R.E. Electrochemical investigation of CrO2.65 doped LiMn2O4 as a cathode material for lithium-ion batteries. J. Power Sources 1998, 76, 81–90. [Google Scholar] [CrossRef]
- Ho, C.; Raistrick, I.D.; Huggins, R.A. Application of A-C Techniques to the Study of Lithium Diffusion in Tungsten Trioxide Thin Films. J. Electrochem. Soc. 1980, 127, 343–350. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Tan, X.; Wang, H.; Holt, C.M.B.; Stephenson, T.; Olsen, B.C.; Mitlin, D. Mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors. Energy Environ. Sci. 2013, 6, 871–878. [Google Scholar] [CrossRef]
- Yu, W.; Wang, H.; Liu, S.; Mao, N.; Liu, X.; Shi, J.; Liu, W.; Chen, S.; Wang, X. N, O-codoped hierarchical porous carbons derived from algae for high-capacity supercapacitors and battery anodes. J. Mater. Chem. A 2016, 4, 5973–5983. [Google Scholar] [CrossRef]
- Kakunuri, M.; Sharma, C.S. Candle Soot derived Fractal-like Carbon Nanoparticles Network as High-Rate Lithium Ion Battery Anode Material. Electrochim. Acta 2015, 180, 353–359. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, J.; Ai, W.; Fan, Z.; Shen, X.; Zou, C.; Liu, J.; Zhang, H.; Yu, T. Evolution of disposable bamboo chopsticks into uniform carbon fibers: A smart strategy to fabricate sustainable anodes for Li-ion batteries. Energy Environ. Sci. 2014, 7, 2670–2679. [Google Scholar] [CrossRef] [Green Version]
- Han, S.W.; Jung, D.W.; Jeong, J.H.; Oh, E.S. Effect of pyrolysis temperature on carbon obtained from green tea biomass for superior lithium ion battery anodes. Chem. Eng. J. 2014, 254, 597–604. [Google Scholar] [CrossRef]
- Guo, H.J.; Li, X.H.; Zhang, X.M.; Wang, H.Q.; Wang, Z.X.; Peng, W. jie Diffusion coefficient of lithium in artificial graphite, mesocarbon microbeads, and disordered carbon. New Carbon Mater. 2007, 22, 7–10. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Lin, C.; Yang, W.; Meng, Y.; Guo, Y.; Li, M.; Xiao, D. Hierarchically porous nitrogen-rich carbon derived from wheat straw as an ultra-high-rate anode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 9684–9690. [Google Scholar] [CrossRef]
- Ru, H.; Bai, N.; Xiang, K.; Zhou, W.; Chen, H.; Zhao, X.S. Porous carbons derived from microalgae with enhanced electrochemical performance for lithium-ion batteries. Electrochim. Acta 2016, 194, 10–16. [Google Scholar] [CrossRef]
- Tao, L.; Huang, Y.; Zheng, Y.; Yang, X.; Liu, C.; Di, M.; Larpkiattaworn, S.; Nimlos, M.R.; Zheng, Z. Porous carbon nanofiber derived from a waste biomass as anode material in lithium-ion batteries. J. Taiwan Inst. Chem. Eng. 2019, 95, 217–226. [Google Scholar] [CrossRef]
- Dou, Y.; Liu, X.; Yu, K.; Wang, X.; Liu, W.; Liang, J.; Liang, C. Biomass porous carbon derived from jute fiber as anode materials for lithium-ion batteries. Diam. Relat. Mater. 2019, 98, 107514. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, T.; Zhang, D.; Zhang, W.; Zhang, H.; Liu, R.; Yao, M.; Liu, B. Remarkable cycle-activated capacity increasing in onion-like carbon nanospheres as lithium battery anode material. Nanotechnology 2017, 28, 035704. [Google Scholar] [CrossRef]
- Hernández-Rentero, C.; Marangon, V.; Olivares-Marín, M.; Gómez-Serrano, V.; Caballero, Á.; Morales, J.; Hassoun, J. Alternative lithium-ion battery using biomass-derived carbons as environmentally sustainable anode. J. Colloid Interface Sci. 2020, 573, 396–408. [Google Scholar] [CrossRef]
- Yu, K.; Li, J.; Qi, H.; Liang, C. Cellulose-Derived Hollow Carbonaceous Nanospheres from Rice Husks as Anode for Lithium-Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance. ChemistrySelect 2017, 2, 3627–3632. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, K.; Park, H.; Shin, J.W.; Ryoo, R. Anomalously High Lithium Storage in Three-Dimensional Graphene-like Ordered Microporous Carbon Electrodes. J. Phys. Chem. C 2018, 122, 4955–4962. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Li, G.D.; Chen, J.S. Microporous carbon derived from pinecone hull as anode material for lithium secondary batteries. Mater. Lett. 2007, 61, 5209–5212. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Heckmann, A.; Winter, M.; Placke, T.; Ramirez-Rico, J. Iron-Catalyzed Graphitic Carbon Materials from Biomass Resources as Anodes for Lithium-Ion Batteries. ChemSusChem 2018, 11, 2776–2787. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Jeong, S.K.; Shin, J.S.; Nahm, K.S.; Stephan, A.M. High capacity disordered carbons obtained from coconut shells as anode materials for lithium batteries. J. Alloys Compd. 2008, 448, 141–147. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Jeong, S.K.; Nahm, K.S.; Shin, J.S.; Manuel Stephan, A. Pyrolytic carbon derived from coffee shells as anode materials for lithium batteries. J. Phys. Chem. Solids 2007, 68, 182–188. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Chaldun, E.R.; Nuryadin, B.W.; Fikriyyah, A.K.; Subhan, A.; Ghozali, M.; Purwasasmita, B.S. Preparation and Characterization of Biomass-Derived Advanced Carbon Materials for Lithium-Ion Battery Applications. J. Electron. Mater. 2018, 47, 4028–4037. [Google Scholar] [CrossRef]
- Stephan, A.M.; Kumar, T.P.; Ramesh, R.; Thomas, S.; Jeong, S.K.; Nahm, K.S. Pyrolitic carbon from biomass precursors as anode materials for lithium batteries. Mater. Sci. Eng. A 2006, 430, 132–137. [Google Scholar] [CrossRef]
- Du, R.; Tong, Z.; Wei, C.; Qin, A. Preparation of activated carbons from sisal fibers as anode materials for lithium ion batteries. Int. J. Electrochem. Sci. 2016, 11, 8418–8429. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K.; Tian, N.; Qin, A.; Liao, L.; Du, R.; Wei, C. Biomass carbon derived from sisal fiber as anode material for lithium-ion batteries. Mater. Lett. 2015, 142, 193–196. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Sharon, M.; Ishihara, T.; Jayabhaye, S.; Afre, R.; Soga, T.; Sharon, M. Carbon Material from Natural Sources as an Anode in Lithium Secondary Battery. Carbon Lett. 2007, 8, 285–291. [Google Scholar] [CrossRef]
- Biegun, M.; Dymerska, A.; Chen, X.; Mijowska, E. Study of the Active Carbon from Used Coffee Grounds as the Active Material for a High-Temperature Stable Supercapacitor with Ionic-Liquid Electrolyte. Materials 2020, 13, 3919. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.; Zhu, W.; Gariépy, V.; Gagnon, C.; Provencher, M.; Laul, D.; Veillette, R.; Trudeau, M.L.; Guerfi, A.; et al. High Capacity and High Efficiency Maple Tree-Biomass-Derived Hard Carbon as an Anode Material for Sodium-Ion Batteries. Materials 2018, 11, 1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | C (%) 1 | N (%) 1 | d002 (nm) | Lc (nm) 2 | La (nm) 3 | N Layers | R Factor | ID/IG Ratio | SBET (m2 g−1) | Vtotal (cm3 g−1) | Vmicro (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPW | 49.9 | 2.45 | - | - | - | - | - | - | 12 | 0.036 | 0.004 | 12.5 |
| BPW@H3PO4 | 86.25 | 1.75 | 0.393 | 1.85 | 4.91 | 4.71 | 1.96 | 0.83 | 815 | 0.420 | 0.332 | 2.08 |
| BPW@ZnCl2 | 85.73 | 1.53 | 0.397 | 1.83 | 4.86 | 4.61 | 2.03 | 0.87 | 348 | 0.198 | 0.154 | 2.31 |
| BPW@KOH | 83.15 | 1.19 | 0.399 | 1.72 | 4.83 | 4.31 | 2.20 | 0.90 | 264 | 0.155 | 0.117 | 2.39 |
| Biomass Precursor | Long-Term Cycling | Rate Capability | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| Initial Capacity (mAh g−1) | Reversible Capacity (mAh g−1) | Cycles | Rate (C) 1 | Average Capacity (mAh g−1) | Cycles | Rate (C) 1 | ||
| Soybean curd | 102 | 90 | 5 | 0.2 | 40 | 5 | 1 | [73] |
| 25 | 5 | 2 | ||||||
| Bamboo wood | 169 | 170 | 300 | 1 | 82 | 3 | 1 | [15] |
| 41 | 3 | 3 | ||||||
| Soap-nut seeds | 650 | 130 | 100 | 0.1 | - | - | - | [77] |
| Tea leaves | 100 | 63 | 100 | 0.1 | - | - | - | [16] |
| Rice straw | 562 | 300 | 5 | 0.07 | 175 | 5 | 0.7 | [18] |
| Sisal fibers | 998 | 250 | 40 | 0.1 | - | - | - | [75] |
| Sisal fibers | 650 | 240 | 30 | 0.1 | - | - | - | [76] |
| Mushroom skin | 771 2 | 260 | 700 | 0.27 | - | - | - | [23] |
| Wood pieces | 466 3 | 231 | 200 | 1 | 243 | 5 | 1 | [70] |
| 150 | 5 | 2 | ||||||
| Pinecone hull | 660 4 | 394 | 8 | 0.02 | - | - | - | [69] |
| Coconut shells | 1714 | 250 | 15 | 0.1 | - | - | - | [71] |
| Olive stones | 615 | 170 | 100 | 0.2 | 130 | 100 | 1 | [21] |
| 105 | 100 | 2 | ||||||
| Cherry stones | 900 | 287 | 100 | 0.1 | 278 | 100 | 1 | [20] |
| 243 | 100 | 2 | ||||||
| Coffee shells | 1200 | 300 | 15 | 0.2 | - | - | - | [72] |
| Walnut shell | 410 | 380 | 50 | 0.08 | 190 | 10 | 2.7 | [63] |
| 180 | 10 | 5.4 | ||||||
| Banana fibers | 921 | 217 | 10 | 0.1 | - | - | - | [74] |
| Banana peels | 942 | 272 | 200 | 0.2 | 149 | 10 | 1 | This work |
| 131 | 10 | 2 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Lama, F.; Morales, J.; Caballero, A. Biomass Porous Carbons Derived from Banana Peel Waste as Sustainable Anodes for Lithium-Ion Batteries. Materials 2021, 14, 5995. https://doi.org/10.3390/ma14205995
Luna-Lama F, Morales J, Caballero A. Biomass Porous Carbons Derived from Banana Peel Waste as Sustainable Anodes for Lithium-Ion Batteries. Materials. 2021; 14(20):5995. https://doi.org/10.3390/ma14205995
Chicago/Turabian StyleLuna-Lama, Fernando, Julián Morales, and Alvaro Caballero. 2021. "Biomass Porous Carbons Derived from Banana Peel Waste as Sustainable Anodes for Lithium-Ion Batteries" Materials 14, no. 20: 5995. https://doi.org/10.3390/ma14205995






