Characterization of Ternary CuNiCo Metallic Nanoparticles Produced by Hydrogen Reduction
Abstract
:1. Introduction
2. Materials and Experimental Procedure
Characterization Methods
3. Results
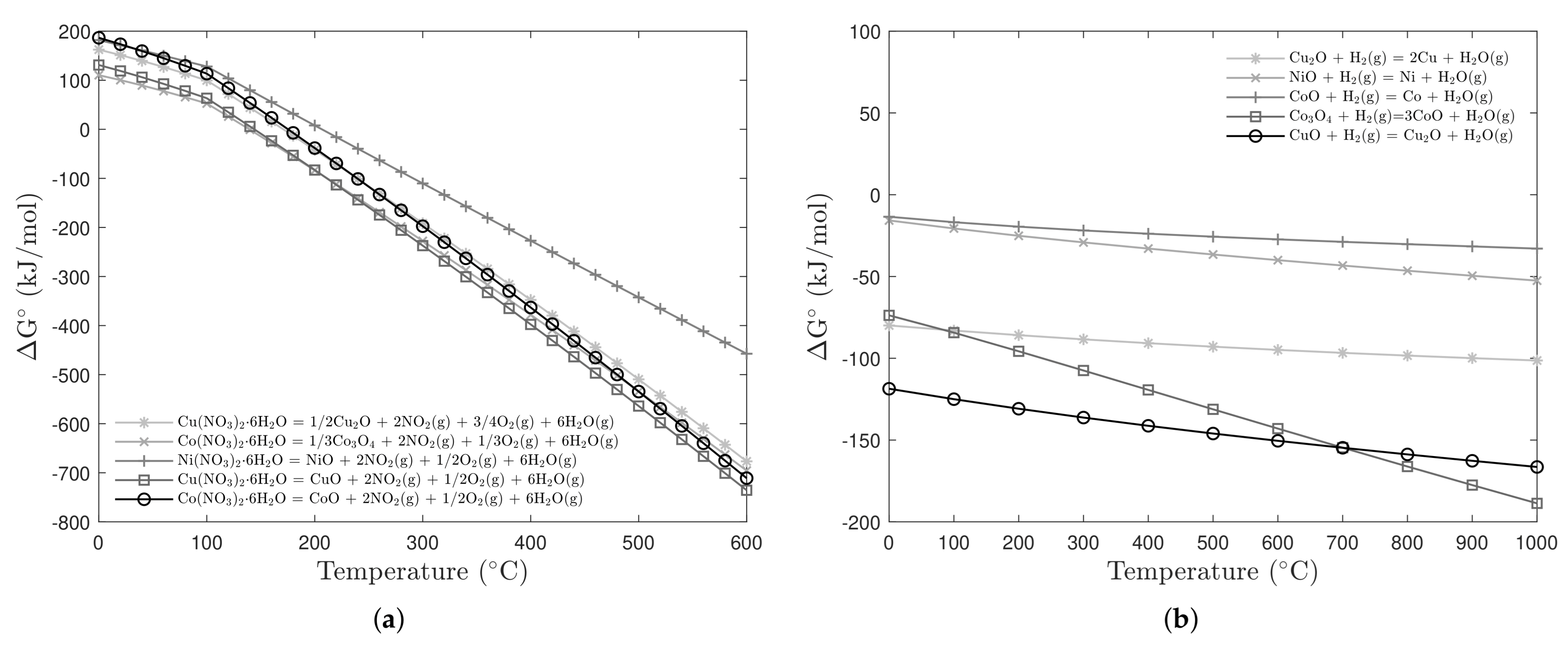
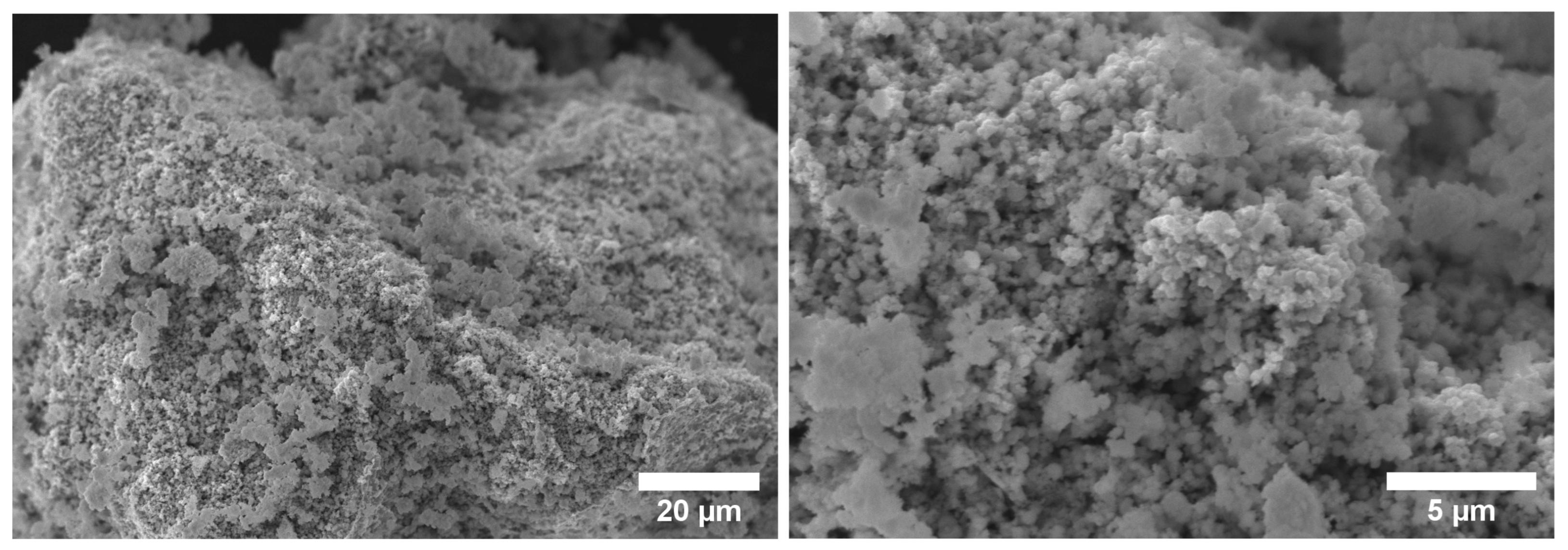
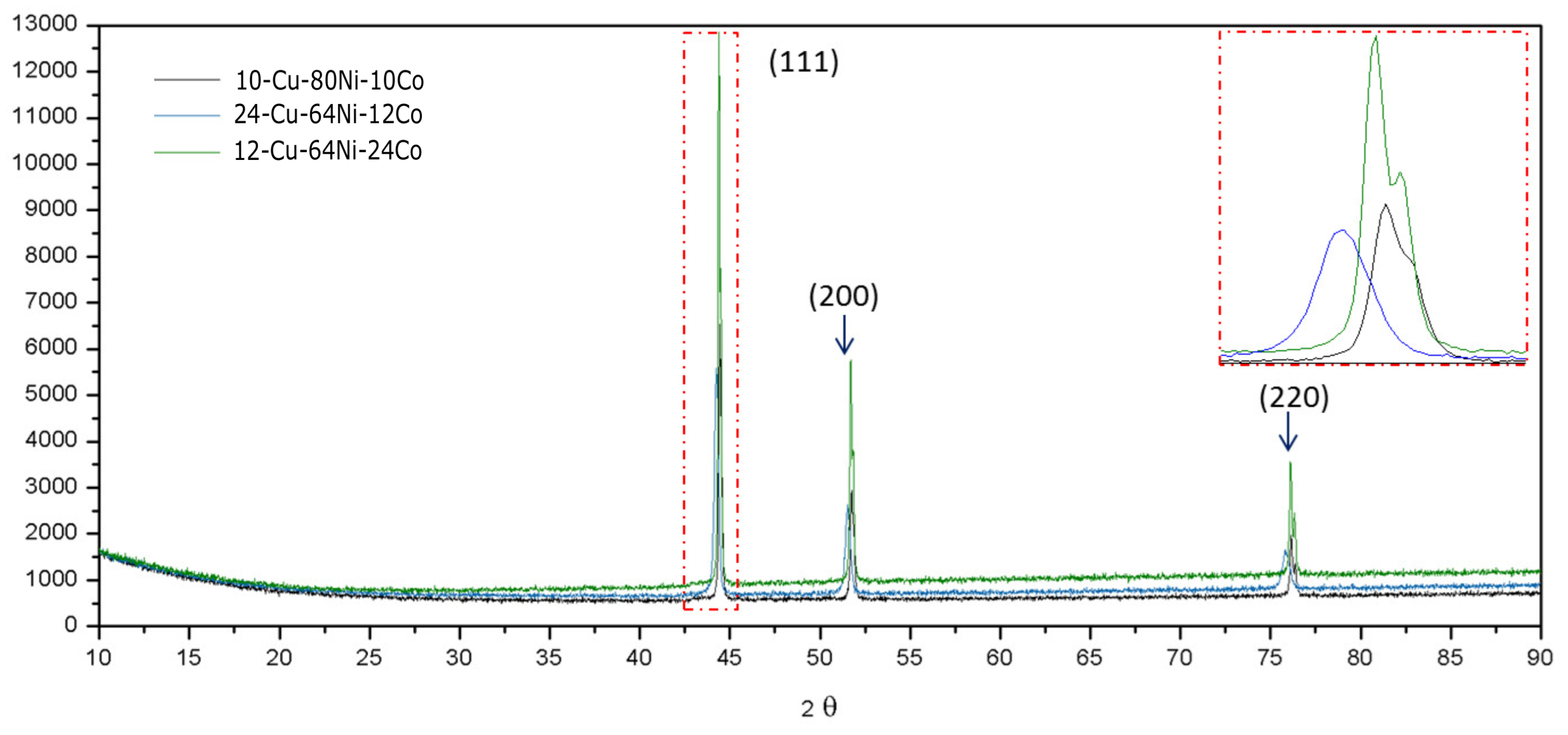
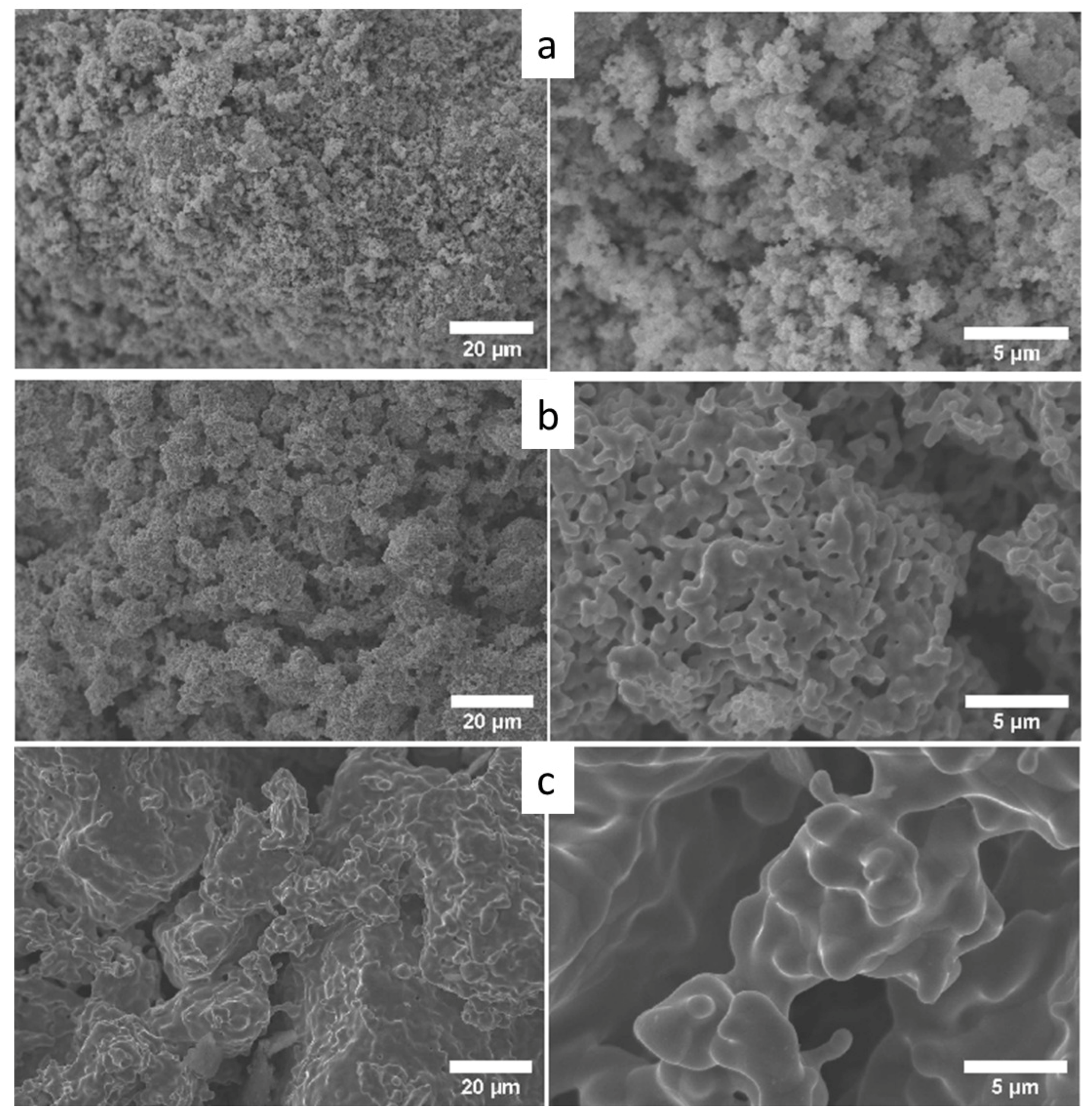
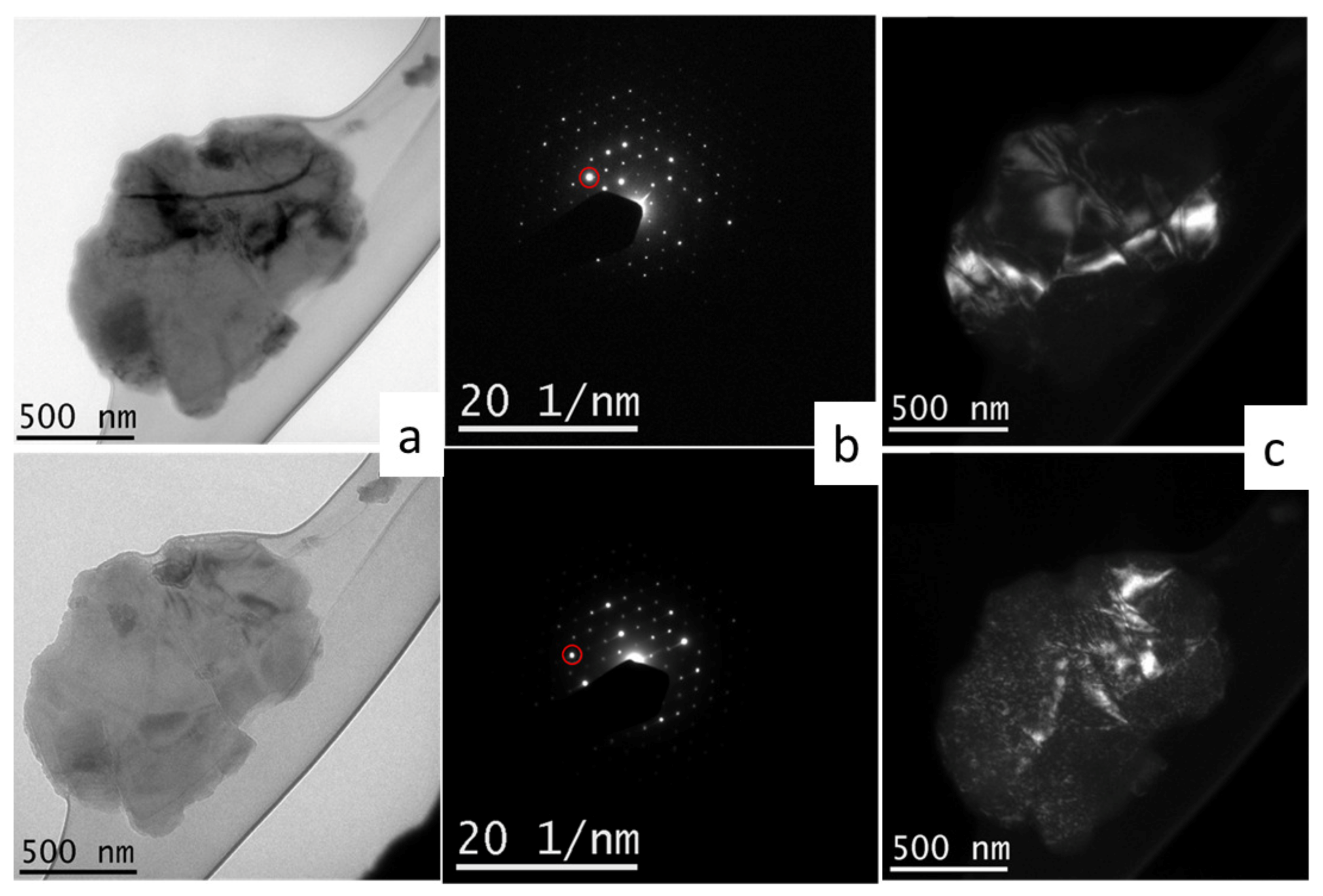
3.1. Characterization of the Powdered Oxides
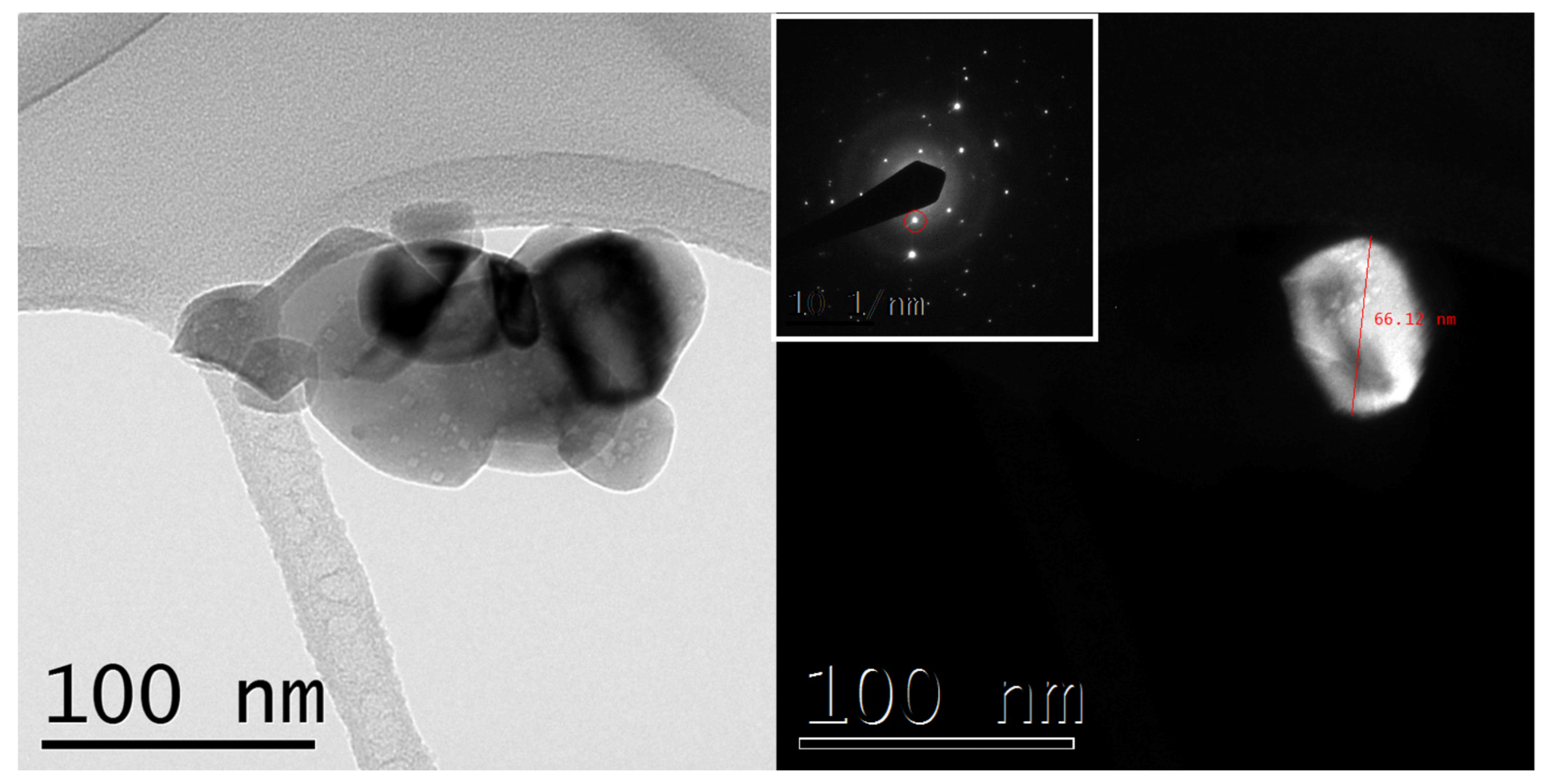
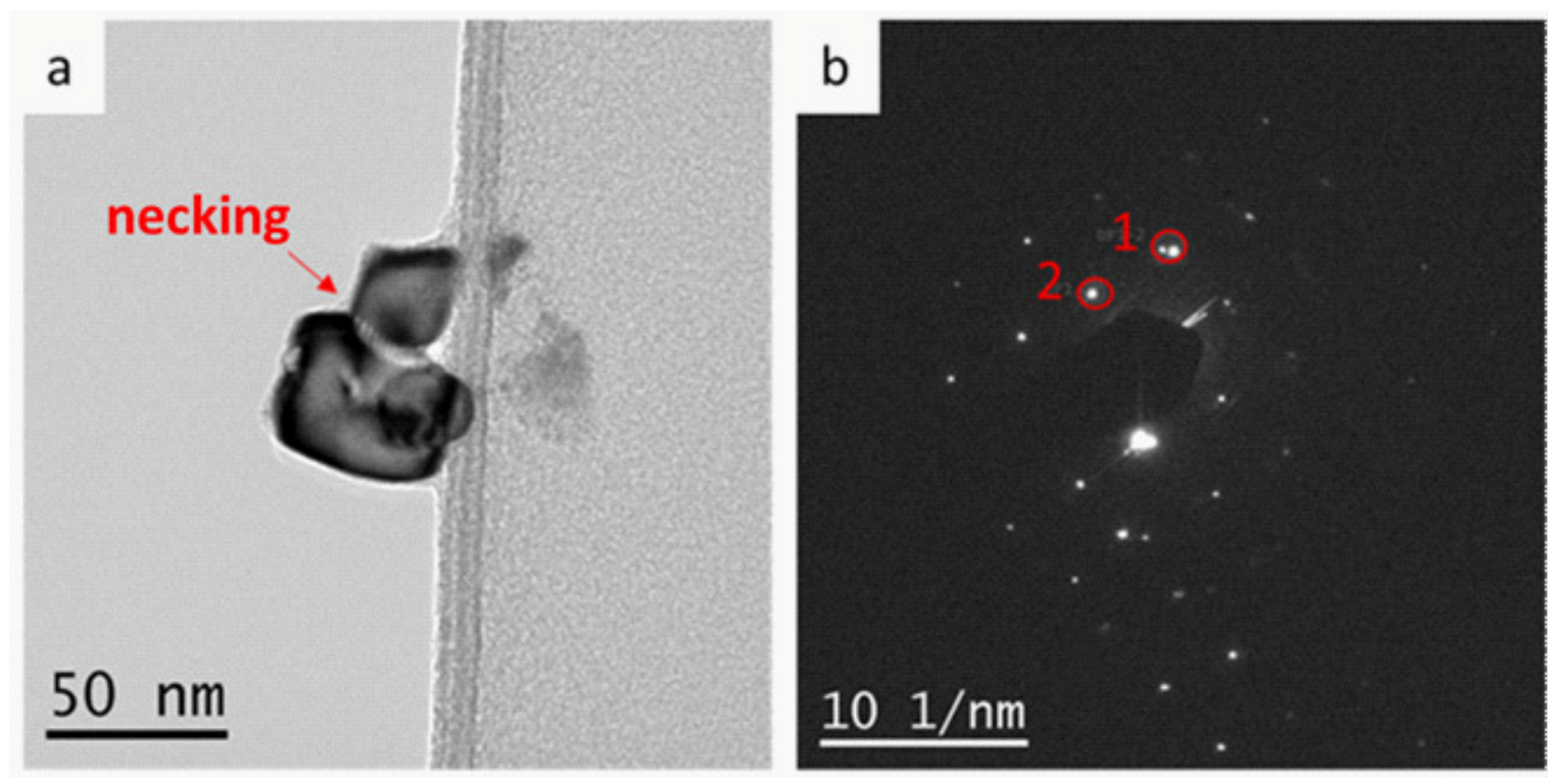
3.2. Characterization of Ternary Alloy Metallic Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zach, M.; Hagglund, C.; Chakarov, D.; Kasemo, B. Nanoscience and nanotechnology for advanced energy systems. Curr. Opin. Solid State Mater. Sci. 2006, 10, 132–143. [Google Scholar] [CrossRef]
- Islam, N.; Miyazaki, K. Nanotechnology innovation system: Understanding hidden dynamics of nanoscience fusion trajectories. Technol. Forecast. Soc. Chang. 2009, 76, 128–140. [Google Scholar] [CrossRef]
- Mondal, B.; Basumallick, A.; Chattopadhyay, P. Magnetic behavior of nanocrystalline Cu-Ni-Co alloys prepared by mechanical alloying and isothermal annealing. J. Alloys Compd. 2008, 457, 10–14. [Google Scholar] [CrossRef]
- Chai, Z.; Jiang, C.; Zhao, Y.; Wang, C.; Zhu, K.; Cai, F. Microstructural characterization and corrosion behaviors of Ni-Cu-Co coatings electrodeposited in sulphate-citrate bath with additives. Surf. Coat. Technol. 2016, 307, 817–824. [Google Scholar] [CrossRef]
- Pané, S.; Gómez, E.; Vallés, E. Magnetoresistive granular Cu-Co-Ni coatings prepared by electrodeposition. J. Electroanal. Chem. 2006, 596, 87–94. [Google Scholar] [CrossRef]
- Gomez, E.; Pane, S.; Valles, E. Electrodeposition of Co–Ni and Co–Ni–Cu systems in sulphate–citrate medium. Electrochim. Acta 2005, 51, 146–153. [Google Scholar] [CrossRef]
- Karpuz, A.; Kockar, H.; Alper, M. Study of electrolyte pH in production of Cu–Co–Ni ternary alloys and its effect on microstructural and magnetic properties. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Ignatova, K.N.; Marcheva, Y.S. Electrodeposition and Structure of Co coatings (CoCu, NiCo and CoNiCu) in potentiostatic and pulse potential modes. Bulg. Chem. Commun. 2013, 45, 57–63. [Google Scholar]
- Bai, A.; Hu, C.C. Effects of electroplating variables on the composition and morphology of nickel–cobalt deposits plated through means of cyclic voltammetry. Electrochim. Acta 2002, 47, 3447–3456. [Google Scholar] [CrossRef]
- Tury, B.; Lakatos-Varsányi, M.; Roy, S. Ni–Co alloys plated by pulse currents. Surf. Coat. Technol. 2006, 200, 6713–6717. [Google Scholar] [CrossRef]
- Cai, F.; Jiang, C.; Fu, P.; Ji, V. Effects of Co contents on the microstructures and properties of electrodeposited NiCo–Al composite coatings. Appl. Surf. Sci. 2015, 324, 482–489. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A. Effect of particle size and co-deposition technique on hardness and corrosion properties of Ni–Co/SiC composite coatings. Surf. Coatings Technol. 2012, 206, 4964–4975. [Google Scholar] [CrossRef]
- Blackman, J.A. Metallic Nanoparticles (Handbook of Metal Physics); Elsevier Science: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Karpuz, A.; Kockar, H.; Alper, M. Microstructure dependence of magnetic properties on electrochemically produced ternary CuCoNi alloys. J. Mater. Sci. Mater. Electron. 2014, 25, 4483–4488. [Google Scholar] [CrossRef]
- Zhanbo, S.; Yaomin, Z.; Xiaoyuan, L.; Xiaoping, S. Compositions, magnetic properties and GMR performances of melt-spun Cu-Co-Ni ribbons. J. Magn. Magn. Mater. 2004, 269, 341–345. [Google Scholar] [CrossRef]
- Hanafi, I.; Daud, A.R.; Radiman, S.; Ghani, M.H.A.; Budi, S. Surfactant Assisted Electrodeposition of Nanostructured CoNiCu Alloys. J. Phys. Conf. Ser. 2013, 431, 012013. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, P.; Singh, G. Synthesis, characterization of Co-Ni-Cu trimetallic alloy nanocrystals and their catalytic properties, Part-91. J. Alloys Compd. 2013, 562, 150–155. [Google Scholar] [CrossRef]
- Budi, S.; Daud, A.; Radiman, S.; Umar, A.A. Effective electrodeposition of Co–Ni–Cu alloys nanoparticles in the presence of alkyl polyglucoside surfactant. Appl. Surf. Sci. 2010, 257, 1027–1033. [Google Scholar] [CrossRef]
- Péter, L.; Pádár, J.; Tóth-Kádár, E.; Cziráki, Á.; Sóki, P.; Pogány, L.; Bakonyi, I. Electrodeposition of Co–Ni–Cu/Cu multilayers. Electrochim. Acta 2007, 52, 3813–3821. [Google Scholar] [CrossRef]
- Spasojević, M.; Spasojević, M.; Mašković, P.; Marković, D.; Ribić-Zelenović, L. Electrodeposition, Microstructure and Magnetic Properties of Nickel-Cobalt-Copper Alloy Powders. J. Electrochem. Soc. 2018, 165, D511–D517. [Google Scholar] [CrossRef]
- Adams, D. Reactive multilayers fabricated by vapor deposition: A critical review. Thin Solid Films 2015, 576, 98–128. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, I.; Narayanan, T.S.; Stephen, A. Pulsed electrodeposition of nanocrystalline Cu–Ni alloy films and evaluation of their characteristic properties. Mater. Lett. 2006, 60, 1990–1995. [Google Scholar] [CrossRef]
- Wen, M.; Liu, Q.Y.; Wang, Y.F.; Zhu, Y.Z.; Wu, Q.S. Positive microemulsion synthesis and magnetic property of amorphous multicomponent Co-, Ni-and Cu-based alloy nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 238–244. [Google Scholar] [CrossRef]
- Brocchi, E.A.; Moura, F.J.; de Macedo, D.W. Synthesis and characterisation of nanostructured Ni-Co alloy Part 1: NiO reduction kinetics. Miner. Process. Extr. Metall. 2009, 118, 35–39. [Google Scholar] [CrossRef]
- Brocchi, E.; Moura, F.; de Macedo, D. Synthesis and characterisation of nanostructured Ni–Co alloy Part 2: Co3O4 reduction kinetics. Miner. Process. Extr. Metall. 2009, 118, 40–43. [Google Scholar] [CrossRef]
- Brocchi, E.; Moura, F.; de Macedo, D. Synthesis and characterisation of nanostructured Ni–Co alloy Part 3: NiO and Co3O4 coformed reduction kinetics. Miner. Process. Extr. Metall. 2009, 118, 44–48. [Google Scholar] [CrossRef]
- Brocchi, E.; Motta, M.; Solórzano, I.; Jena, P.; Moura, F. Chemical Route Processing and Structural Characterization of Cu-Al2O3 and Ni-Al2O3 Nano-Composites. J. Metastable Nanocryst. Mater. 2004, 22, 77–82. [Google Scholar] [CrossRef]
- Motta, M.; Jena, P.; Brocchi, E.; Solórzano, I. Characterization of Cu–Al2O3 nano-scale composites synthesized by in situ reduction. Mater. Sci. Eng. C 2001, 15, 175–177. [Google Scholar] [CrossRef]
- Ramos, M.I.; Suguihiro, N.M.; Brocchi, E.A.; Navarro, R.; Solorzano, I.G. Microstructure Investigation of Cu-Ni Base Al2O3 Nanocomposites: From Nanoparticles Synthesis to Consolidation. Metall. Mater. Trans. A 2017, 48, 2643–2653. [Google Scholar] [CrossRef]
- Jena, P.; Brocchi, E.; Motta, M. In-situ formation of Cu-Al2O3 nano-scale composites by chemical routes and studies on their microstructures. Mater. Sci. Eng. A 2001, 313, 180–186. [Google Scholar] [CrossRef]
- Jena, P.K.; Brocchi, E.A.; Motta, M.S. Preparation of Cu-Ni alloys through a new chemical route. Metall. Mater. Trans. B 2004, 35, 1107–1112. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A. A fundamental parameters approach to X-ray line-profile fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]


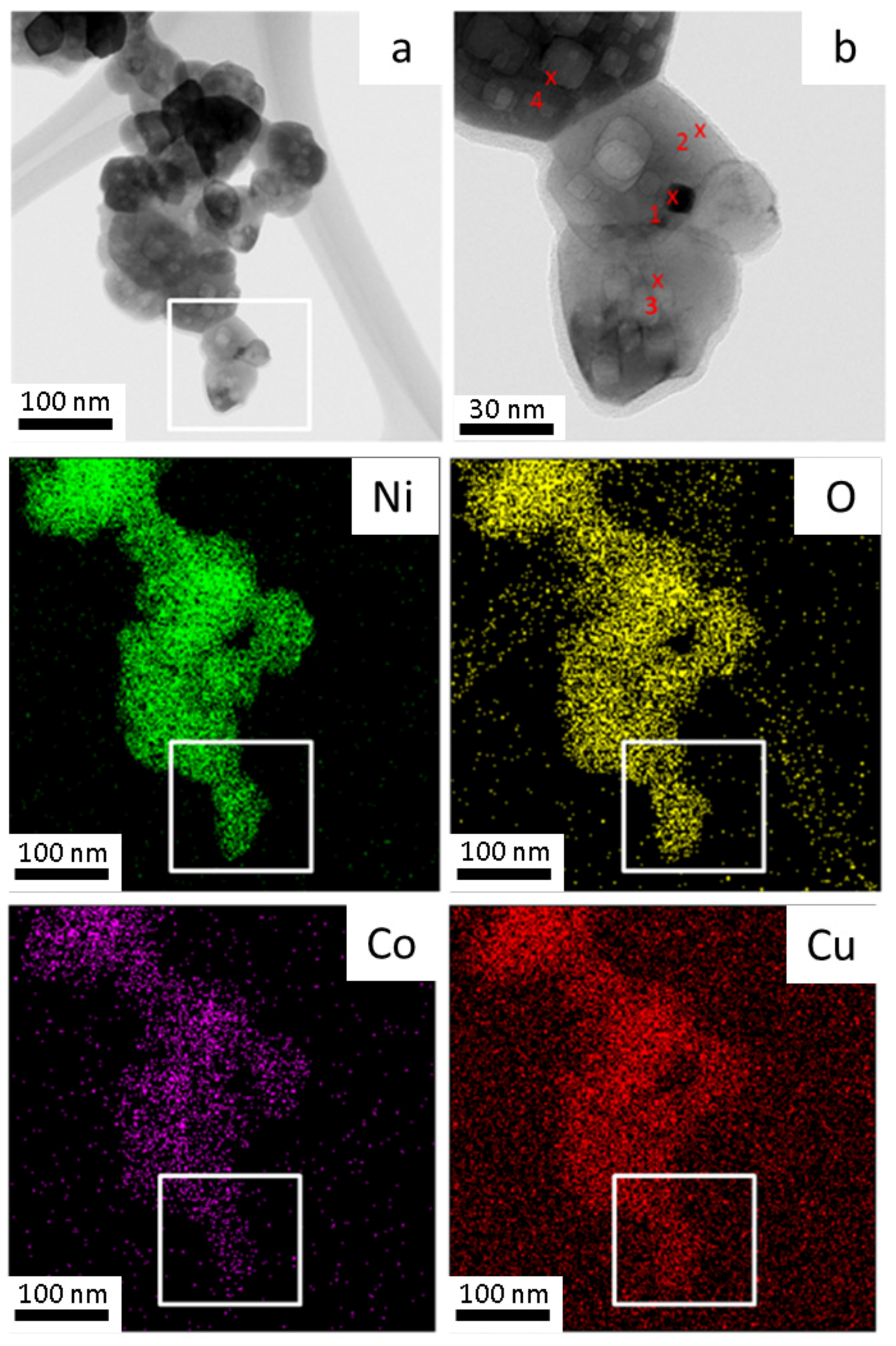
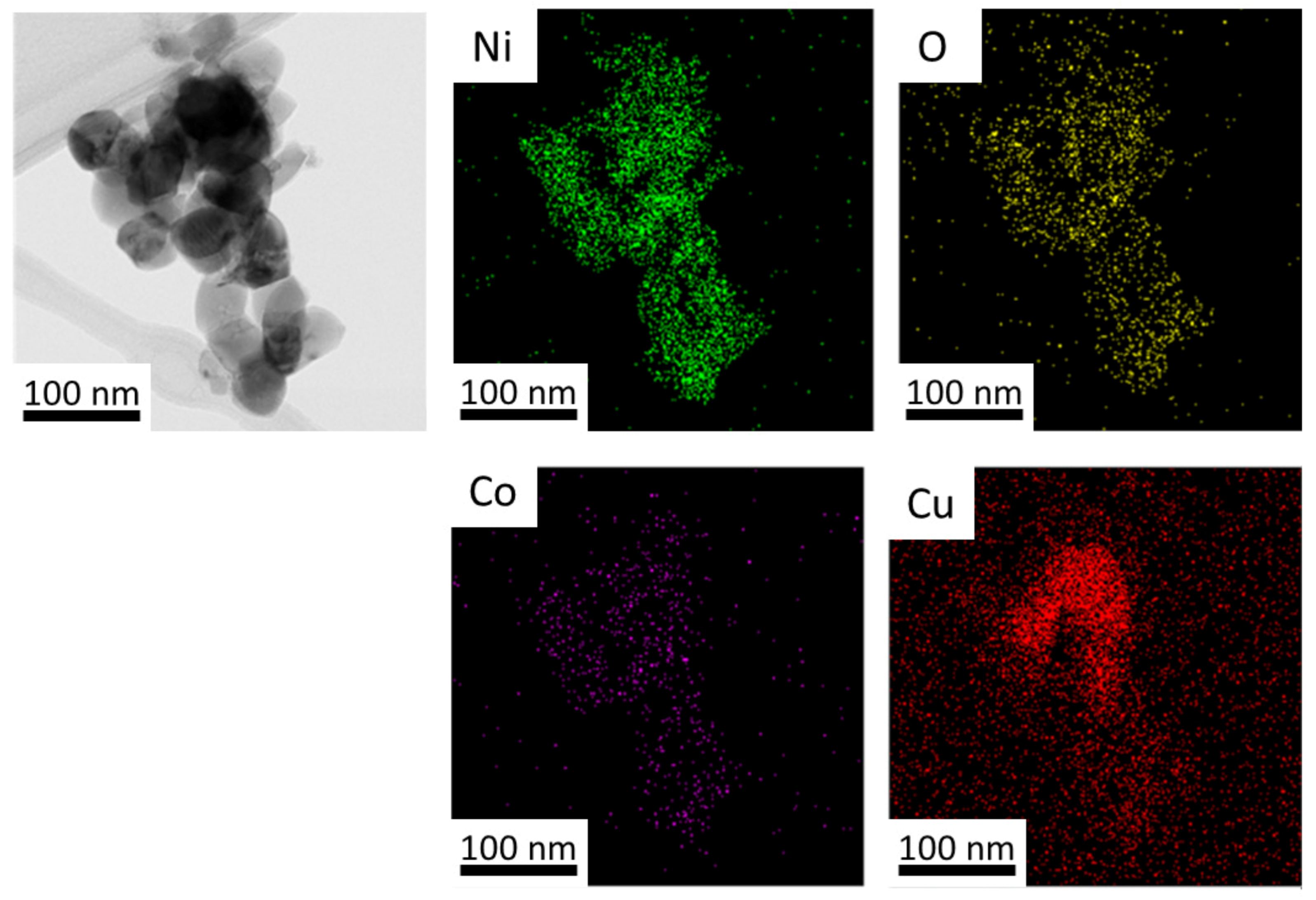

| O | Cu | Ni | Co | ||||
|---|---|---|---|---|---|---|---|
| %wt | %at | %wt | %at | Ni %wt | %at | Co %wt | %at |
| 20.12 | 48.27 | 8.73 | 5.52 | 61.96 | 40.52 | 9.14 | 5.69 |
| Point ↓ | O | Cu | Ni | Co | ||||
|---|---|---|---|---|---|---|---|---|
| %wt | %at | %wt | %at | Ni %wt | %at | Co %wt | %at | |
| 1 | 93.448 | 98.14 | 0.290 | 0.08 | 3.739 | 1.07 | 2.523 | 0.72 |
| 2 | 93.244 | 98.07 | 0.300 | 0.08 | 4.000 | 1.15 | 2.457 | 0.71 |
| 3 | 94.611 | 98.48 | 0.224 | 0.06 | 3.006 | 0.85 | 2.160 | 0.61 |
| 4 | 85.663 | 95.66 | 0.612 | 0.17 | 7.867 | 2.39 | 5.858 | 1.78 |
| Temperature ↓ | O | Cu | Ni | Co | ||||
|---|---|---|---|---|---|---|---|---|
| %wt | %at | %wt | %at | Ni %wt | %at | Co %wt | %at | |
| 300 C | 2.66 | 9.34 | 12.11 | 10.71 | 72.99 | 69.87 | 10.57 | 10.08 |
| 600 C | 1.26 | 4.51 | 10.75 | 9.69 | 76.11 | 74.26 | 11.88 | 11.54 |
| 900 C | 1.94 | 6.80 | 10.68 | 9.42 | 75.51 | 72.11 | 12.28 | 11.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín Castaño, E.P.; Campos, J.B.d.; Solórzano-Naranjo, I.G.; Brocchi, E.d.A. Characterization of Ternary CuNiCo Metallic Nanoparticles Produced by Hydrogen Reduction. Materials 2021, 14, 6006. https://doi.org/10.3390/ma14206006
Marín Castaño EP, Campos JBd, Solórzano-Naranjo IG, Brocchi EdA. Characterization of Ternary CuNiCo Metallic Nanoparticles Produced by Hydrogen Reduction. Materials. 2021; 14(20):6006. https://doi.org/10.3390/ma14206006
Chicago/Turabian StyleMarín Castaño, Eliana Paola, José Brant de Campos, Ivan Guillermo Solórzano-Naranjo, and Eduardo de Albuquerque Brocchi. 2021. "Characterization of Ternary CuNiCo Metallic Nanoparticles Produced by Hydrogen Reduction" Materials 14, no. 20: 6006. https://doi.org/10.3390/ma14206006






