Effect of Microstructure on Thermophysical Properties of Heat-Treated Duplex Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The 1.4462 DSS Phase Diagram
2.3. Heat Treatment
- (a)
- raw material (reference sample);
- (b)
- solution annealed at 900 °C for 1 h followed by slow cooling in the furnace;
- (c)
- solution annealed at 1200 °C for 1 h followed by water quenching;
- (d)
- solution annealed at 1200 °C for 1 h followed by water quenching then aging at 800 °C for 4 h followed by slow cooling in the furnace.
2.4. Sample Preparation
2.5. Surface Morphology Analysis and Vickers Micro-Hardness Measurements
2.6. Thermal Analysis
2.6.1. LFA
2.6.2. DIL
2.6.3. DSC
3. Results and Discussion
3.1. Microstructural Analysis
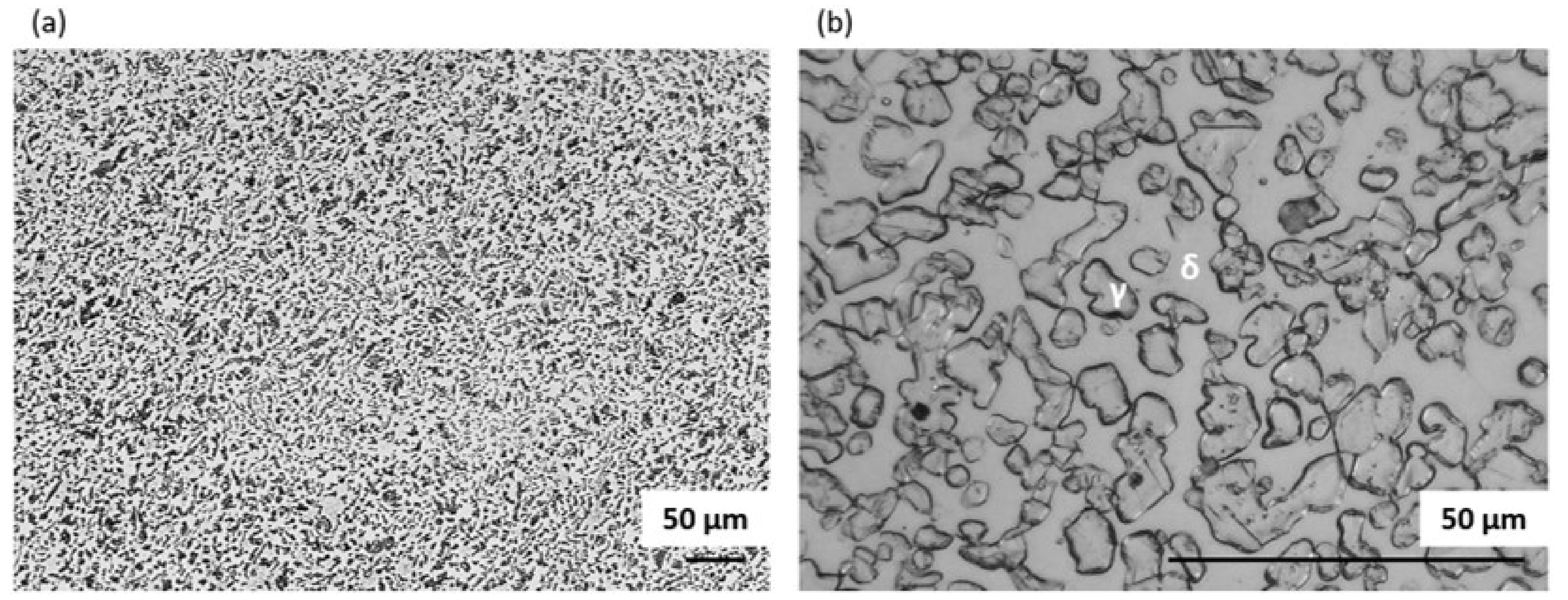
3.1.1. Raw Material
3.1.2. Solution Annealing at 900 °C for 1 h Followed by Slow Cooling in the Furnace
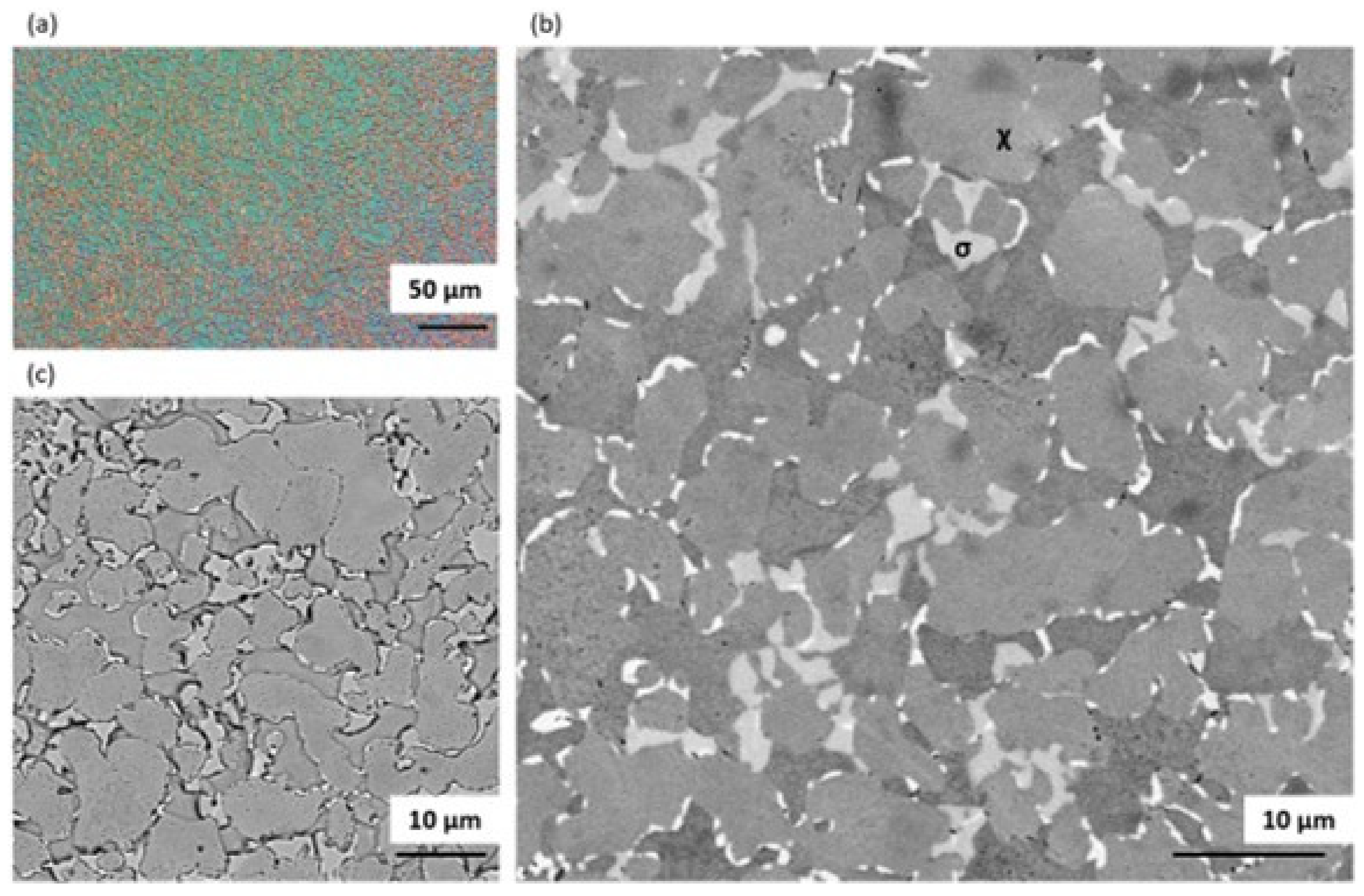
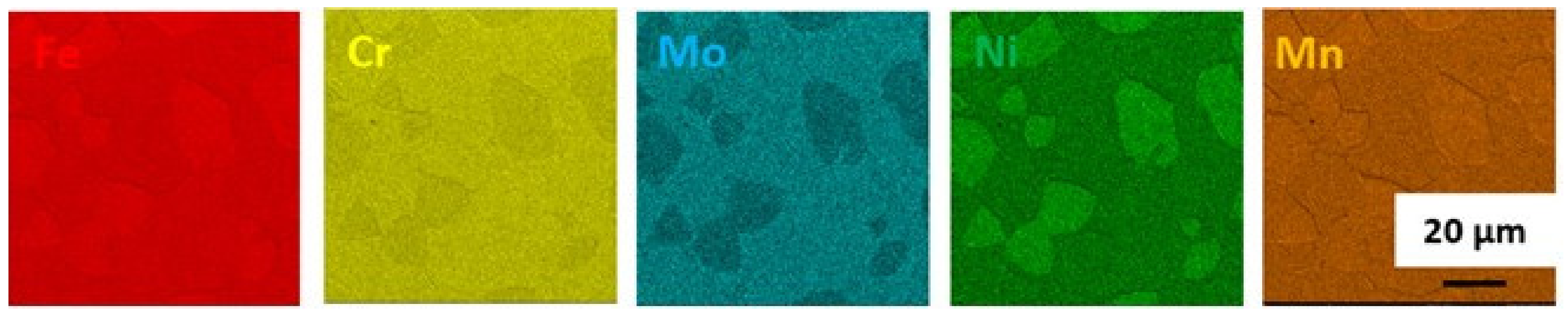
3.1.3. Solution Annealing at 1200 °C for 1 h Followed by Water Quenching
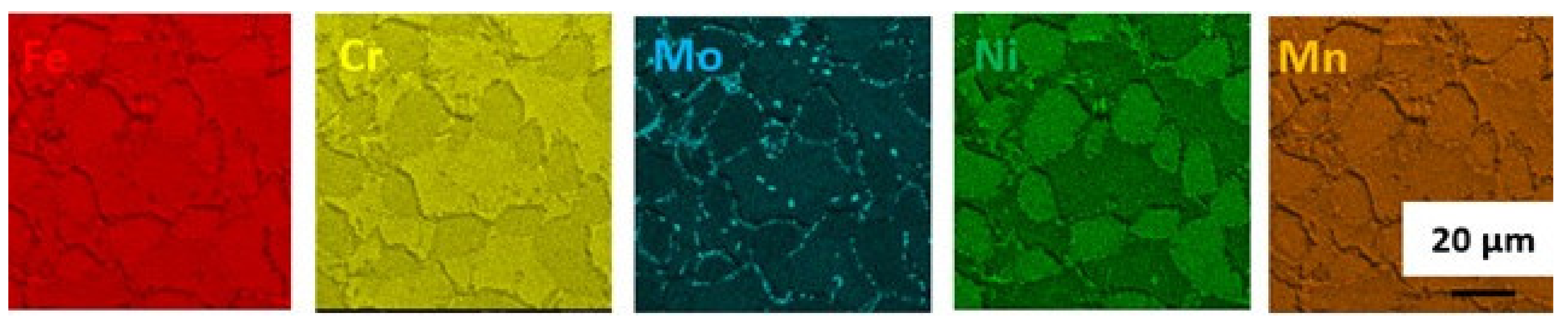
3.1.4. Solution Annealing at 1200 °C for 1 h Followed by Water Quenching Then Aging at 800 °C for 4 h Followed by Slow Cooling in the Furnace
3.1.5. Mechanical Properties
3.1.6. Discussion on Changes in the Microstructure
3.2. Thermal Properties Investigations
3.2.1. LFA Investigations
3.2.2. DIL Investigations
3.2.3. DSC Investigations
4. Conclusions
- (1)
- The analysis of LFA thermograms for the first heating using LFA 427 in the range RT–1000 °C allows us to conclude that:
- (a)
- near 500 °C, the chromium-rich ferrite, i.e., the α′ phase, dissolved and a local minimum of thermal diffusivity for all samples appeared. This effect was greatest for the 75:25 ferrite/austenite sample. In the entire measuring temperature range, i.e., RT–1000 °C, LFA thermograms differed within 5%;
- (b)
- as the ferrite content decreased, the local minimum moved towards a higher temperature, most of all for the 44:56 ferrite/austenite sample (Table 2);
- (2)
- The analysis of LFA thermograms from the first heating using LFA 467 in the range −50–480 °C shows that:
- (a)
- with respect to the specific heat determined from LFA 467 investigations for the first heating using the comparative method:
- an increase in the specific heat value was observed within the temperature range of −50–480 °C, and this was comparable for all samples, i.e., for ferrite/austenite ratios of 75:25, 65:35, 44:56 and for the raw sample.
- (b)
- with respect to the thermal conductivity from LFA 467 investigations for the first heating using the comparative method:
- in the temperature range of −50–480 °C, thermal conductivity increased linearly for all samples from 14 W⋅m−1⋅K−1 to approximately 22 W⋅m−1⋅K−1;
- (3)
- The analysis of DIL thermograms using DIL 402 C for the first heating in the range 200–1000 °C shows that:
- (a)
- at a temperature of approximately 500 °C, the chromium-rich ferrite, or α′ phase, dissolved. The CLTE change effect was greatest for the 75:25 ferrite/austenite sample and decreased with ferrite content;
- (b)
- at approximately 720 °C, there was a peak which corresponds to the calculated Curie temperature (706 °C). The peak was greatest with a ferrite content of 75%. The effect occurred with any ferrite content;
- (c)
- above 900 °C, there was a rapid reduction in thermal expansion. This was caused by the disappearance of austenite as a result of the transformation reaction of the austenitic phase into ferrite. It was particularly visible in the sample with the highest content of austenite, i.e., 56%, as it already appeared at 940 °C;
- (4)
- DIL thermograms using DIL 402 Expedis for the first heating in the range 50–500 °C revealed:
- no precipitation effects were observed in the temperature range 50–480 °C.
- (5)
- The analysis of DSC thermograms using DSC 404 F1 Pegasus for the first heating in the range of 25–1000 °C showed that:
- (a)
- at a temperature of approximately 500 °C, the chromium-rich ferrite, i.e., the α’ phase, dissolved and a first peak in the apparent specific heat temperature characteristic appeared for all 1.4462 DSS samples;
- (b)
- at approximately 900–950 °C, for all but one sample, a second peak appeared, which was connected with the transformation of the austenitic phase into ferrite. For the 75:25 ferrite/austenite sample, this peak was shifted towards higher temperatures out of the measured range.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Charles, J. Composition and properties of duplex stainless steels. Weld. World 1995, 36, 89–97. [Google Scholar]
- Rosso, M.; Peter, I.; Suani, D. About heat treatment and properties of Duplex Stainless Steels. J. Achiev. Mater. Manuf. Eng. 2013, 59, 26–36. [Google Scholar]
- Knyazeva, M.; Pohl, M. Duplex Steels. Part II Carbides and Nitrides. Metallogr. Microstruct. Anal. 2013, 2, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Knyazeva, M.; Pohl, M. Duplex Steels: Part I: Genesis, Formation, Structure. Metallogr. Microstruct. Anal. 2013, 2, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Li, S.L.; Zhang, H.L.; Wang, Y.L.; Li, S.X.; Zheng, K.; Xue, F.; Wang, X.T. Annealing induced recovery of long-term thermal aging embrittlement in a duplex stainless steel. Mater. Sci. Eng. A 2013, 564, 85–91. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, B.; Huang, H.; Li, J.; Wang, J. The effect of large heat input on the microstructure and corrosion behaviour of simulated heat affected zone in 2205 duplex stainless steel. Corros. Sci. 2011, 53, 3756–3763. [Google Scholar] [CrossRef]
- Topolska, S.; Łbanowski, J. Effect of microstructure on impact thougness of duplex and superduplex stainless steels. J. Achiev. Mater. Manuf. Eng. 2009, 36, 142–149. [Google Scholar]
- Hwang, H.; Park, Y. Effects of Heat Treatment on the Phase Ratio and Corrosion Resistance of Duplex Stainless Steel. Mater. Trans. 2009, 50, 1548–1552. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.K.; Mondal, S. High temperature ageing behaviour of a duplex stainless steel. Mater. Charact. 2008, 59, 1776–1783. [Google Scholar] [CrossRef]
- Sieurin, H.; Snadstrom, R. Sigma phase precipitation in duplex stainless steel 2205. Mater. Sci. Eng. A 2007, 444, 271–276. [Google Scholar] [CrossRef]
- Phol, M.; Storz, O.; Glogowski, T. Effect of intermetallic precipitations on the properties of duplex stainless steel. Mater. Charact. 2007, 58, 65–71. [Google Scholar]
- Lecomte-Beckers, J.; Moureaux, P.; Carton, M.; Habraken, A.M. Characterization of Duplex steel Uranus 76N during deformation and heat treatment. Phys. Stat. Sol. 2006, 203, 3651–3664. [Google Scholar] [CrossRef]
- Klancnik, G.; Steiner Petrovic, D.; Medved, J. Thermodynamic Calculation of Phase Equalibria in Stainless Steels. J. Min. Metall. Sec. B Metall. 2012, 48, 383–390. [Google Scholar] [CrossRef]
- Riad, H.; Nabil, K. Experimental Study of the Thermal Diffusivity and Heat Capacity Concerning Some Duplex Stainless Steel. Khwarizmi Eng. J. 2015, 11, 51–61. [Google Scholar]
- Certificate No 2018/02/027146, Grade: 1.4462, ROLDAN, S.A.—Stainless Steels, Spain. 7 August 2018, Delivery Note No 2018/004688.
- Practical Guidelines for the Fabrication Duplex Stainless Steels; International Molybdenum Association: London, UK, 2001.
- Netzsch Proteus ver. 7.1 Software Manual. Available online: https://www.netzsch-thermal-analysis.com/en/products-solutions/software/proteus/ (accessed on 2 March 2020).
- Mészáros, I. Possibility of Indirect Hardness Testing of Duplex Stainless Steel. Available online: http://anyagokvilaga.hu/tartalom/2004/dec/06_MI.pdf (accessed on 16 June 2021).
- Li, J.; Shen, W.; Lin, P.; Wang, F.; Yang, Z. Effect of Solution Treatment Temperature on Microstructural Evolution, Precipitation Behavior, and Comprehensive Properties in UNS S32750 SuperDuplex Stainless Steel. Materials 2020, 10, 1481. [Google Scholar]
- Bugat, S.; Besson, J.; Gourgues, A.F.; Guyrn, F.; Pineau, A. Microstructure and damage initation in duplex stainless steels. Mater. Sci. Eng. A 2001, 317, 32–36. [Google Scholar] [CrossRef]
- Leber, H.J.; Niffenegger, M.; Tirbonod, B. Microstuctural aspects of low cycle fatigued austenitic stainless tube and pipe steel. Mater. Sci. Eng. A 2001, 314, 32–36. [Google Scholar]
- Man, J.; Obrtlik, K.; Polak, J. Study of surface relief evoluetion in fatigued 316L austenitic stainless steel by AFM. Mater. Sci. Eng. A 2003, 351, 123–132. [Google Scholar] [CrossRef]
- Massol, K.; Vogt, J.B.; Foct, J. Fatigue behavior of new duplex stainless steels upgraded by nitrogen alloying. ISIJ Int. 2002, 42, 310–315. [Google Scholar] [CrossRef]
- Willis, C.F.; Gronsky, R.; Devine, T.M. Carbide precipitation in welds of two-phase austenitic–ferritic stainless steel. Metall. Trans. 1991, 22, 2289–2902. [Google Scholar] [CrossRef]
- Elmer, J.W.; Allen, S.M.; Eagar, T.W. The influence of cooling rate on the ferrite content of stainless steel alloys. In Proceedings of the 2nd International Conference on Trends in Welding Resarch, Gatlinburg, TN, USA, 15 May 1989; pp. 165–170. [Google Scholar]
- Schaeffler, A. Constitution Diagram for Stainless Steel Weld Metal. Metal Prog. 1949, 56, 680. [Google Scholar]
- Delong, W.T. Ferrite in Austenitic Weld Metal. Weld. J. 1974, 53, 273s–286s. [Google Scholar]
- ASTM A800/A800M Standard practice for Steel Casting, Austenitic Alloy, Estimating Ferrite Content Thereof.
- Kotechi, D.J.; Siewert, T.A. WRC-1992 Constitution Diagram for stainless steel weld metals: A modification of the WRC-1988 Diagram. Weld. Res. Suppl. 1992, 71, 171s–178s. [Google Scholar]
- Cortie, B.; Jackson, M. Simulation of the precipitation of sigma phase in duplex stainless steels. Metall. Mater. Trans. A 1997, 28, 2477–2484. [Google Scholar] [CrossRef]
- Villanueva, D.M.E.; Junior, F.C.P.; Plaut, R.L.; Padilha, A.F. Comparative study on sigma phase precipitation of three types of stainless steels: Austenitic, superferritic and duplex. Mater. Sci. Technol. 2006, 22, 1098–1104. [Google Scholar] [CrossRef]
- Roscoe, C.V.; Gradwell, K.J.; Lorimer, G.W. Stainless Steels’84: Proceedings of the Conference. Institute of Metals: London, UK, 1985. [Google Scholar]
- Pohl, M.; Storz, O. Sigma phase in duplex stainless steels. Zeit Met. 2004, 95, 7. [Google Scholar] [CrossRef]
- Mola, M. Numerische Legierungsentwicklung eines Nickelreduzierten Duplex-Stahls; European University: Bochum, Germany, 2005. [Google Scholar]
- Strutt, A.J.; Lorimer, G.N.; Roscoe, C.V.; Gradwell, K.J. Structure property relationships of Zeron 100. In Proceedings of the International Conference on Duplex Stainless Steels, The Hague, The Netherlands, 26–28 October 1986; pp. 310–318. [Google Scholar]
- Escriba, D.M.; Materna-Morris, E.; Plaut, R.L.; Padilha, A.F. Chi-phase precipitation in a duplex stainless steel. Mater. Charact. 2009, 60, 1214–1219. [Google Scholar] [CrossRef]
- Marchewka, J. Measurements of Thermal Expansion of Selected Alloy Steels. Master’s Thesis, Military University of Technology, Warsaw, Poland, 2018. [Google Scholar]
- Jóźwiak, S. Analysis of Thermally Activated Changes in the Structure and Their Influence on the Corrosion Resistance and Mechanical Properties of the OOH2ON5M1 Two-Phase Steel. Ph.D. Thesis, Military University of Technology, Warsaw, Poland, 1993. (In Polish). [Google Scholar]
- Shamanth, V.; Ravishankar, K.S. Dissolution of alpha—prime precipitates in thermally embrittled S2205—duplex steels during reversion-heat treatment. Results Phys. 2015, 5, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Elmer, J.W.; Palmer, T.A.; Specht, E.D. In suit observations of sigma phase in 2205 duplex stainless steel using synchrotron X-ray diffraction. Mater. Sci. Eng. A 2007, 497, 151–155. [Google Scholar] [CrossRef] [Green Version]














| Component | C | Mn | Cr | Ni | Mo | N | Cu | Si | Ti |
|---|---|---|---|---|---|---|---|---|---|
| Concentration [wt.%] | 0.023 | 1.605 | 22.310 | 4.745 | 3.135 | 0.172 | 0.141 | 0.364 | 0.017 |
| Component | P | S | Fe | ||||||
| Concentration [wt.%] | 0.025 | 0.001 | Balance | ||||||
| Initial | 900 °C | 1200 °C | 1200 °C + 800 °C | |
|---|---|---|---|---|
| HRC (HV1) | 32.58 (323) | 37.51 (367) | 25.48 (269) | 27.88 (285) |
| Ferrite/Austenite | Raw | 75:25 | 65:35 | 44:56 |
|---|---|---|---|---|
| Minimum [°C] | 508.6 | 514.6 | 524.0 | 525.4 |
| (a) Ferrite/austenite in the ratio 75:25 | |||
| Coefficient | Value | Coefficient | Value |
| 5.39 × 10−1 | −2.36 × 10−8 | ||
| 1.71 × 10−4 | −2.54 × 10−1 | ||
| (b) Ferrite/austenite in the ratio 65:35 | |||
| Coefficient | Value | Coefficient | Value |
| 5.51 × 10−1 | 1.29 × 10−7 | ||
| 4.95 × 10−5 | −2.01 × 10−1 | ||
| (c) Ferrite/austenite in the ratio 44:56 | |||
| Coefficient | Value | Coefficient | Value |
| 4.56 × 10−1 | −8.23 × 10−8 | ||
| 3.07 × 10−4 | −1.55 × 10−2 | ||
| (d) Raw sample | |||
| Coefficient | Value | Coefficient | Value |
| 5.39 × 10−1 | −1.74 × 10−7 | ||
| 3.25 × 10−4 | −0.31 × 10−9 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koniorczyk, P.; Sienkiewicz, J.; Zmywaczyk, J.; Dębski, A.; Zieliński, M.; Preiskorn, M. Effect of Microstructure on Thermophysical Properties of Heat-Treated Duplex Steel. Materials 2021, 14, 6043. https://doi.org/10.3390/ma14206043
Koniorczyk P, Sienkiewicz J, Zmywaczyk J, Dębski A, Zieliński M, Preiskorn M. Effect of Microstructure on Thermophysical Properties of Heat-Treated Duplex Steel. Materials. 2021; 14(20):6043. https://doi.org/10.3390/ma14206043
Chicago/Turabian StyleKoniorczyk, Piotr, Judyta Sienkiewicz, Janusz Zmywaczyk, Andrzej Dębski, Mateusz Zieliński, and Marek Preiskorn. 2021. "Effect of Microstructure on Thermophysical Properties of Heat-Treated Duplex Steel" Materials 14, no. 20: 6043. https://doi.org/10.3390/ma14206043







