Tribological Behavior of Ionic Liquid with Nanoparticles
Abstract
:1. Introduction
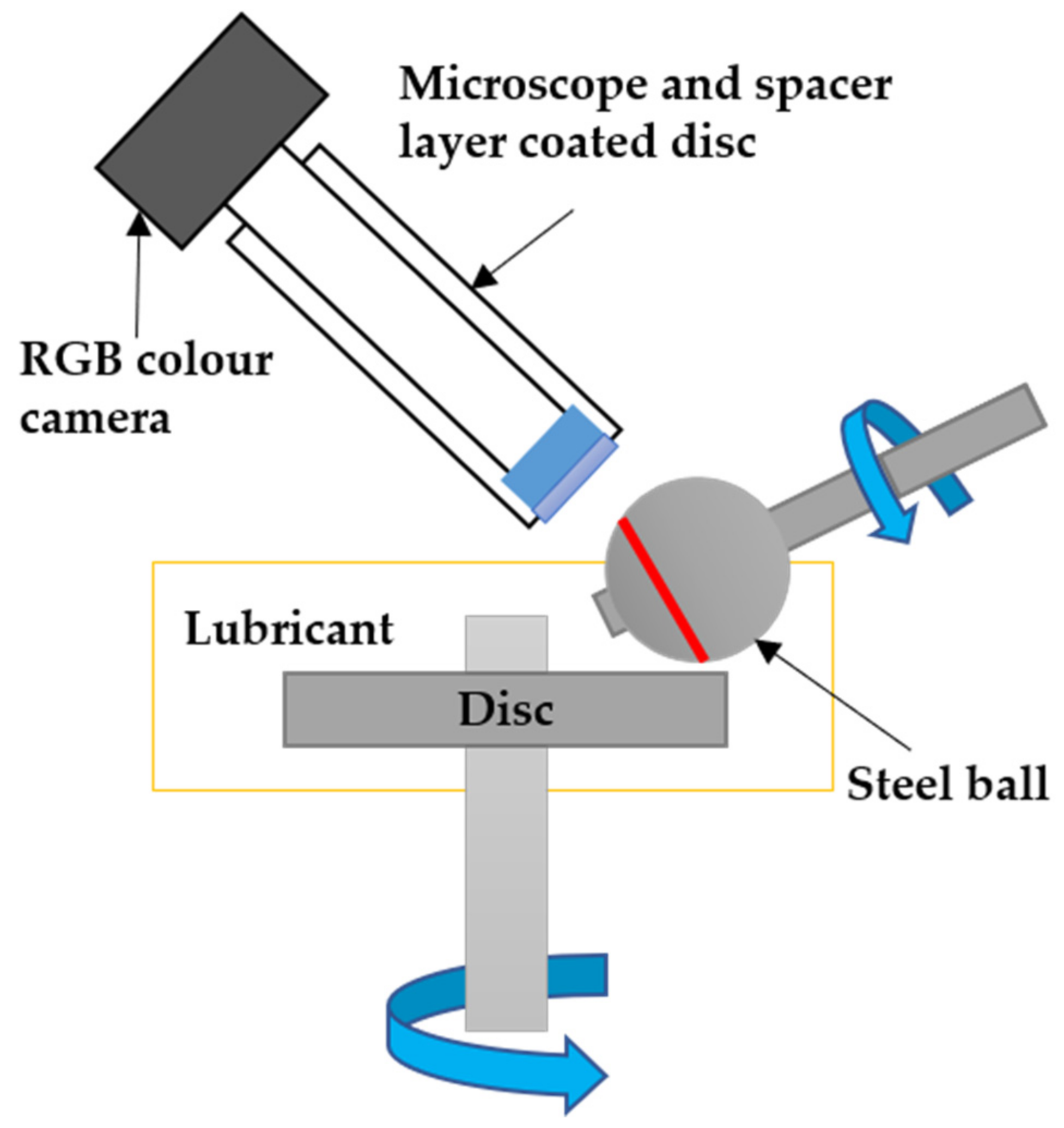
2. Experimental Description
3. Results and Discussion
3.1. Friction and Wear Behaviors
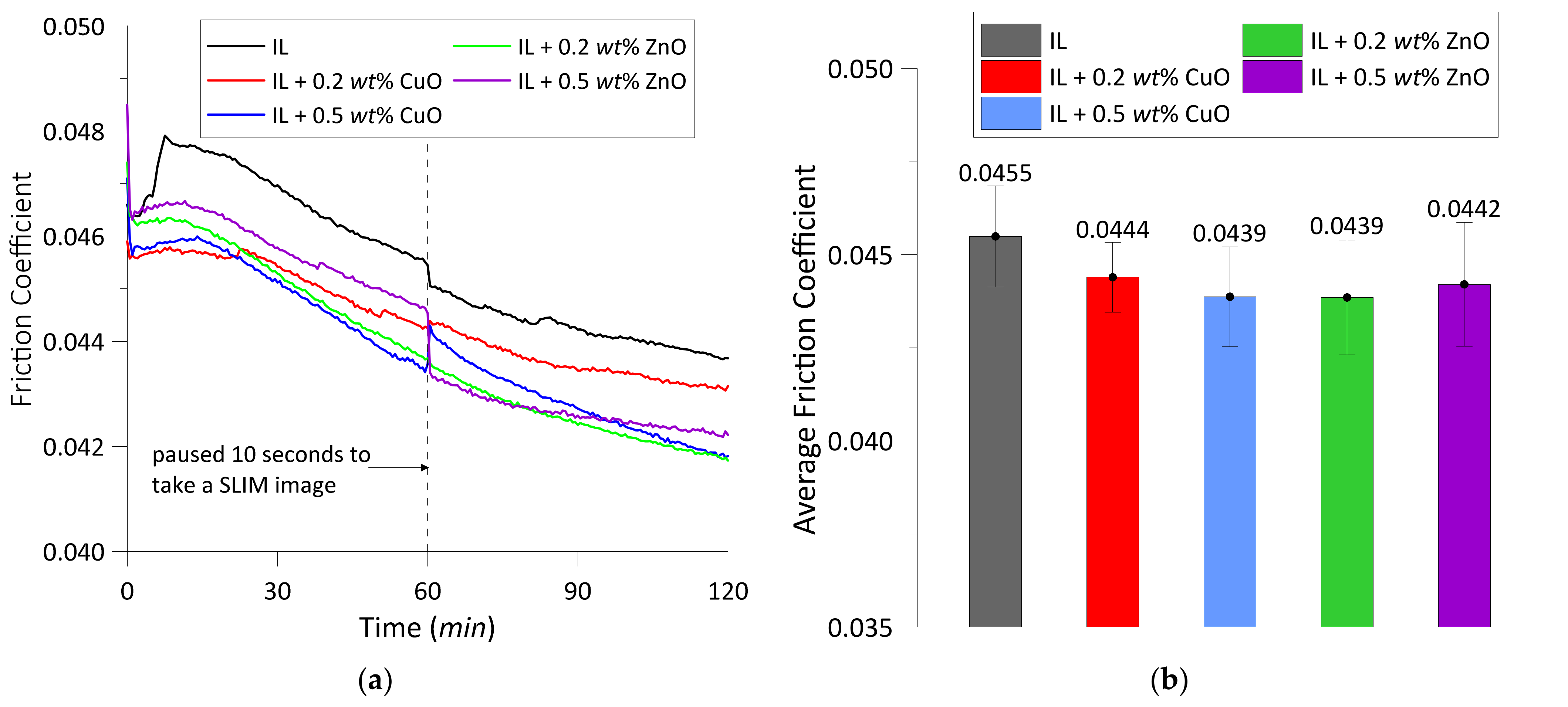
3.1.1. Friction Coefficients
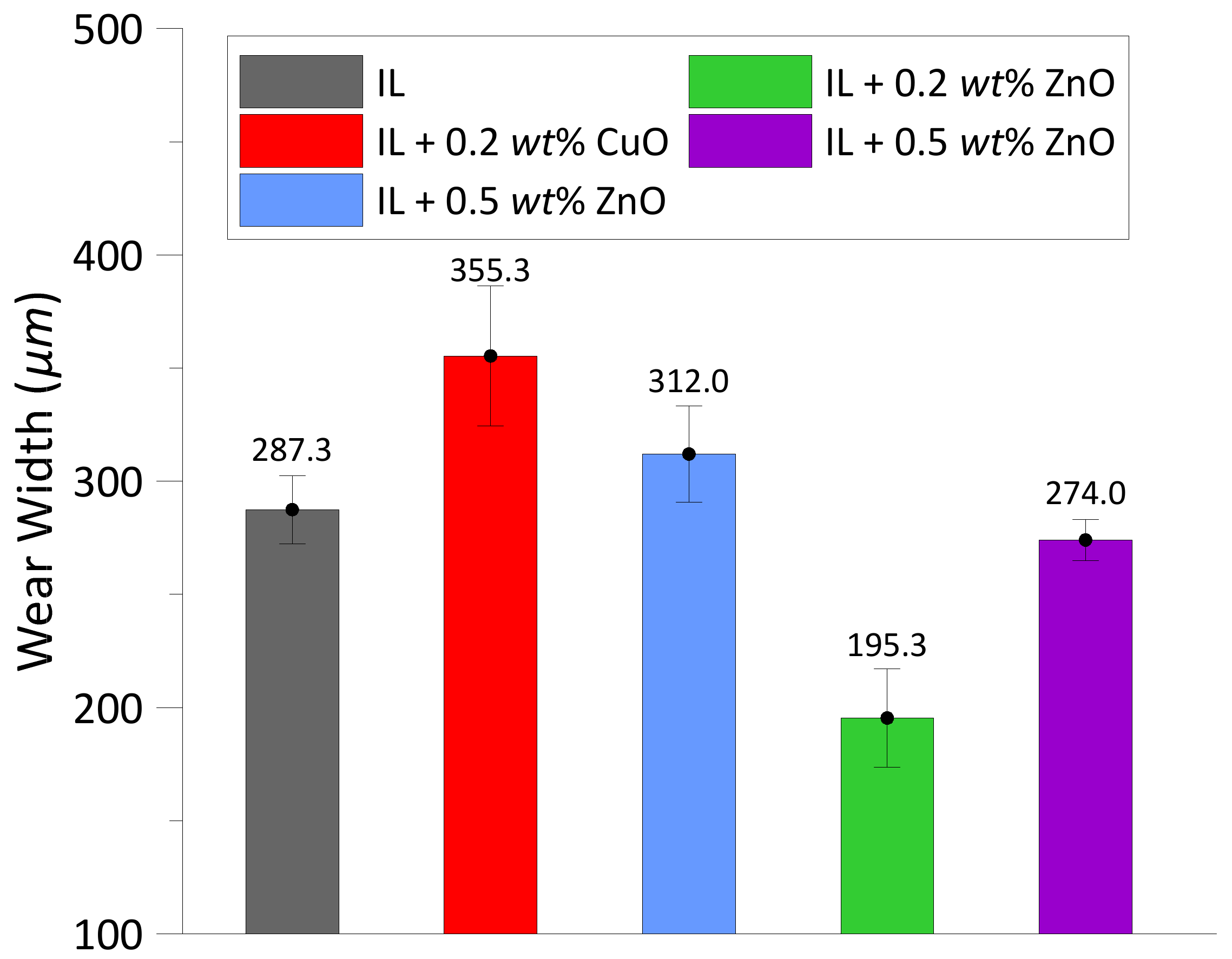
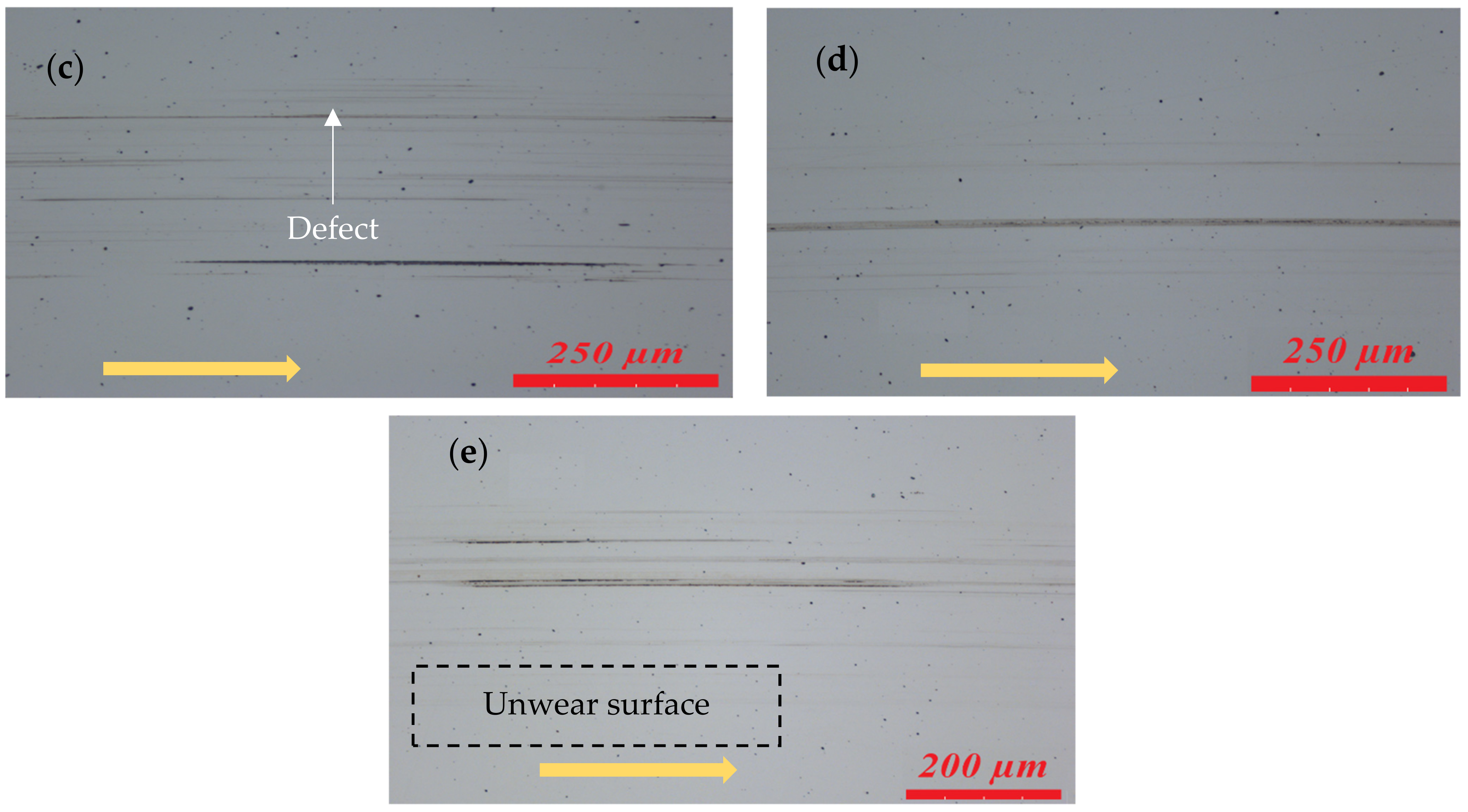
3.1.2. Wear
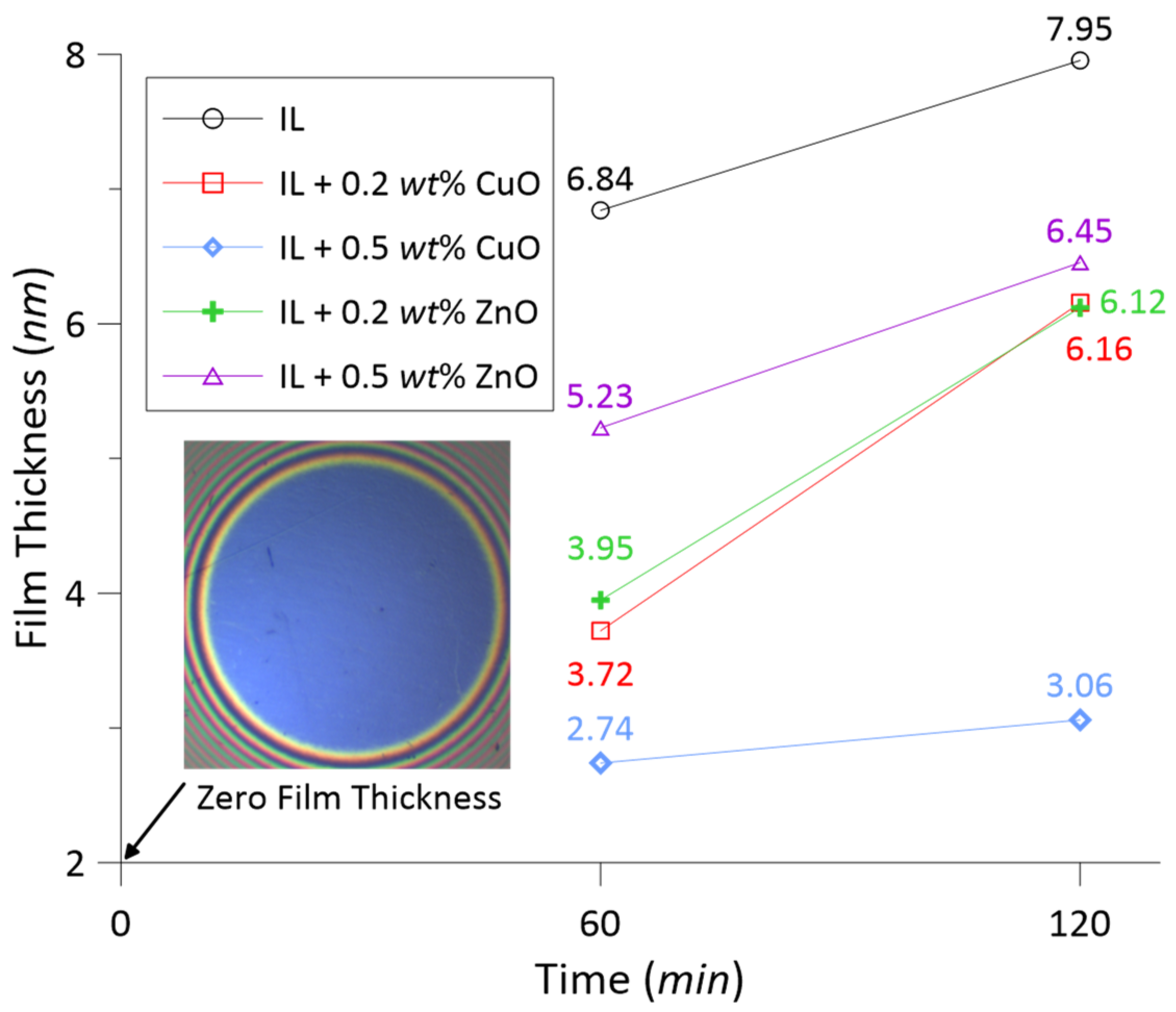
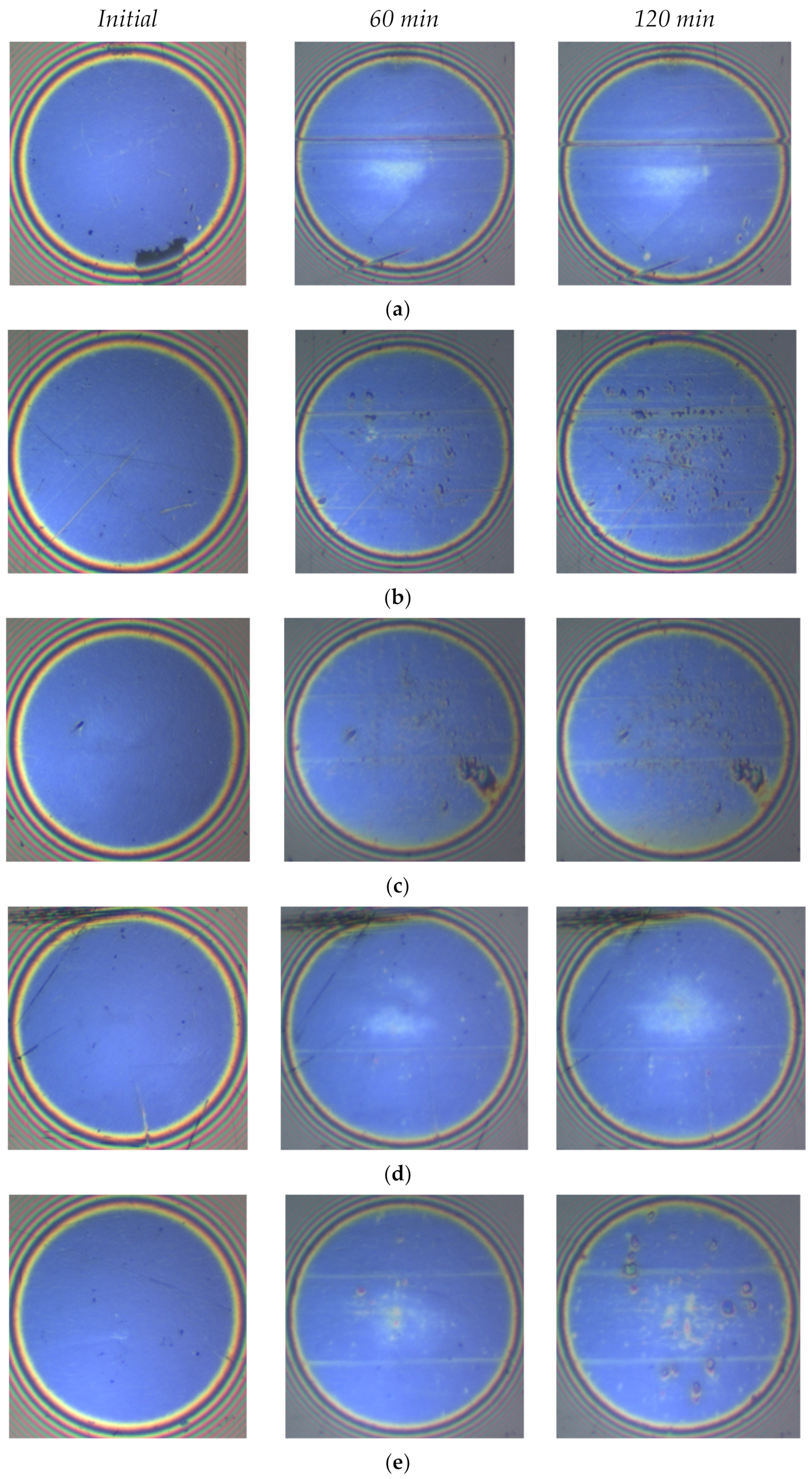
3.2. Tribofilm Thickness
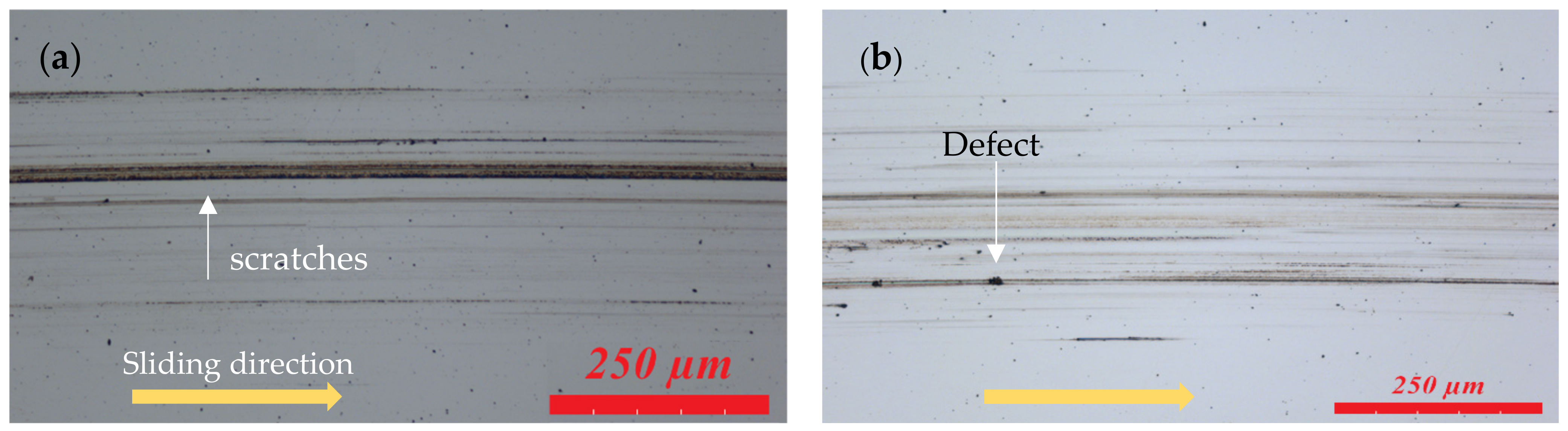
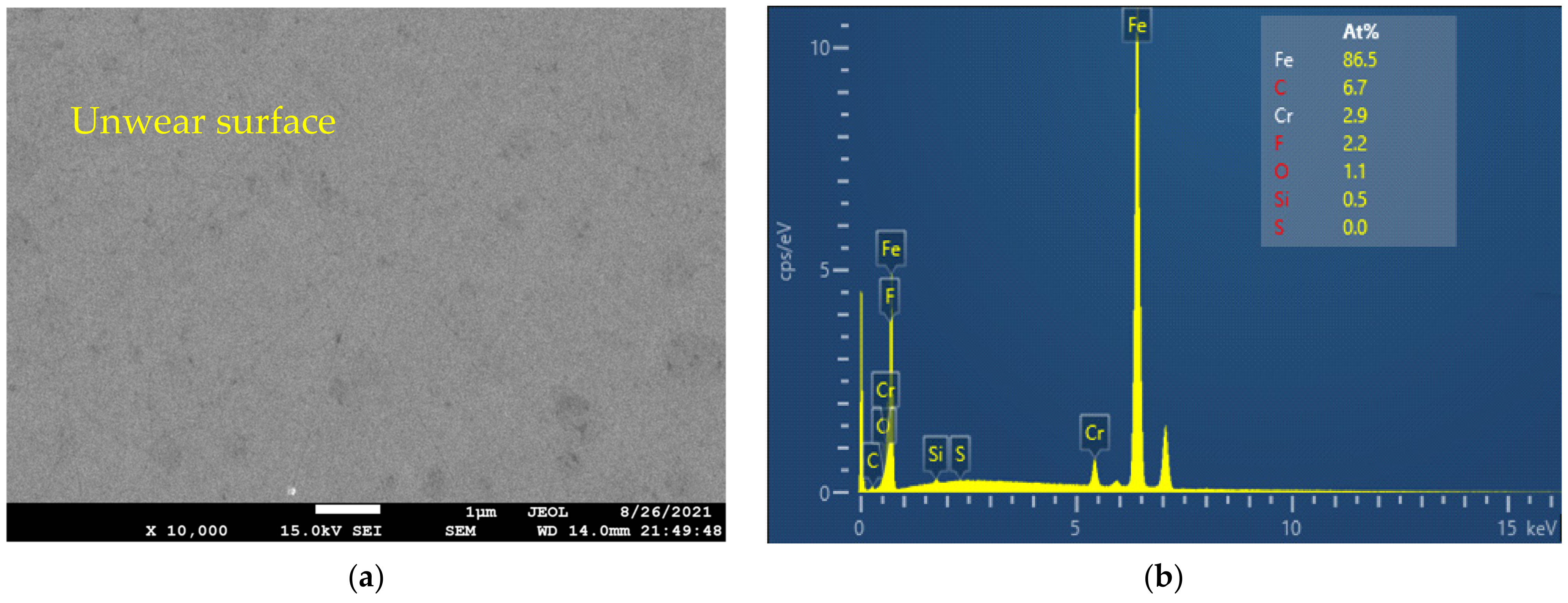
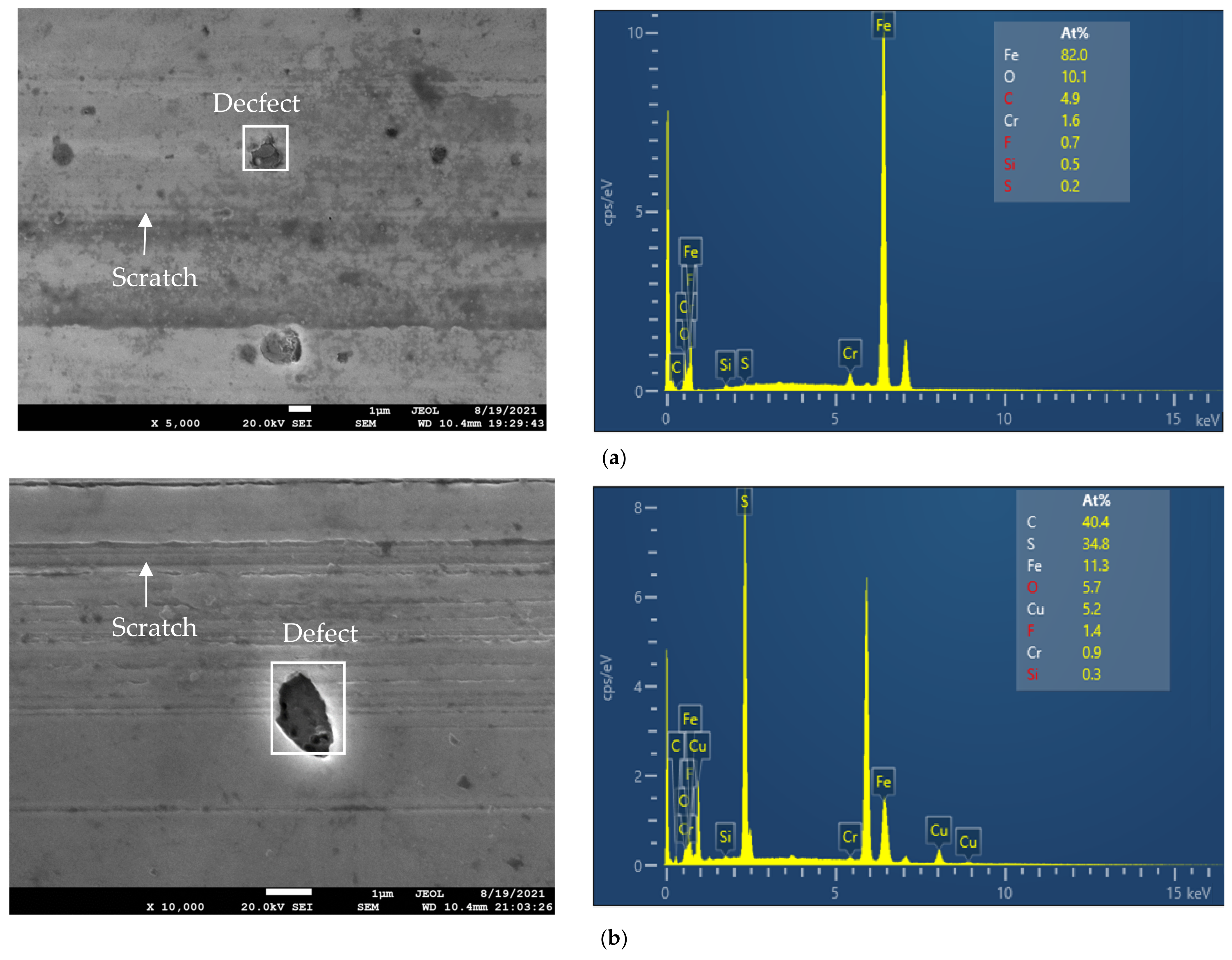
3.3. Surface Analysis
4. Conclusions
- (1)
- At the same concentrations of 0.2 and 0.5 wt%, the ZnO nanoparticles enhanced the tribological behavior of IL better than the CuO nanoparticles.
- (2)
- Compared to the test lubricated with the IL, the addition of both oxide nanoparticles in the IL could reduce the friction coefficient by approximately 2.4% to 3.5% depending on their concentrations. However, the wear width was decreased by 4.6% to 32% in the experiments with ZnO, while it was increased by 8.6% to 23.7% in the experiments with CuO. Hence, ZnO nanoparticles in the IL show a better result in reducing wear width than the CuO nanoparticles. This behavior could be related to the fact that CuO nanoparticles have a lower bulk hardness than ZnO and are thus more likely to be deposited and flattened at the contact area, resulting in a larger interface for friction [34].
- (3)
- From the EDX analysis results, it can be seen that the lubrication mechanisms of CuO and ZnO, when used as additives in the IL, are tribo-sintering and third body with pure rolling mechanisms, respectively.
- (4)
- Out of the two types of nanoparticles and two concentrations tested, the best tribological performance was observed at the concentration of 0.2 wt% ZnO.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, C.F.; Liu, W.M.; Chen, Y.X.; Yu, L.G. Room-temperature ionic liquids: A novel versatile lubricant. Chem. Commun. 2001, 2244–2245. [Google Scholar] [CrossRef]
- Minami, I. Ionic liquids in tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, T.; Rahid, R.; Mufti, R.A.; Waqas, M.; Hanif, M.T. A review on tribological performance of ionic liquids as additives to bio lubricants. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2021, 235, 1782–1806. [Google Scholar] [CrossRef]
- Khemchandani, B.; Somers, A.; Howlett, P.; Jaiswal, A.K.; Sayanna, E. A biocompatible ionic liquid as an antiwear additive for biodegradable lubricants. Tribol. Int. 2014, 77, 171–177. [Google Scholar] [CrossRef]
- Barnhill, W.C.; Gao, H.; Kheireddin, B.; Papke, B.L.; Luo, H.; West, B.H.; Qu, J. Tribological bench and engine dynamometer tests of a low viscosity SAE 0W-16 engine oil using a combination of ionic liquid and ZDDP as anti-wear additives. Front. Mech. Eng. 2015, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Battez, A.H.; Blanco, D.; Gonzalez, A.F.; Mallada, M.T.; Gonzalez, R.; Viesca, J.L. Friction, wear and tribofilm formation with a [NTf2] anion-based ionic liquid as neat lubricant. Tribol. Int. 2016, 103, 73–86. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbyte, M.; Kupcinskas, A.; Kazancev, K.; Ta, T.N.; Horng, J.H. Investigation of tribological properties of two protic ionic liquids as additives in water for steel-steel and alumina-steel contacts. Wear 2020, 456–457, 203390. [Google Scholar] [CrossRef]
- Nainaparampil, J.J.; Eapen, K.C.; Sanders, J.H.; Voevodin, A.A. Ionic-liquid lubrication of sliding MEMS contacts: Comparison of AFM liquid cell and device-level tests. J. Microelectromech. Syst. 2007, 16, 836–843. [Google Scholar] [CrossRef]
- Kumara, C.; Speed, L.; Viola, M.B.; Luo, H.; Qu, J. Using ionic liquid additive to enhance lubricating performance for low-viscosity engine oil. ACS Sustain. Chem. Eng. 2021, 9, 21. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, S.R. Ionic liquids as environmental friendly cutting fluids-a review. Mater. Proc. 2021, 37, 2121–2125. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. A review of ionic liquid lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Alves, S.M.; Mello, V.S.; Faria, E.A.; Camargo, A.P.P. Nanolubricants developed from tiny CuO nanoparticles. Tribol. Int. 2016, 100, 263–271. [Google Scholar] [CrossRef]
- Kumar, G.; Garg, H.C.; Gijawara, A. Experimental investigation of tribological effect on vegetable oil with CuO nanoparticles and ZDDP additives. Ind. Lub. Tribol. 2019, 71, 499–508. [Google Scholar] [CrossRef]
- Thottackkad, M.V.; Perikinalil, R.K.; Kumarapillai, P.N. Experimental evaluation on the tribological properties of coconut oil by the addition of CuO nanoparticles. Int. J. Precis. Eng. Manuf. 2012, 13, 111–116. [Google Scholar] [CrossRef]
- Azman, N.F.; Samion, S.; Amirrul, M.; Moen, A.; Hamid, M.K.A.; Musa, M.N. The anti-wear and extreme pressure performances of CuO and graphite nanoparticles as an additive in palm oil. Int. J. Struct. Integr. 2019, 10, 714–725. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanola, A.; Garg, H.C.; Kumar, G. Improving the tribological performance of canola oil by adding CuO nanoadditives for steel/steel contact. Mater. Today Proc. 2020, 28, 1392–1396. [Google Scholar] [CrossRef]
- Jain, A.K.; Kumar, M.; Thakre, G.D. Potential of CuS and CuO nanoparticles for friction reduction in piston ring-line contact. Tribol.-Mater. Surf. Interfaces 2021. [Google Scholar] [CrossRef]
- Gupta, H.; Rai, S.K.; Krishna, N.S.; Anand, G. The effect of copper oxide nanoparticle additives on the rheological and tribological properties of engine oil. J. Dispers. Sci. Technol. 2021, 42, 622–632. [Google Scholar] [CrossRef]
- Abdel-Rehim, A.A.; Akl, S.; Elsoudy, S. Investigation of the tribological behavior of mineral lubricant using copper oxide nano additives. Lubricants 2021, 9, 16. [Google Scholar] [CrossRef]
- Wu, C.; Xiong, R.; Ni, J.; Yao, L.; Chen, L.; Li, X. Effects of CuO nanoparticles on friction and vibration behaviors of grease on rolling bearing. Tribol. Int. 2020, 152, 106552. [Google Scholar] [CrossRef]
- Mana, A.P.; Shah, A.B.; Kothari, K. A comparative tribological performance of lubricating oils with Zinc Dialkyl Dithiophosphate and zinc oxide nanoparticles as additives. SAE Technol. Pap. 2019, 2688–3627. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Estelle, P. Experimental comparison between ZnO and MoS2 nanoparticles as additives on performance of diesel oil-based nano lubricant. Sci. Rep. 2020, 10, 5813. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.B.; Heris, S.Z. Experimental investigation of ZnO nanoparticles effects on thermophysical and tribological properties of diesel oil. Int. J. Hydrog. Energy 2020, 45, 23603–23614. [Google Scholar] [CrossRef]
- Mello, S.M.; Trajano, M.F.; Guedes, A.E.D.S.; Alves, S.M. Comparison between the action of nano-oxides and conventional EP additives in boundary lubrication. Lubricants 2020, 8, 54. [Google Scholar] [CrossRef]
- Vyavhare, K.; Timmons, R.B.; Erdemir, A.; Edwards, B.L.; Aswath, P.B. Tribochemistry of fluorinated ZnO nanoparticles and ZDDP lubricated interface and implications for enhanced anti-wear performance at boundary lubricated contacts. Wear 2021, 474–475, 203717. [Google Scholar] [CrossRef]
- Verbic, A.; Sala, M.; Jerman, I.; Gorjanc, M. Novel green in situ synthesis of ZnO nanoparticles on cotton using pomegranate peel extract. Materials 2021, 14, 4472. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Rahman, A.; Tajuddin; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Chang, B.P.; Akil, H.M.; Nasir, R.B.M.; Bandara, M.C.C.D.; Rajapakse, S. The effect of ZnO nanoparticles on the mechanical, tribological and antibacterial properties of ultra-high molecular weight polyethylene. J. Reinf. Plast. Comp. 2013, 1–13. [Google Scholar] [CrossRef]
- Sinha, M.K.; Madarkar, R.; Ghosh, S.; Rao, P.V. Application of eco-friendly nanofluids during grinding of Inconel 718 through small quantity lubrication. J. Clean. Prod. 2017, 141, 1359–1375. [Google Scholar] [CrossRef]
- AZO Materials. Available online: https://www.azom.com/article.aspx?ArticleID=6704 (accessed on 7 September 2021).
- Sigma-Aldrich. Available online: https://www.sigmaaldrich.com (accessed on 30 August 2021).
- Akbarzadeh, A.; Mehdizadeh, M.; Akbarzadeh, S.; Shams, K. Effect of Nanoparticles on the Running-in Behavior in Lubricated Point Contact. In Proceedings of the STLE 70th Annual Meeting & Exhibition, Dallas, TX, USA, 17–21 May 2015. [Google Scholar]
- Battez, A.H.; Gonzalez, R.; Viesca, J.L.; Fernandez, J.E.; Fernandez, J.M.D.; Machado, A.; Chou, R.; Riba, J. CuO, ZrO2, and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 2008, 265, 422–428. [Google Scholar] [CrossRef]
- Darabi, L.; Zare, M. High correlate simple equations for temperature and pressure dependence of the viscosity of ionic liquids. Chem. Phys. 2020, 539, 110933. [Google Scholar] [CrossRef]
- Luo, Z.; Zuo, J.; Jiang, H.; Geng, W.; Zhou, Y.; Lian, Z.; Wei, W. Inhibition effect of fluoride ion on corrosion of 304 stainless steel in occluded cell corrosion stage in the presence of chloride ion. Metals 2021, 11, 350. [Google Scholar] [CrossRef]
- Richter, J.; Ruck, M. Dissolution of metal oxides in task-specific ionic liquid. RSC Adv. 2019, 9, 29699–29710. [Google Scholar] [CrossRef] [Green Version]
- Almira, A.; Chen, Y.Y.; Horng, J.H. Effect of rolling on the friction coefficient in three-body contact. Adv. Mech. Eng. 2019, 11, 1687814019872303. [Google Scholar] [CrossRef] [Green Version]
- Bhaumik, S.; Pathak, S.D. Analysis of anti-wear properties of CuO nanoparticles as friction modifiers in mineral oil (460cSt viscosity) using pin-on-disk tribometer. Tribol. Ind. 2015, 37, 196–203. [Google Scholar]
- Chung, Y.W.; Homola, A.M.; Street, G.B. Surface Science Investigations in Tribology: Experimental Approaches; American Chemical Society: Washington, DC, USA, 1992; pp. 15–42. [Google Scholar]
- Master Organic Chemistry. Available online: https://www.masterorganicchemistry.com/2011/08/01/oxidation-and-reduction-in-organic-chemistry/ (accessed on 30 September 2021).


| Material Properties | Specimens | |
|---|---|---|
| Ball | Disc | |
| Material | AISI 52100 steel | AISI 52100 steel |
| Hardness | 800–920 HV | 720–780 HV |
| Surface roughness | Ra 0.02 μm | Ra 0.02 μm |
| Element | Fe | Cr | C | Mn | Si |
|---|---|---|---|---|---|
| Weight (%) | 96.50–97.32 | 1.30–1.60 | 0.98–1.10 | 0.25–0.45 | 0.15–0.30 |
| Name (CAS Number) | Methyltrioctylammonium Bis(Trifluoromethylsulfonyl)imide (375395-33-8) | ||||
|---|---|---|---|---|---|
| Properties | Cation [N1888] | Anion [NTf2] | Purity (%) | Viscosity 40 °C (cSt) | Molecular Weight |
C25H54N+ | C2F6S2O4N−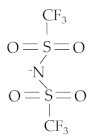 | 99 | 200.7 | 648.85 | |
| Element | IL | IL + 0.2 wt% CuO | IL + 0.5 wt% CuO | IL + 0.2 wt% ZnO | IL + 0.5 wt% ZnO |
|---|---|---|---|---|---|
| Fe | 83.8 | 81.4 | 81.4 | 83.3 | 85.0 |
| C | 10.9 | 11.5 | 13.6 | 12.9 | 10.6 |
| O | 2.0 | 3.2 | 2.1 | 1.1 | 1.0 |
| F | 1.8 | 1.7 | 0.5 | 0.8 | 1.7 |
| Cr | 1.1 | 1.2 | 1.1 | 1.3 | 1.3 |
| Si | 0.4 | 0.5 | 0.6 | 0.4 | 0.5 |
| S | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cu | 0.0 | 0.4 | 0.7 | 0.0 | 0.0 |
| Zn | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ta, T.-N.; Chern, S.-Y.; Horng, J.-H. Tribological Behavior of Ionic Liquid with Nanoparticles. Materials 2021, 14, 6318. https://doi.org/10.3390/ma14216318
Ta T-N, Chern S-Y, Horng J-H. Tribological Behavior of Ionic Liquid with Nanoparticles. Materials. 2021; 14(21):6318. https://doi.org/10.3390/ma14216318
Chicago/Turabian StyleTa, Thi-Na, Shin-Yuh Chern, and Jeng-Haur Horng. 2021. "Tribological Behavior of Ionic Liquid with Nanoparticles" Materials 14, no. 21: 6318. https://doi.org/10.3390/ma14216318






