Effects of TiAl Alloy as a Binder on Cubic Boron Nitride Composites
Abstract
:1. Introduction
2. Materials and Methods
3. Results
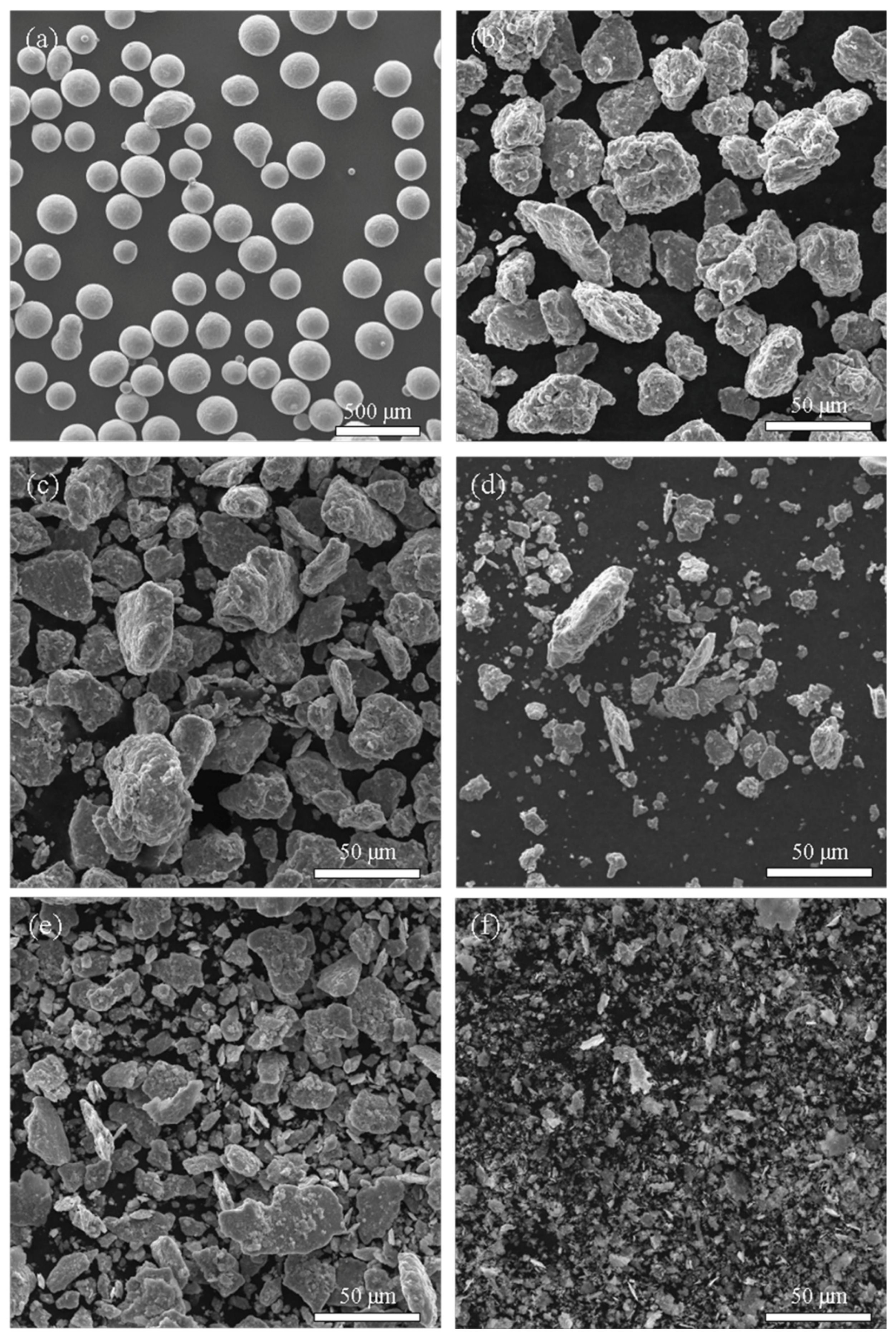
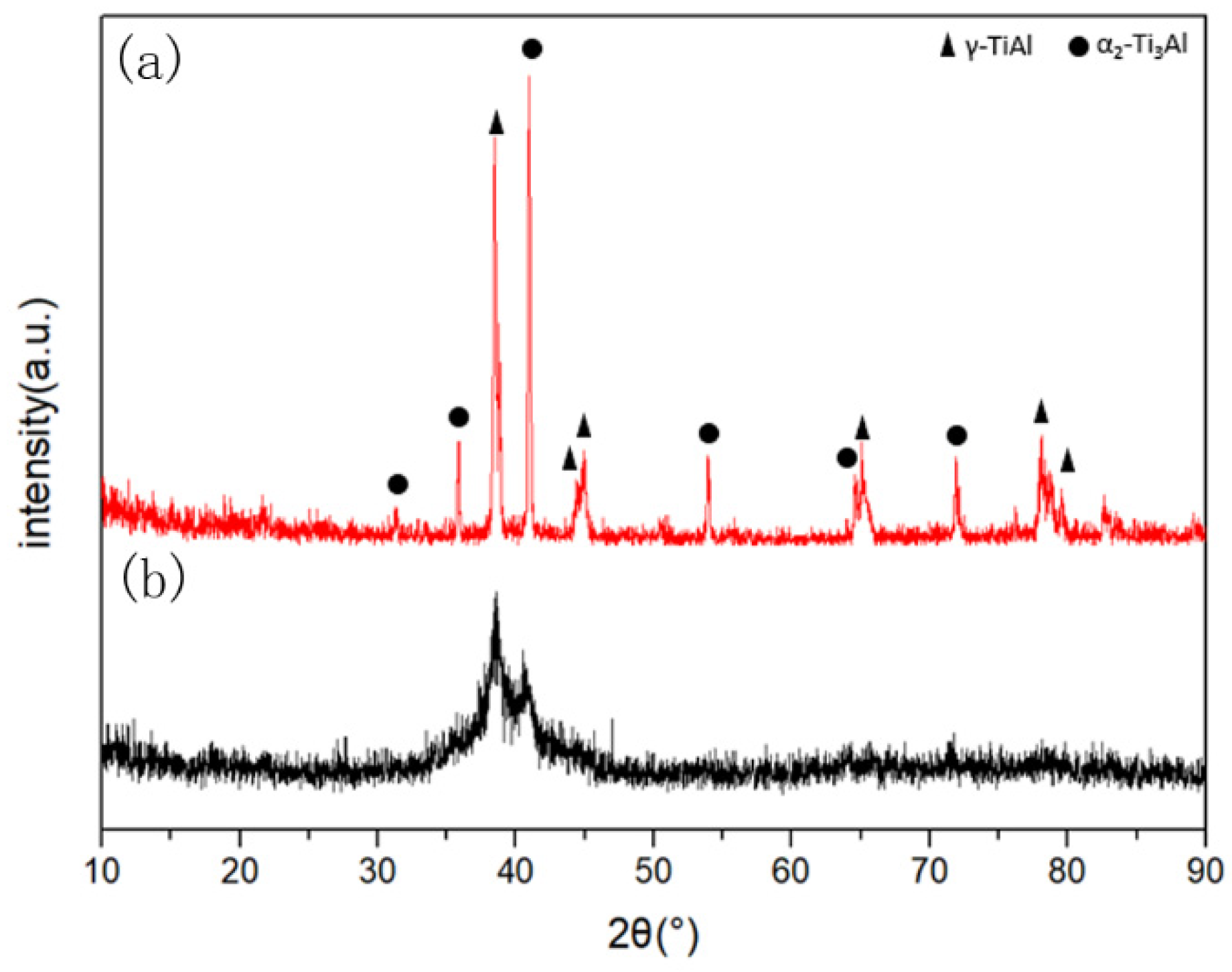
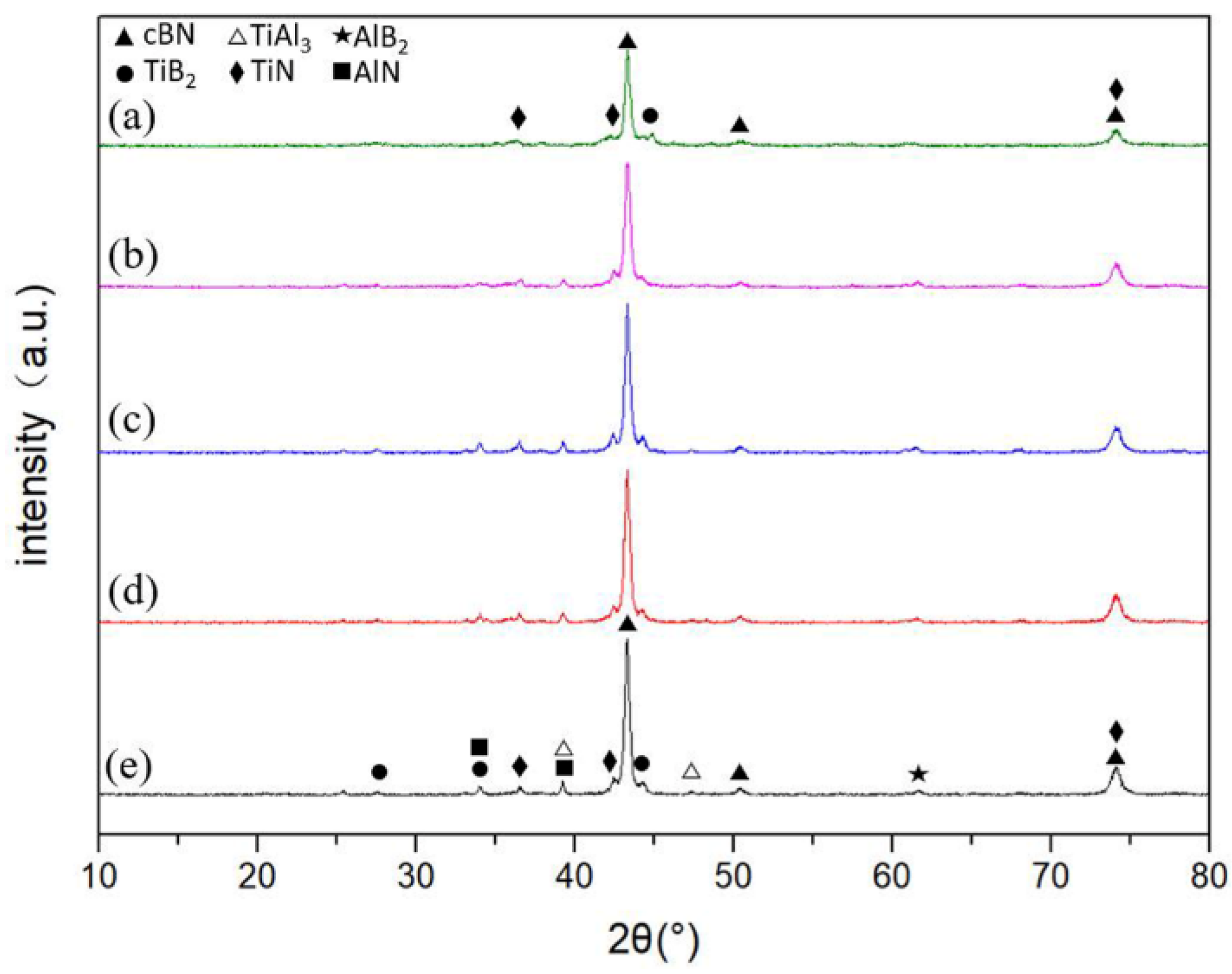
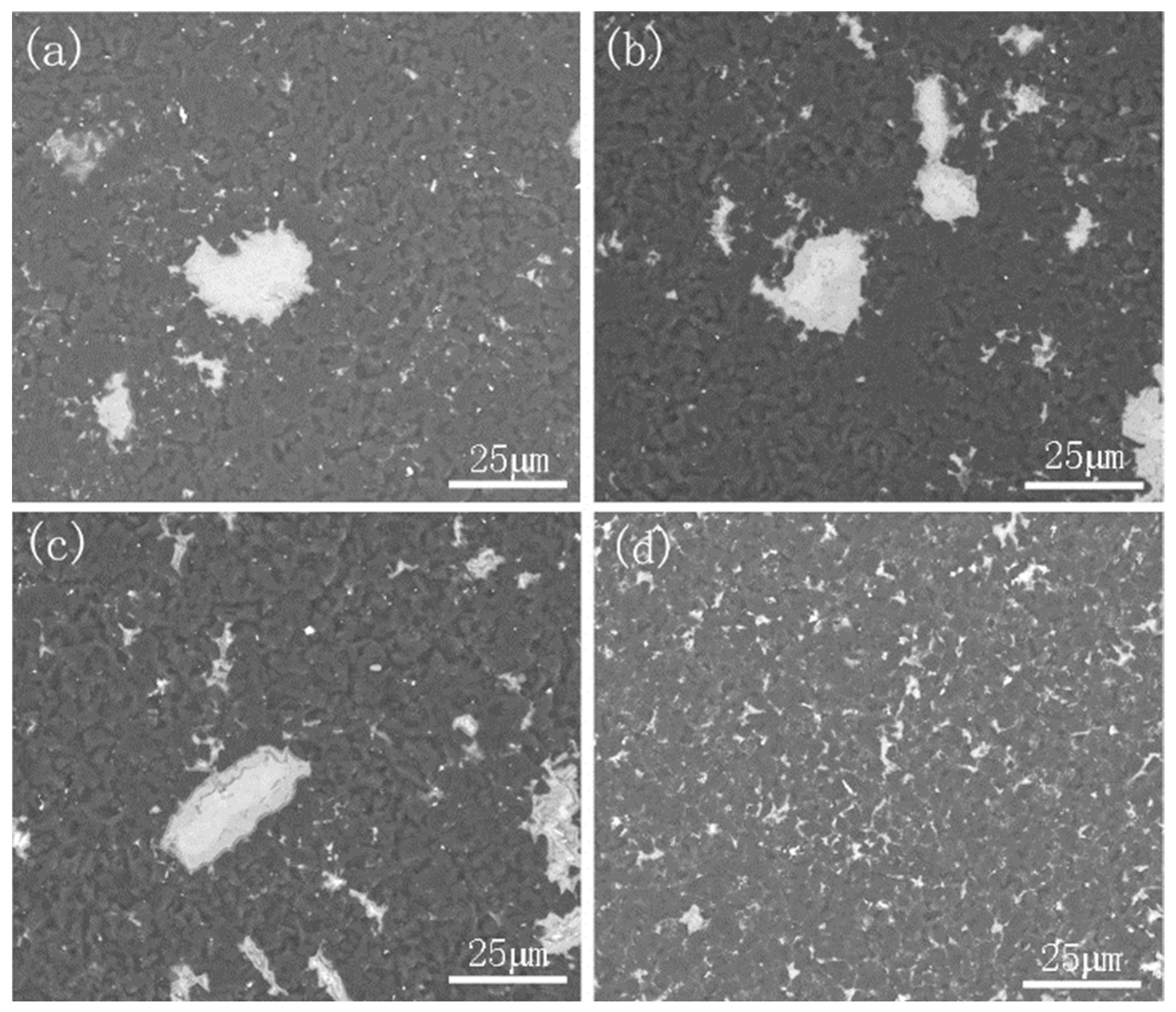
3.1. Phase and Microstructure
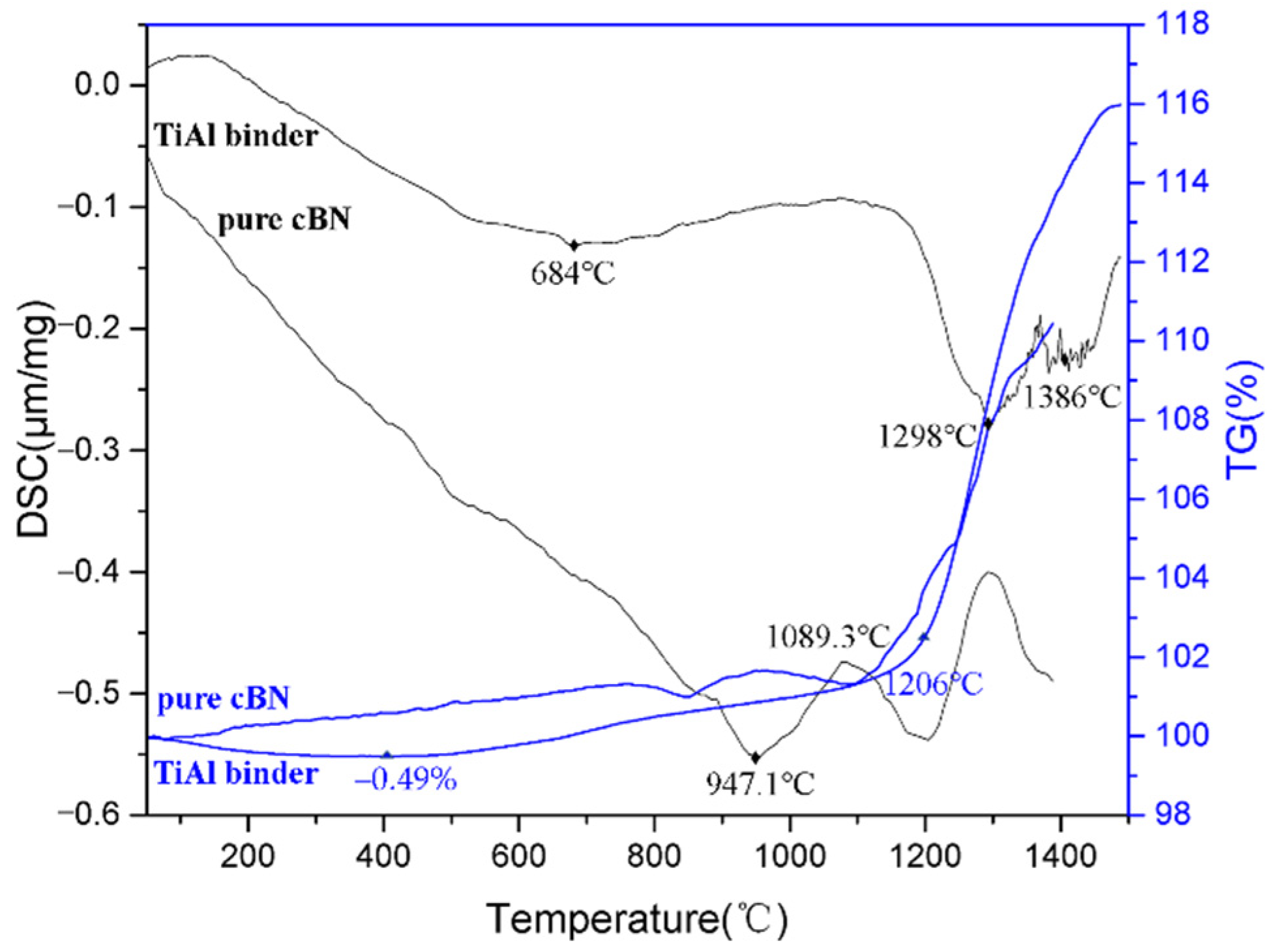
3.2. Sintering Mechanism
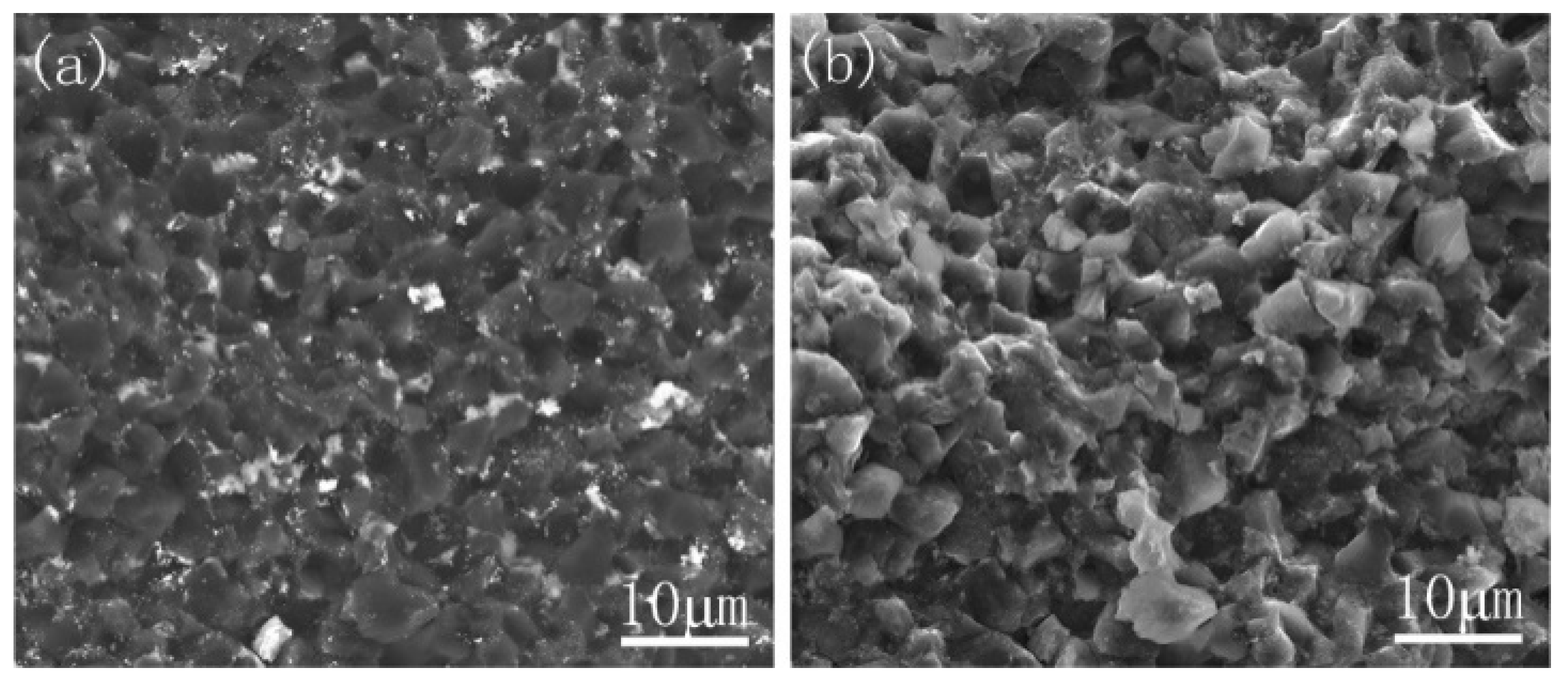
3.3. Properties Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, H.; Wang, M.; He, D.; Zou, Q.; Ke, Y.; Zhao, Y. Synthesis of nano-polycrystalline diamond in proximity to industrial conditions. Carbon 2016, 108, 1–6. [Google Scholar] [CrossRef]
- Poulachon, G.; Bandyopadhyay, B.P.; Jawahir, I.S.; Pheulpin, S.; Seguin, E. Wear behavior of CBN tools while turning various hardened steels. Wear 2004, 256, 302–310. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; Sun, K.; Ji, H.; Feng, D.; Li, Z. Composition, microstructure and mechanical properties of cBN-based composites sintered with AlN-Al-Ni binder. Ceram Int. 2018, 44, 16915–16922. [Google Scholar] [CrossRef]
- Barsoum, M.W. The MN+ 1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Xie, H.; Deng, F.; Wang, H.; Liu, J.; Han, S.; Feng, F. Study of the proportioning design method and mechanical properties of a cBN–TiN composite. Int. J. Refract Met. Hard. Mater. 2020, 89, 105209. [Google Scholar] [CrossRef]
- Li, L.; Zhou, A.; Wang, L.; Li, S.; Wu, D.; Yan, C. In situ synthesis of cBN–Ti3AlC2 composites by high-pressure and high-temperature technology. Diam. Relat. Mater. 2012, 29, 8–12. [Google Scholar] [CrossRef]
- Yue, Z.; Yang, L.; Gong, J.; Gao, J. Synthesis and comparison of Two cBN composites with starting ternary carbide binders. J. Mater. Eng. Perform. 2018, 27, 2124–2130. [Google Scholar] [CrossRef]
- Benko, E.; Klimczyk, P.; Mackiewicz, S.; Barr, T.L.; Piskorska, E. cBN–Ti3SiC2 composites. Diam. Relat. Mater. 2004, 13, 521–525. [Google Scholar] [CrossRef]
- Semiatin, S.L.; Seetharaman, V.; Weiss, I. Hot workability of titanium and titanium aluminide alloys—An overview. Mater. Sci. Eng. A 1998, 243, 1–24. [Google Scholar] [CrossRef]
- KIM, Y.Y. Microstructural evolution and mechanical properties of a forged gamma titanium aluminide alloy. Acta Metall. Mater. 1992, 40, 1121–1134. [Google Scholar] [CrossRef]
- Perrut, M.; Caron, P.; Thomas, M.; Couret, A. High temperature materials for aerospace applications: Ni-based superalloys and γ-TiAl alloys. R. Phys. 2018, 19, 657–671. [Google Scholar] [CrossRef]
- Liang, B.; Wang, Z.; Zhang, Y. Effect of c-BN size and content on the self-propagating high-temperature synthesis of c-BN composites bonded with Ti-Al-C system multiphase products. High Temp. Mater. Proc. 2016, 35, 369–374. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, A.; Zhong, S.; Mo, P.; Wu, Y. Effect of AlTi content on mechanical properties of in-situ synthesized PcBN composites. Diam. Relat. Mater. 2020, 109, 108068. [Google Scholar] [CrossRef]
- Yuan, Y.; Cheng, X.; Chang, R.; Li, T.; Zang, J.; Wang, Y.; Yu, Y.; Lu, J.; Xu, X. Reactive sintering cBN-Ti-Al composites by spark plasma sintering. Diam. Relat. Mater. 2016, 69, 138–143. [Google Scholar] [CrossRef]
- Bača, Ľ.; Stelzer, N. Adapting of sol–gel process for preparation of TiB2 powder from low-cost precursors. J. Eur. Ceram. Soc. 2008, 28, 907–911. [Google Scholar] [CrossRef]
- Carlone, C.; Lakin, K.M.; Shanks, H.R. Optical phonons of aluminum nitride. J. Appl. Phys. 1984, 55, 4010–4014. [Google Scholar] [CrossRef]
- Jallad, K.N.; Ben-Amotz, D. Raman chemical imaging of tribological nitride coated (TiN, TiAlN) surfaces. Wear 2020, 252, 956–969. [Google Scholar] [CrossRef]
- Deng, W.; Deng, F.; Liu, R.; Peng, Z.; Ma, X.; Xu, Z. Interfacial bonding mechanism of high-pressure sintered Al-Ti-cBN composites. Diam. Relat. Mater. 2019, 91, 29–33. [Google Scholar]
- Li, Y.; Li, S.; Lv, R.; Qin, J.; Zhang, J.; Wang, J.; Wang, F.; Kou, Z.; He, D. Study of high-pressure sintering behavior of cBN composites starting with cBN–Al mixtures. J. Mater. Res. 2008, 23, 2366–2372. [Google Scholar] [CrossRef]
- Rong, X.Z.; Yano, T. TEM investigation of high-pressure reaction-sintered cBN-Al composites. J. Mater. Sci. 2004, 39, 4705–4710. [Google Scholar] [CrossRef]
- Rong, X.Z.; Tsurumi, T.; Fukunaga, O.; Yano, T. High-pressure sintering of cBN-TiN-Al composite for cutting tool application. Diam. Relat. Mater. 2002, 11, 280–286. [Google Scholar] [CrossRef]
- Deng, F.; Liu, R. The process and mechanism of high pressure sintering of PcBN. Superhard. Mater. Eng. 2008, 3, 5–8. [Google Scholar]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances: Supplement; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; p. 33. [Google Scholar]
- Walmsley, J.C.; Lang, A.R. A transmission electron microscope study of a cubic boron nitride-based compact material with AIN and AIB2 binder phases. J. Mater. Sci. 1987, 22, 4093–4102. [Google Scholar] [CrossRef]
- Zhang, A.; Li, Z.; Li, Z.; Zhu, Y. Preparation and characterization of SiO2–Al2O3–Na2O glass coated cBN abrasive particles via sol–gel route. J. Solgel. Sci. Technol. 2009, 49, 6–11. [Google Scholar] [CrossRef]

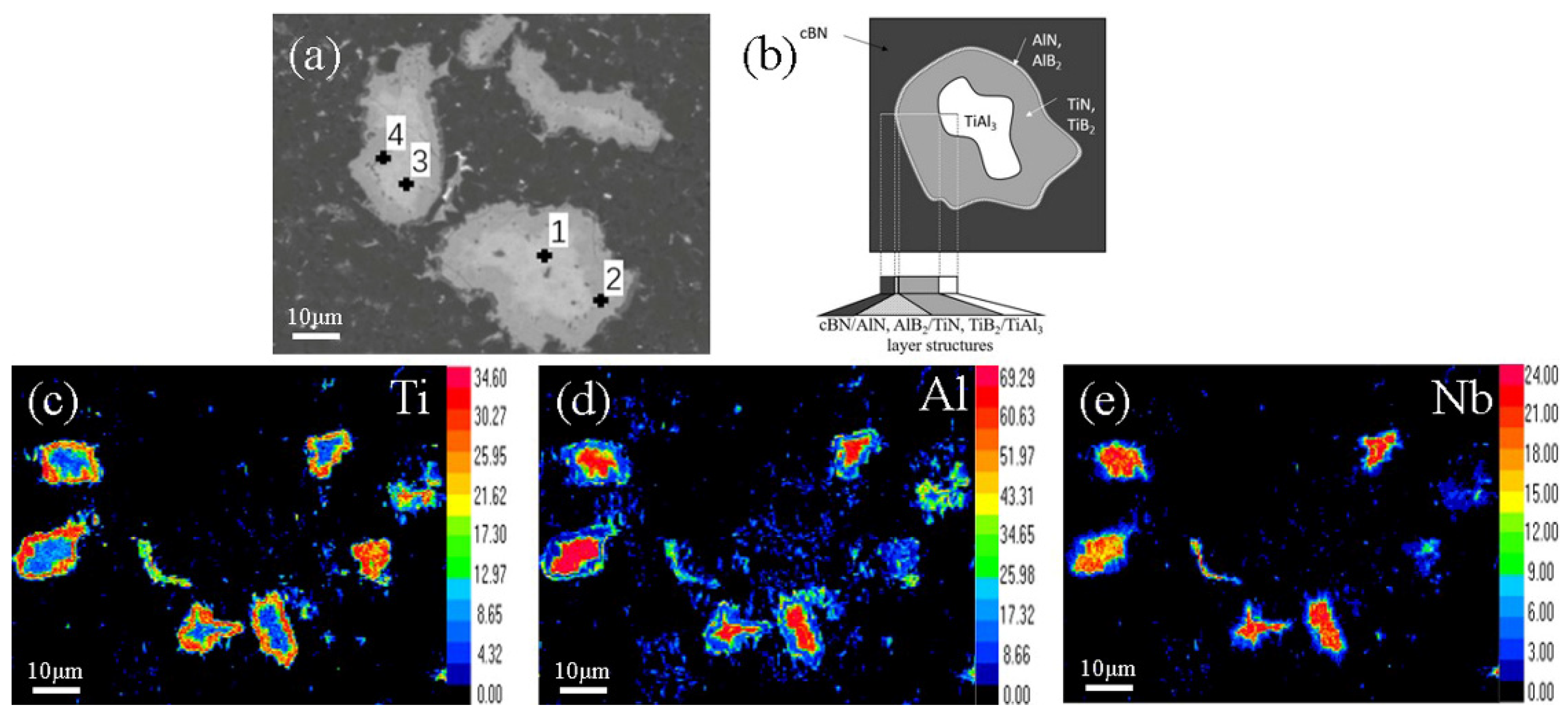

| Point | Ti | Al | B | N | Nb |
|---|---|---|---|---|---|
| 1 | 11.67 | 53.10 | 19.20 | 3.47 | 12.56 |
| 2 | 27.18 | 15.49 | 31.60 | 24.18 | 1.55 |
| 3 | 12.13 | 67.29 | 0.00 | 6.22 | 14.35 |
| 4 | 32.14 | 8.92 | 22.43 | 34.90 | 1.60 |
| Binder Type | Preferred Element | Sintering Type | Local Heating |
|---|---|---|---|
| Ti + Al | Al | liquid-state | √ |
| Ti3AlC2 | Al | solid-state | × |
| TiAl | Ti | solid-state | × |
| NO. | TiAl Particle Size/μm | Ball Milling Method | Flexural Strength/MPa |
|---|---|---|---|
| 1 | 29 | dry | 99 |
| 2 | 26 | dry | 101 |
| 3 | 17 | dry | 182 |
| 4 | 15 | wet | 170 |
| 5 | 5 | wet | 360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, W.; Peng, Y.; Fan, G.; Liu, B. Effects of TiAl Alloy as a Binder on Cubic Boron Nitride Composites. Materials 2021, 14, 6335. https://doi.org/10.3390/ma14216335
Liu Y, Zhang W, Peng Y, Fan G, Liu B. Effects of TiAl Alloy as a Binder on Cubic Boron Nitride Composites. Materials. 2021; 14(21):6335. https://doi.org/10.3390/ma14216335
Chicago/Turabian StyleLiu, Yuxi, Wei Zhang, Yingbo Peng, Guojiang Fan, and Bin Liu. 2021. "Effects of TiAl Alloy as a Binder on Cubic Boron Nitride Composites" Materials 14, no. 21: 6335. https://doi.org/10.3390/ma14216335






