Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Physicochemical Properties
2.3. Enzymatic Activity
2.4. Microbial Biomass Carbon Content
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Properties and Microbial Biomass Carbon (MBC)
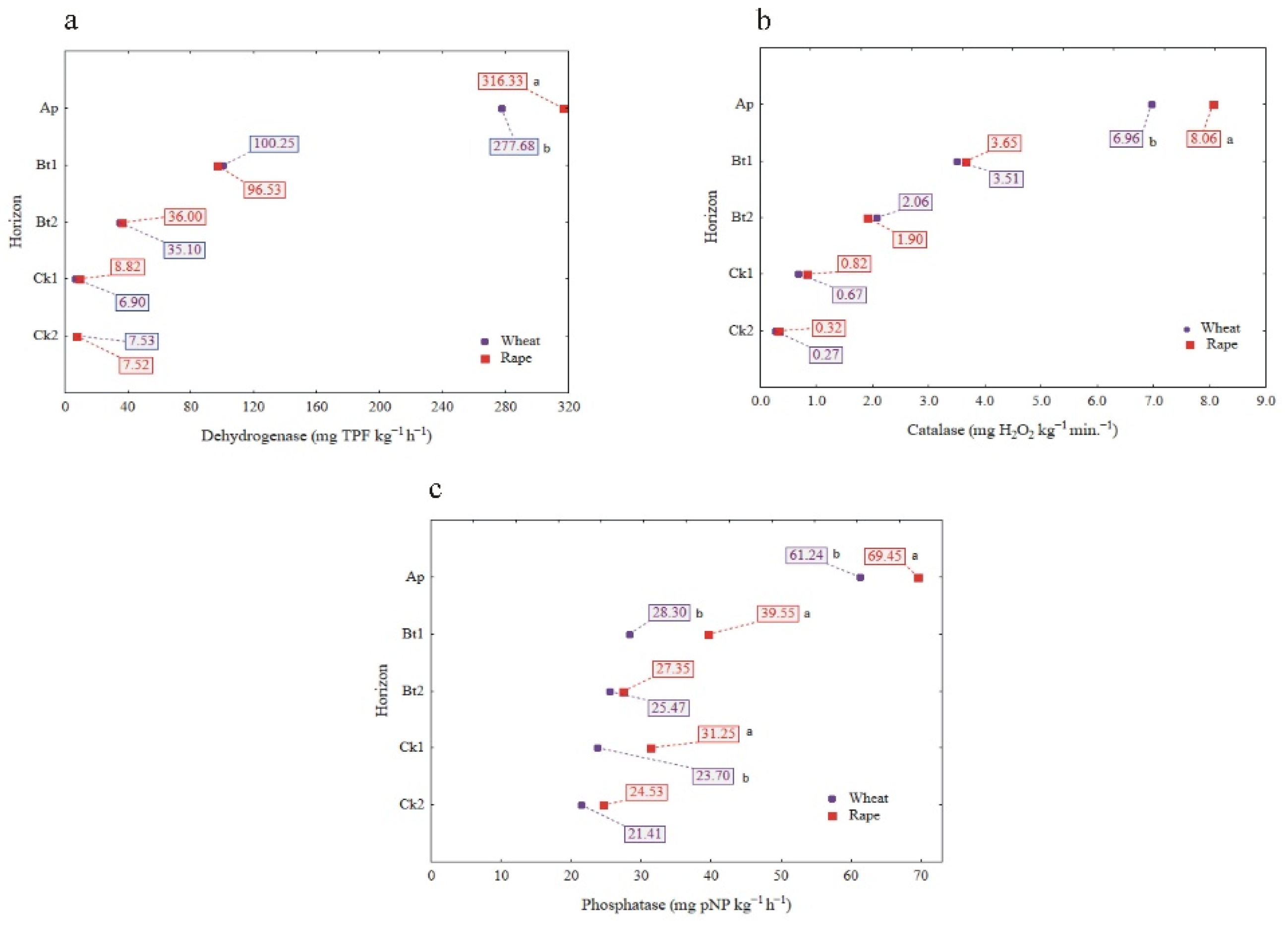
3.2. Soil Enzymatic Activity
3.2.1. Enzymatic Activity Expressed Per Soil Unit
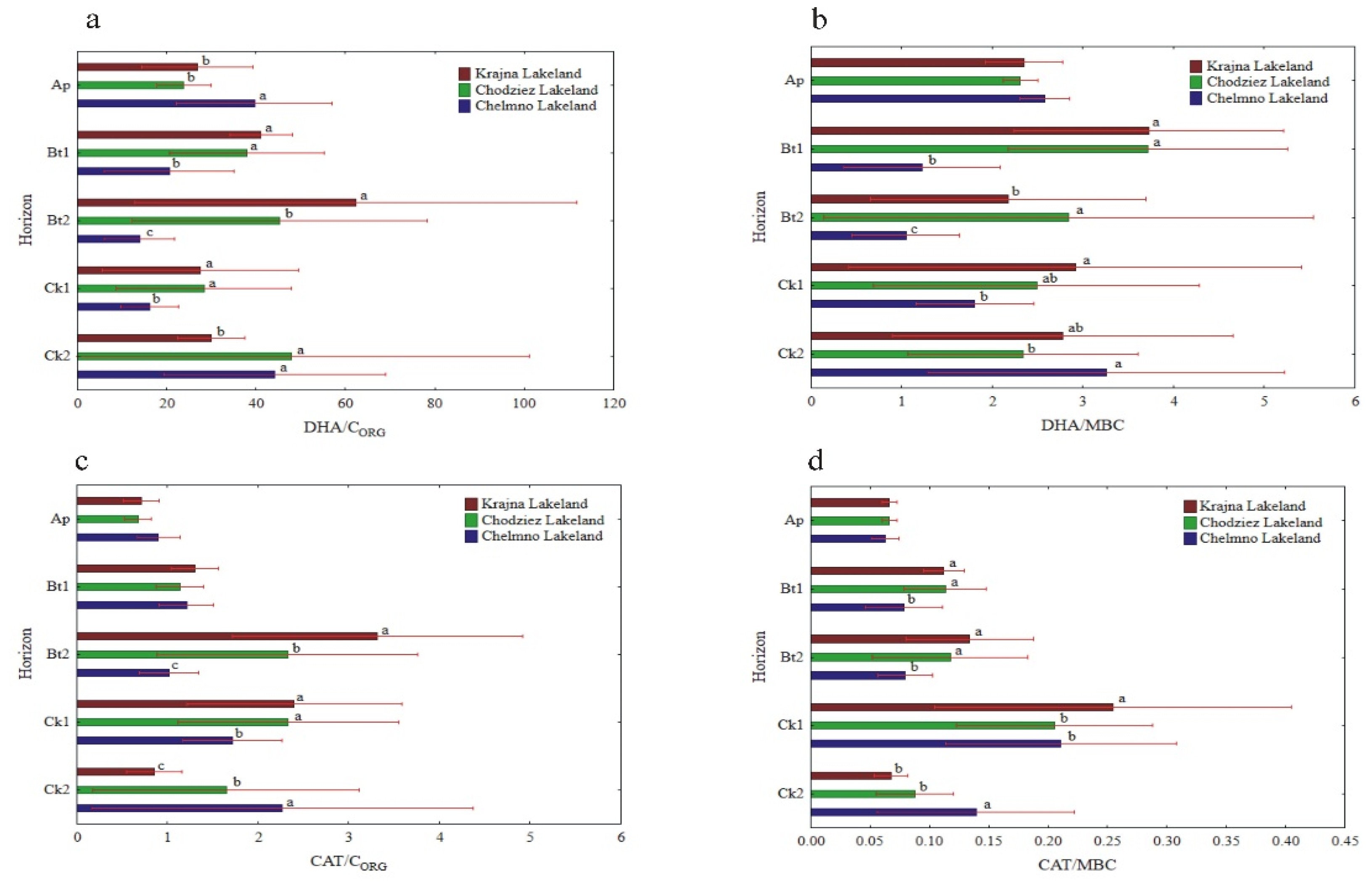
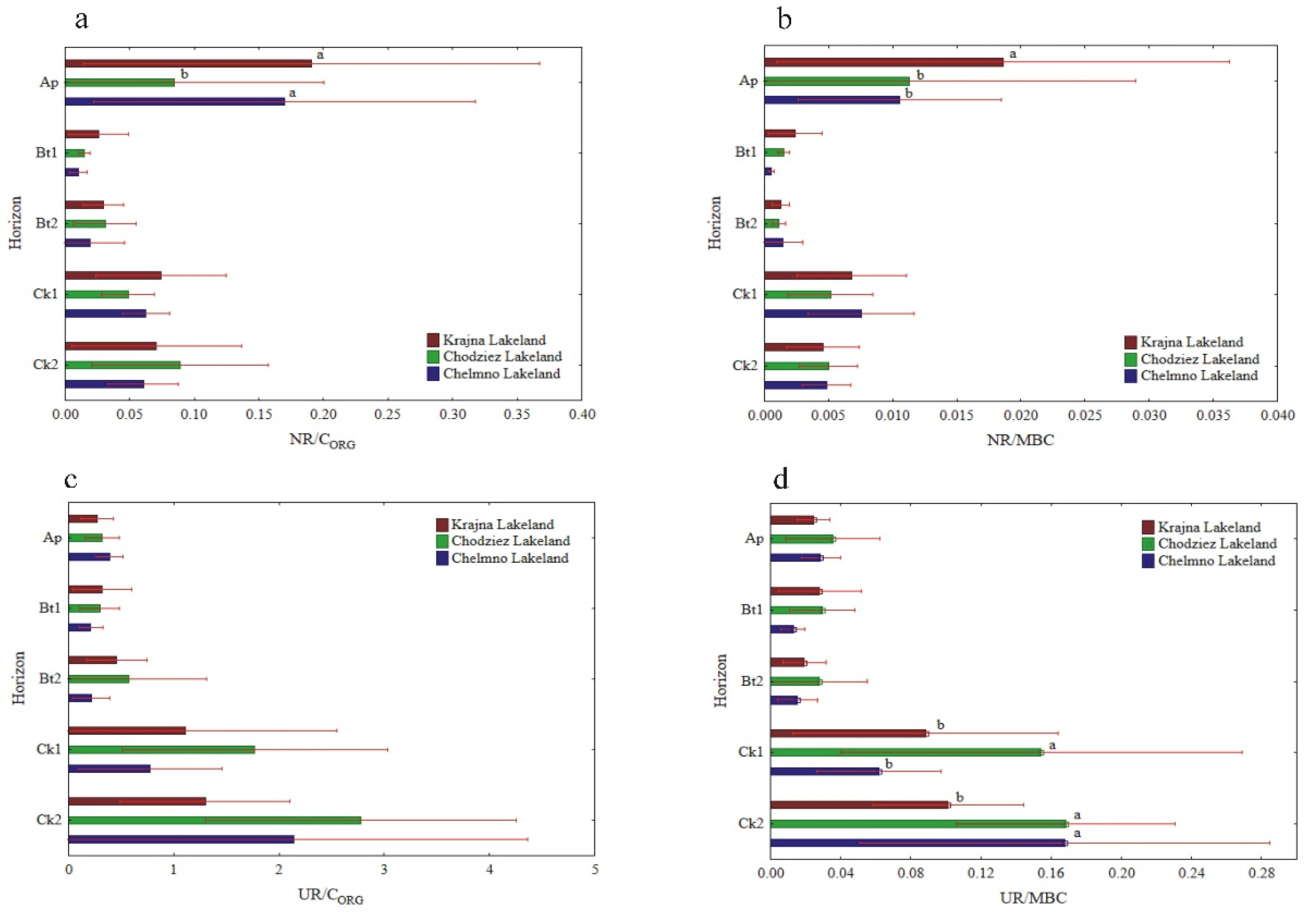
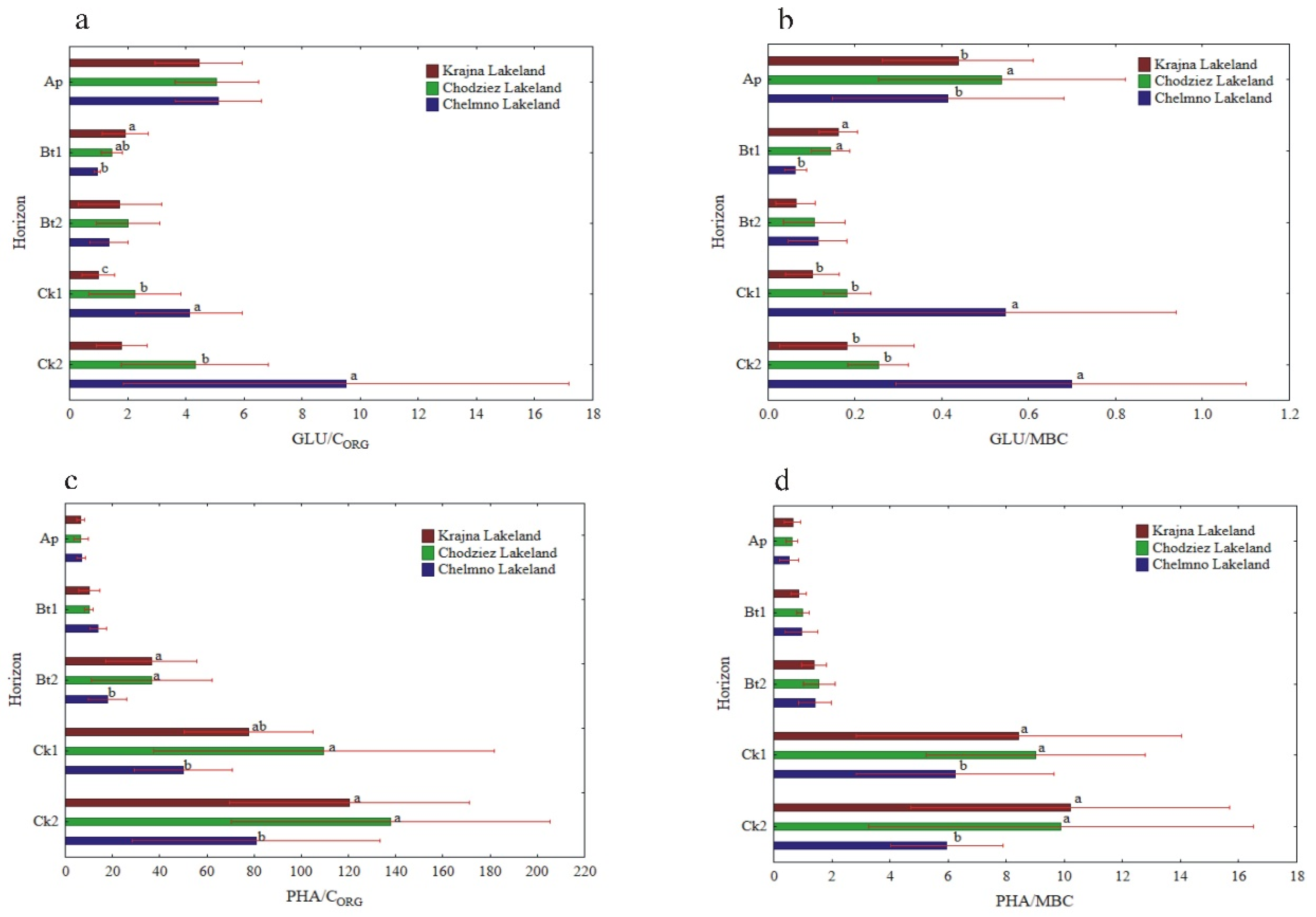
3.2.2. Enzymatic Activity Expressed on an Organic Carbon (CORG) and Microbial Biomass Carbon (MBC) Basis
3.2.3. Correlation between the Studied Properties
4. Discussion
4.1. Physicochemical Properties
4.2. Enzyme Activity in the Soil Profile
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallenstein, M.D.; Burns, R.G. Ecology of extracellular enzyme activities and organic matter degradation in soil: A complex community-driven process. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 35–55. [Google Scholar]
- Stone, M.M.; De Forest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Baldrian, P. Microbial enzyme-catalyzed processes in soil and their analysis. Plant Soil Environ. 2009, 55, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Loeppmann, S.; Blagodatskaya, E.; Pausch, J. Enzyme properties down the soil profile—A matter of substrate quality in rhizosphere and detritusphere. Soil Biol. Biochem. 2016, 103, 274–283. [Google Scholar] [CrossRef]
- Gianfreda, L.; Ruggiero, P. Enzyme Activities in Soil. In Nucleic Acids and Proteins in Soil; Nannipieri, P., Smalla, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 20–25. [Google Scholar]
- Venkatesan, S.; Senthurpandian, V.K. Comparison of enzyme activity with depth under tea plantations and forested sites in south India. Geoderma 2006, 137, 212–216. [Google Scholar] [CrossRef]
- Marinari, S.; Antisari, L.V. Effect of lithological substrate on microbial biomass and enzyme activity in brown soil profiles in the northern Apennines (Italy). Pedobiologia 2010, 53, 313–320. [Google Scholar] [CrossRef]
- Herold, N.; Schöning, I.; Berner, D.; Haslwimmer, H.; Kandeler, E.; Michalyik, B.; Schrumpf, M. Vertical gradient of potential enzymes activities in soil profiles of European beech, Norwaz spruce and Scots pine dominated forest sites. Pedobiologia—J. Soil Ecol. 2014, 57, 181–189. [Google Scholar] [CrossRef]
- Senga, Y.; Hiroki, M.; Nakamura, Y.; Watarasi, Y.; Watanabe, Y.; Nohara, S. Vertical profiles of DIN, DOC, and microbial activities in the wetland soil of Kushiro Mire, northeastern Japan. Limnology 2011, 12, 17–23. [Google Scholar] [CrossRef]
- Kramer, S.; Marhan, S.; Haslwimmer, H.; Ruess, L.; Kandeler, E. Temporal variation in surface and subsoil abundance and function of the soil microbial community in an arable soil. Soil Biol. Biochem. 2013, 61, 76–85. [Google Scholar] [CrossRef]
- Wang, Z.; Van Oost, K.; Govers, G. Predicting the long-term fate of buried organic carbon in colluvial soils. Glob. Biogeochem. 2015, 29, 65–79. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic C in deep layers controlled by fresh C supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Niemi, R.M.; Vepsäläinen, M.; Wallenius, K.; Simpanen, S.; Alakukku, L.; Pietola, L. Temporal and soil depth-related variation and soil enzyme activities and in root growth of red clover (Trifolium pratense) and timothy (Phleum pratense) in the field. Appl. Soil Ecol. 2005, 30, 113–125. [Google Scholar] [CrossRef]
- Kabała, C.; Musztyfaga, E. Clay-illuvial soil in the Polish and international soil classifications. Soil Sci. Ann. 2015, 66, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Świtoniak, M. Use of soil profile truncation to estimate influence of accelerated erosion on soil cover transformation in young morainic landscapes, North-Eastern Poland. Catena 2014, 116, 173–184. [Google Scholar] [CrossRef]
- Świtoniak, M.; Mroczek, P.; Bednarek, R. Luvisols or Cambisols? Micromorphological study of soil truncation in young morainic landscapes—Case study: Brodnica and Chełmno Lake Districts (North Poland). Catena 2016, 137, 583–595. [Google Scholar] [CrossRef]
- Dreibrodt, S.; Lomax, J.; Nelle, O.; Lubos, C.; Fischer, P.; Mitusov, A.; Reiss, S.; Radtke, U.; Nadeau, M.; Grootes, P.M.; et al. Are mid-latitude slopes sensitive to climatic oscillations? Implications from an Early Holocene sequence of slope deposits and buried soils from eastern Germany. Geomorphology 2010, 122, 351–369. [Google Scholar] [CrossRef]
- Kittel, P. Slope deposits as an indicator of anthropopressure in the light of research in Central Poland. Quat. Int. 2014, 324, 34–55. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World References Base for Soil Resources; World Soil Resources Reports, No 106; FAO: Rome, Italy, 2015; p. 132. [Google Scholar]
- Kobierski, M. Morphology, Properties and Mineralogical Composition of Eroded Luvisols in Selected Morainic Areas of the Kujavian and Pomeranian Province. Habilitation Thesis, University of Technology and Life Sciences, Bydgoszcz, Poland, 2013; pp. 1–121. (In Polish). [Google Scholar]
- Polish Norm PN-ISO 11277. Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation; Polish Committee for Standardization: Warsaw, Poland, 2005. [Google Scholar]
- Polish Norm PN-ISO 10390. Soil Quality—Determination of Soil pH; Polish Committee for Standardization: Warsaw, Poland, 1997. [Google Scholar]
- Burt, R. Soil Survey Laboratory Methods Manual; Soil Survey Investigations Report 469 No. 42, version 4.0; USDA-NRCS: Lincoln, NE, USA, 2004.
- Egnér, H.; Riehm, H.; Domingo, W.R. Studies concerning the chemical analysis of soils as background for soil nutrient assessment II: Chemical extracting methods to determinate the phosphorous and potassium content of soil. Kungl. Lantbr. Ann. 1960, 26, 199–215. (In German) [Google Scholar]
- Hao, X.; Ball, B.C.; Culley, J.L.B.; Carter, M.R.; Parkin, G.W. Soil Density and Porosity. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Thalmann, A. Zur Methodik der Bestimmung der Dehydrodgenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Kandeler, E. Enzymes Involved in Nitrogen Metabolism. In Methods in Soil Biology; Scinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 163–184. [Google Scholar]
- Johnson, J.L.; Temple, K.L. Some variables affecting measurement of catalase activity in soil. Soil Sci. Soc. Am. Proc. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenylophosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonia. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinsen, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]
- Wilding, L.P. Spatial variability: Its documentation, accommodation, and implication to soil surveys. In Soil Spatial Variability; Nielsen, D.R., Bouma, J., Eds.; Pudoc: Wageningen, The Netherlands, 1985; pp. 166–194. [Google Scholar]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Till. Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Flowers, M.; Lal, R. Axle load and tillage effect on soil physical properties and soybean grain yield on a mollic ochraqualf in Northwest Ohio. Soil Till. Res. 1998, 48, 21–35. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Lapen, D.R.; Ma, B.L.; McLaughlin, N.B.; VandenBygaart, A.J. Soil and crop response to varying levels of compaction, nitrogen fertilization, and clay content. Soil Sci. Soc. Am. J. 2011, 75, 1483–1492. [Google Scholar] [CrossRef]
- Lipiec, J. Crop responses to soil compaction. In Proceedings of the NJF Seminar 448 on Soil Compaction—Effects on Soil Functions and Strategies for Prevention, Helsinki, Finland, 6–8 March 2012; pp. 27–36. [Google Scholar]
- Jobbágy, E.; Jackson, R.B. The distribution of soil nutrient with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Smeck, N.E.; Sajf, H.T.; Bigham, J.M. Formation of a transient magnesium-aluminium double hydroxide in soils of southeastern Ohio. Soil Sci. Soc. Am. J. 1994, 58, 470–476. [Google Scholar] [CrossRef]
- Sajf, H.T.; Smeck, N.E.; Bigham, J.M. Pedogenic influence on base saturation and calcium/magnesium ratios in soils of southeastern Ohio. Soil Sci. Soc. Am. J. 1997, 61, 509–515. [Google Scholar]
- Callesen, I.; Raulund-Rasmussen, K.; Westman, C.J.; Tau-Strand, L. Nitrogen pools and C:N ratios in well-drained Nordic forest soils related to climate and soil texture. Environ. Res. 2007, 12, 681–692. [Google Scholar]
- Kizilkaya, R.; Dengiz, O. Variation of land use and land cover effects on some soil physic-chemical characteristics and soil enzyme activity. Zemdirbystre-Agriculture 2010, 9, 15–24. [Google Scholar]
- Steinweg, J.M.; Dukes, J.S.; Paul, E.A.; Wallenstein, M.D. Microbial responses to multi-factor climate change: Effects on soil enzymes. Front. Microbiol. 2013, 4, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goberna, M.; Sánchez, J.; Pascual, J.A.; García, C. Surface and subsurface organic carbon, microbial biomass and activity in a forest soil sequence. Soil Biol. Biochem. 2006, 38, 2233–2243. [Google Scholar] [CrossRef]
- Taylor, J.P.; Wilson, M.S.; Mills, M.S.; Burns, R.G. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol. Biochem. 2002, 34, 387–401. [Google Scholar] [CrossRef]
- Ge, C.R.; Xue, D.; Yao, H.Y. Microbial biomass, community diversity, and enzyme activities in response to urea application in tea orchard soils. Commun. Soil Sci. Plant Anal. 2010, 41, 797–810. [Google Scholar] [CrossRef]
- Brewer, T.E.; Aronson, E.L.; Arogyaswamy, K.; Billings, S.A.; Botthoff, J.K.; Campbell, A.N.; Dove, N.C.; Fairbanks, D.; Gallery, R.E.; Hart, S.C.; et al. Ecological and genomic attributes of novel bacterial taxa that thrive in subsurface soil horizons. mBio 2019, 10, e1318-19. [Google Scholar] [CrossRef] [Green Version]
- Lemanowicz, J. Dynamics of phosphorus content and the activity of phosphatase in forest soil in the sustained nitrogen compounds emissions zone. Environ. Sci. Pollut. Res. 2018, 25, 33773–33782. [Google Scholar] [CrossRef] [Green Version]
- Lemanowicz, J.; Siwik-Ziomek, A.; Koper, J. Enzymatic variation of soils exposed to the impact of the soda plant in terms of biochemical parameters. Int. J. Environ. Sci. Technol. 2019, 16, 3309–3316. [Google Scholar] [CrossRef] [Green Version]
- Gelsomino, A.; Azzellino, A. Multivariate analysis of soils: Microbial biomass, metabolic activity, and bacterial-community structure and their relationships with soil depth and type. J. Plant Nutr. Soil Sci. 2011, 174, 381–394. [Google Scholar] [CrossRef]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology; Shukla, G.C., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 229–243. [Google Scholar]
- Allison, S.D. Soil minerals and humic acids alter enzyme stability: Implications for ecosystem processes. Biogeochemistry 2006, 81, 361–373. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, M. Stabilizing enzymes as synthetic complexes. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 319–369. [Google Scholar]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic stoichiometry and ecological theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef] [Green Version]
- Pupin, B.; da Silva Freddi, O.; Nahas, E. Microbial alteration of the soil influenced by induced compaction. Rev. Bras. Ciênc. Solo 2009, 33, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Marinari, S.; Masciandaro, G.; Ceccanti, B.; Grego, S. Influence of organic and mineral fertilizers on soil biological and physical properties. Bioresour. Technol. 2000, 72, 9–17. [Google Scholar] [CrossRef]
- Pagliai, M.; De Nobili, M. Relationship between soil porosity, root development and soil enzyme activity in cultivated soils. Geoderma 1993, 56, 243–256. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Guber, A.K.; Razavi, B.S.; Koestel, J.; Quigley, M.Y.; Robertson, G.P. Microbial spatial footprint as a driver of soil carbon stabilization. Nat. Commun. 2019, 10, 3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobierski, M.; Wojtasik, M. Organic and inorganic carbon densities in arable and orchard soils in selected mesoregions of the South-Baltic Lakeland. Soil Sci. Ann. 2009, 60, 57–64. [Google Scholar]
- Egamberdieva, D.; Renella, G.; Wirth, S.; Islam, R. Enzyme activities in the Rhizosphere of Plants. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 149–166. [Google Scholar]
- Zanphorlin, L.M.; de Giuseppe, P.O.; Honorato, R.V.; Costa Tonoli, C.C.; Fattori, J.; Crespin, E.; Lopes de Oliveira, P.S.; Ruller, R.; Murakami, M.T. Oligomerization as a strategy for cold adaptation: Structure and dynamics of the GH1 β-glucosidase from Exiguobacterium antarcticum B7. Sci. Rep. 2016, 6, 23776. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adopted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [Green Version]



| Horizon | CORG | NTOT | C/N | MBC (mg kg−1) | MBC/CORG | Clay (%) | Bulk Density (g cm−3) | Porosity (%) |
| (g kg−1) | ||||||||
| Ap | 9.79 ± 1.06 a* | 1.03 ± 0.08 a | 9.50 ± 0.54 | 115.6 ± 38.7 a | 1.22 ± 0.44 b | 14.1 ± 1.9 c | 1.64 ± 0.07 c | 35.9 ± 2.2 a |
| Bt1 | 2.98 ± 0.66 b | 0.34 ±0.08 b | 8.76 ± 0.94 | 38.3 ± 11.6 b | 1.35 ± 0.56 b | 22.4 ± 2.8 a | 1.77 ± 0.05 b | 31.3 ± 2.0 ab |
| Bt2 | 1.11 ± 0.50 c | 0.27 ± 0.07 bc | 4.11 ± 1.20 | 19.4 ± 6.3 c | 2.12 ± 1.07 a | 19.8 ± 2.0 b | 1.77 ± 0.03 b | 31.5 ± 1.4 ab |
| Ck1 | 0.36 ± 0.09 d | 0.10 ± 0.04 c | 3.60 ± 0.32 | 3.92 ± 1.9 d | 1.15 ± 0.74 b | 15.3 ± 2.4 c | 1.85 ± 0.02 a | 28.6 ± 0.6 b |
| Ck2 | 0.21 ± 0.07 d | 0.07 ± 0.02 c | 3.00 ± 0.28 | 2.79 ± 0.7 d | 1.47 ± 0.75 b | 14.9 ± 1.9 c | 1.87 ± 0.01 a | 28.3 ± 0.5 b |
| Horizon | pH in KCl | Hh [cmol kg−1] | Basic Saturation [%] | PAVAIL | KAVAIL | MgAVAIL | ||
| [mg kg−1] | ||||||||
| Ap | 6.65 ± 0.09 b | 0.71 ± 0.36 a | 95.2 ± 2.9 b | 80.4 ± 21.4 a | 152.7 ± 84.7 a | 92.9 ± 27.2 b | ||
| Bt1 | 6.38 ± 0.03 b | 0.98 ± 0.94 a | 94.6 ± 4.9 b | 29.9 ± 14.6 b | 95.8 ± 30.2 b | 120.7 ± 25.7 ab | ||
| Bt2 | 6.57 ± 0.08 b | 0.57 ±0.28 a | 96.5 ± 1.6 b | 22.8 ± 19.4 b | 60.5 ± 14.6 c | 127.9 ± 43.0 a | ||
| Ck1 | 7.21 ± 0.02 a | 0.06 ± 0.01 b | 99.6 ± 0.3 a | 3,9 ± 2.3 c | 47.3 ± 16.7 c | 105.0 ± 35.5 ab | ||
| Ck2 | 7.25 ± 0.03 a | 0.07 ± 0.01 b | 99.7 ± 0.3 a | 2.2 ± 1.2 c | 49.6 ± 17.4 c | 113.6 ± 33.7 ab | ||
| Enzyme | Region | Horizon | Mean | Range | SD | CV (%) |
|---|---|---|---|---|---|---|
| Dehydrogenase (mg TPF kg−1 h−1) | Krajna | Ap | 265.1 A^b* | 112.9–457.1 | 129.4 | 48.1 |
| Bt1 | 131.5 Ba | 102.4–166.1 | 26.9 | 20.4 | ||
| Bt2 | 43.1 Ca | 9.9–96.3 | 34.5 | 80.0 | ||
| Ck1 | 8.14 Dab | 2.0–14.3 | 5.27 | 64.7 | ||
| Ck2 | 6.66 Da | 4.8–10.9 | 2.42 | 36.3 | ||
| Chodzież | Ap | 238.3 Ab | 160.7–313.6 | 63.6 | 26.7 | |
| Bt1 | 123.7 Ba | 68.7–176.3 | 44.0 | 35.5 | ||
| Bt2 | 44.2 Ca | 10.2–73.2 | 31.5 | 71.4 | ||
| Ck1 | 9.57 Da | 3.0–14.5 | 5.07 | 53.0 | ||
| Ck2 | 7.63 Da | 3.3–14.0 | 4.63 | 60.7 | ||
| Dobrzyń | Ap | 368.2 Aa | 181.6–560.8 | 154.7 | 42.0 | |
| Bt1 | 45.8 Bb | 14.6–86.6 | 29.6 | 64.6 | ||
| Bt2 | 20.9 Cb | 7.2–40.9 | 14.7 | 71.0 | ||
| Ck1 | 5.82 Db | 3.6–8.4 | 1.90 | 32.7 | ||
| Ck2 | 8.30 Da | 1.50–11.9 | 4.66 | 56.1 | ||
| Catalase (mg H2O2 kg−1 min−1) | Krajna | Ap | 7.02 Aa | 4.52–9.52 | 1.98 | 28.2 |
| Bt1 | 4.12 Ba | 3.21–4.82 | 0.63 | 15.4 | ||
| Bt2 | 2.43 Ca | 1.15–3.25 | 0.85 | 34.8 | ||
| Ck1 | 0.75 Dab | 0.25–1.10 | 0.31 | 41.9 | ||
| Ck2 | 0.18 Dc | 0.13–0.22 | 0.04 | 21.2 | ||
| Chodzież | Ap | 6.76 Aa | 4.86–8.15 | 1.53 | 22.7 | |
| Bt1 | 3.84 Ba | 3.11–4.65 | 0.76 | 19.7 | ||
| Bt2 | 2.04 Cab | 0.85–3.21 | 0.96 | 47.2 | ||
| Ck1 | 0.84 Da | 0.53–1.11 | 0.27 | 32.9 | ||
| Ck2 | 0.29 Db | 0.13–0.42 | 0.14 | 47.3 | ||
| Dobrzyń | Ap | 8.38 Aa | 6.05–10.9 | 1.97 | 23.47 | |
| Bt1 | 2.80 Bb | 2.11–3.24 | 0.45 | 16.1 | ||
| Bt2 | 1.51 Cb | 1.02–2.81 | 0.73 | 48.7 | ||
| Ck1 | 0.63 Db | 0.42–0.86 | 0.21 | 32.5 | ||
| Ck2 | 0.41 Da | 0.10–0.89 | 0.36 | 86.9 |
| Enzyme | Region | Horizon | Mean | Range | SD | CV |
|---|---|---|---|---|---|---|
| Urease (mg N-NH4+ kg−1 h−1) | Krajna | Ap | 2.68 A^b* | 1.15–4.59 | 1.57 | 58.7 |
| Bt1 | 1.06 Ba | 0.47–2.69 | 0.95 | 89.7 | ||
| Bt2 | 0.34 Cb | 0.12–0.52 | 0.16 | 48.3 | ||
| Ck1 | 0.36 Cb | 0.07–0.99 | 0.38 | 106.6 | ||
| Ck2 | 0.26 Cb | 0.17–0.35 | 0.07 | 26.8 | ||
| Chodzież | Ap | 3.27 Aa | 1.18–5.21 | 1.77 | 54.2 | |
| Bt1 | 1.00 Ba | 0.41–1.59 | 0.56 | 56.4 | ||
| Bt2 | 0.51 Ca | 0.11–1.36 | 0.58 | 114.5 | ||
| Ck1 | 0.63 Ca | 0.11–0.92 | 0.36 | 56.7 | ||
| Ck2 | 0.51 Ca | 0.41–0.62 | 0.09 | 16.9 | ||
| Dobrzyń | Ap | 3.76 Aa | 1.92–5.94 | 1.50 | 39.8 | |
| Bt1 | 0.52 Bb | 0.12–0.77 | 0.28 | 54.5 | ||
| Bt2 | 0.27 Bb | 0.05–0.40 | 0.16 | 58.2 | ||
| Ck1 | 0.25 Bb | 0.02–0.51 | 0.20 | 80.1 | ||
| Ck2 | 0.36 Bb | 0.13–0.67 | 0.21 | 57.6 | ||
| Nitroreductase (mg N-NO2 kg−1 h−1) | Krajna | Ap | 1.98 Aa | 0.02–4.07 | 1.83 | 92.0 |
| Bt1 | 0.09 Ba | 0.02–0.21 | 0.08 | 90.4 | ||
| Bt2 | 0.02 Ba | 0.01–0.04 | 0.01 | 23.7 | ||
| Ck1 | 0.03 Ba | 0.01–0.08 | 0.03 | 101.5 | ||
| Ck2 | 0.01 Ba | 0.00–0.03 | 0.01 | 83.3 | ||
| Chodzież | Ap | 0.88 Ab | 0.11–2.67 | 1.22 | 137.9 | |
| Bt1 | 0.05 Ab | 0.04–0.07 | 0.01 | 27.2 | ||
| Bt2 | 0.02 Ba | 0.01–0.04 | 0.01 | 63.0 | ||
| Ck1 | 0.02 Ba | 0.01–0.04 | 0.01 | 66.2 | ||
| Ck2 | 0.01 Ba | 0.01–0.02 | 0.01 | 37.6 | ||
| Dobrzyń | Ap | 1.59 Aa | 0.11–3.10 | 1.37 | 86.1 | |
| Bt1 | 0.02 Bb | 0.01–0.04 | 0.02 | 68.0 | ||
| Bt2 | 0.02 Ba | 0.00–0.05 | 0.02 | 89.5 | ||
| Ck1 | 0.02 Ba | 0.02–0.03 | 0.01 | 24.1 | ||
| Ck2 | 0.01 Ba | 0.01–0.02 | 0.01 | 55.4 |
| Enzyme | Region | Horizon | Mean | Range | SD | CV |
|---|---|---|---|---|---|---|
| Phosphstase (mg pNP kg−1 h−1) | Krajna | Ap | 63.7 A^a* | 48.2–91.3 | 17.6 | 27.6 |
| Bt1 | 31.1 Ba | 24.0–44.7 | 8.01 | 25.8 | ||
| Bt2 | 25.6 Ba | 16.7–36.7 | 7.19 | 28.1 | ||
| Ck1 | 26.2 Bb | 17.4–38.4 | 8.48 | 32.4 | ||
| Ck2 | 25.0 Ba | 16.5–37.7 | 9.93 | 38.2 | ||
| Chodzież | Ap | 67.0 Aa | 26.6–92.4 | 28.0 | 42.5 | |
| Bt1 | 35.8 Ba | 22.3–25.8 | 13.1 | 36.5 | ||
| Bt2 | 29.6 Ba | 22.9–49.1 | 13.0 | 44.1 | ||
| Ck1 | 39.3 Ba | 14.4–58.2 | 18.4 | 46.8 | ||
| Ck2 | 29.5 Ba | 11.3–49.2 | 17.7 | 59.9 | ||
| Dobrzyń | Ap | 64.8 Aa | 34.4–88.4 | 19.8 | 30.4 | |
| Bt1 | 34.0 Ba | 21.4–41.3 | 8.36 | 25.3 | ||
| Bt2 | 24.4 Bc | 18.3–33.5 | 5.75 | 23.6 | ||
| Ck1 | 17.8 Cb | 10.1–23.3 | 5.23 | 29.3 | ||
| Ck2 | 14.2 Cb | 10.2–18.5 | 3.22 | 22.7 | ||
| β-glucosidase (mg pNP kg−1 h−1) | Krajna | Ap | 43.3 Aa | 28.4–57.7 | 11.5 | 26.6 |
| Bt1 | 5.85 Ba | 4.31–8.06 | 1.30 | 22.2 | ||
| Bt2 | 1.20 Cb | 0.40–2.59 | 0.91 | 75.6 | ||
| Ck1 | 0.31 Cc | 0.08–0.47 | 0.16 | 50.8 | ||
| Ck2 | 0.42 Cc | 0.16–0.82 | 0.29 | 68.0 | ||
| Chodzież | Ap | 51.0 Aa | 31.0–67.0 | 15.7 | 30.8 | |
| Bt1 | 5.18 Ba | 2.42–6.55 | 1.90 | 36.7 | ||
| Bt2 | 1.80 Ca | 0.95–2.69 | 0.80 | 44.4 | ||
| Ck1 | 0.75 Cb | 0.40–1.28 | 0.38 | 48.2 | ||
| Ck2 | 0.78 Cb | 0.64–0.86 | 0.10 | 12.5 | ||
| Dobrzyń | Ap | 48.0 Aa | 30.2–67.1 | 14.0 | 29.1 | |
| Bt1 | 2.25 Bb | 1.77–2.67 | 0.33 | 14.5 | ||
| Bt2 | 1.87 Ca | 0.69–2.40 | 0.71 | 38.1 | ||
| Ck1 | 1.51 Ca | 0.51–2.33 | 0.65 | 43.2 | ||
| Ck2 | 1.59 Ca | 0.82–2.42 | 0.70 | 43.8 |
| Landlake | CORG | NTOT | MBC | pH KCl | Hh | Bulk Density | Porosity | Clay | Available | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Mg | ||||||||||
| DHA | Krajna | 0.848 *** | 0.825 *** | 0.963 *** | −0.500 * | 0.435 * | 0.815 *** | 0.788 *** | - | 0.773 *** | 0.900 *** | - |
| Chodzież | 0.914 *** | 0.921 *** | 0.936 *** | −0.730 ** | ^- | −0.884 *** | 0.870 *** | - | 0.774 *** | 0.859 *** | - | |
| Chełmno | 0.890 *** | 0.869 *** | 0.973 *** | −0.450 * | 0.400 * | −0.591 ** | 0.528 ** | - | 0.730 ** | 0.681 ** | - | |
| CAT | Krajna | 0.877 *** | 0.904 *** | 0.954 *** | −0.636 ** | 0.609 ** | −0.872 *** | 0.839 *** | - | 0.811 *** | 0.905 *** | -- |
| Chodzież | 0.918 *** | 0.929 *** | 0.942 *** | −0.806 *** | 0.502 * | −0.893 *** | 0.904 *** | - | 0.788 *** | 0.846 *** | -- | |
| Chełmno | 0.947 *** | 0.942 *** | 0.973 *** | −0.581 ** | 0.536 ** | −0.721 ** | 0.647 ** | - | 0.832 *** | 0.738 *** | - | |
| UR | Krajna | 0.775 *** | 0.768 *** | 0.835 *** | - | - | −0.790 *** | 0.802 *** | - | 0.739 *** | 0.841 *** | - |
| Chodzież | 0.810 *** | 0.796 *** | 0.704 ** | - | - | −0.556 ** | 0.456 * | - | 0.760 *** | 0.623 ** | −0.459 * | |
| Chełmno | 0.925 *** | 0.888*** | 0.894 *** | −0.483 * | 0.407 * | −0.682 ** | 0.642 ** | - | 0.736 *** | 0.578 ** | - | |
| NR | Krajna | 0.746 *** | 0.698 *** | 0.760 *** | - | - | −0.704 ** | 0.719 ** | - | 0.692 ** | 0.696 ** | −0.399 * |
| Chodzież | 0.589 ** | 0.558 ** | - | - | - | - | - | - | 0.717 ** | 0.573 ** | −0.483 * | |
| Chełmno | 0.736 *** | 0.709 ** | 0.838 *** | - | - | −0.530 ** | 0.488 * | - | 0.673 ** | 0.680 ** | −0.399 * | |
| GLU | Krajna | 0.922 *** | 0.906 *** | 0.893 *** | - | - | −0.914 *** | 0.922 *** | −0.421 * | 0.904 *** | 0.874 *** | - |
| Chodzież | 0.931 *** | 0.909 *** | 0.863 *** | - | - | −0.722 ** | 0.637 ** | −0.502 * | 0.889 *** | 0.721 ** | - | |
| Chełmno | 0.932 *** | 0.915 *** | 0.760 *** | −0.668** | 0.521 ** | −0.634 ** | 0.572 ** | - | 0.704 ** | 0.639 ** | - | |
| PHA | Krajna | 0.825 *** | 0.792 *** | 0.763 *** | −0.464 * | 0.428 * | −0.769 *** | 0.762 ** | −0.473 * | 0.621 ** | 0.663 ** | - |
| Chodzież | 0.574 ** | 0.565 * | 0.690 ** | - | - | −0.669 ** | 0.675 ** | −0.449 * | 0.494 * | - | - | |
| Chełmno | 0.906 *** | 0.886 *** | 0.785 *** | −0.622 ** | 0.546 ** | −0.809 *** | 0.753 ** | - | 0.773 *** | *** | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska-Długosz, A.; Kobierski, M.; Długosz, J. Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols. Materials 2021, 14, 6364. https://doi.org/10.3390/ma14216364
Piotrowska-Długosz A, Kobierski M, Długosz J. Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols. Materials. 2021; 14(21):6364. https://doi.org/10.3390/ma14216364
Chicago/Turabian StylePiotrowska-Długosz, Anna, Mirosław Kobierski, and Jacek Długosz. 2021. "Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols" Materials 14, no. 21: 6364. https://doi.org/10.3390/ma14216364






