The Electrospray (ESI) and Flowing Atmosphere-Pressure Afterglow (FAPA) Mass Spectrometry Studies of Nitrophenols (Plant Growth Stimulants) Removed Using Strong Base-Functionalized Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. The Synthesis of the Molecular Receptors and the Functional Materials
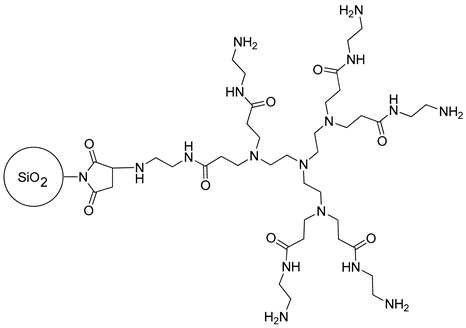
2.2.1. The Synthesis of PAMAM-Type Dendrimers
2.2.2. The Synthesis of Dendrimer-Functionalized Materials
2.3. Instruments
2.4. The Sorption of Nitrophenols-Containing Biostimulator Using the Tested Functional Materials
2.4.1. Adsorption Experiments
2.4.2. The Release of the Nitrophenols from the Functional Sorbents
2.4.3. Analysis of the Nitrophenols’ Affinity towards Soil
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K.; Martinez-Manez, R. The Supramolecular Chemistry of Organic–Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2010, 45, 5924–5948. [Google Scholar]
- Kickelbick, G. Hybrid Materials-Past, Present and Future. Hybrid. Mater. 2014, 1, 39–51. [Google Scholar] [CrossRef]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Kayan, A. Inorganic-organic hybrid materials and their adsorbent properties. Adv. Compos. Hybrid. Mater. 2019, 2, 34–45. [Google Scholar] [CrossRef]
- Lu, K. Hybrid materials-a review on co-dispersion, processing, patterning, and properties. Int. Mater. Rev. 2020, 65, 463–501. [Google Scholar] [CrossRef]
- Silina, Y.E.; Gernaey, K.V.; Semenova, D.; Iatsunskyi, I. Application of Organic-Inorganic Hybrids in Chemical Analysis, Bio- and Environmental Monitoring. Appl. Sci. 2020, 10, 1458. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Przybysz, A.; Gawrońska, H.; Gajc-Wolska, J. Biological mode of action of a nitrophenolates-based biostimulant: Case study. Front. Plant Sci. 2014, 5, 713–727. [Google Scholar] [CrossRef]
- EPA, USA. EPA’s Report on the Environment; Technical Report; US EPA: Washington, DC, USA, 2008.
- Superplon, K. Safety Date; Update: 2 January 2020. Available online: https://www.target.com.pl/files/125/41/1/superplonk_01_2020pl.pdf (accessed on 24 October 2021).
- Serjeant, E.P.; Dempsey, B. Ionisation Constants of Organic Acids in Aqueous Solution. In IUPAC Chemical Data Series No. 23; Pergamon Press, Inc.: New York, NY, USA, 1979. [Google Scholar]
- Chowdhury, M.A. The Controlled Release of Drugs and Bioactive Compounds from Mesoporous Silica Nanoparticles. Curr. Drug Deliv. 2016, 13, 839–856. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Cegłowski, M.; Kurczewska, J.; Ruszkowski, P.; Liberska, J.; Schroeder, G. The influence of cross-linking agent onto adsorption properties, release behavior and cytotoxicity of doxorubicin-imprinted microparticles. Colloids Surf. B Biointerfaces 2019, 182, 110379. [Google Scholar] [CrossRef]
- Gościanska, J.; Olejnik, A. Removal of 2,4-D herbicide from aqueous solution by aminosilane grafted mesoporous carbons. Adsorption 2019, 25, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Olejnik, A.; Nowak, I.; Schroeder, G. Functionalized polystyrene beads as carriers in release studies of two herbicides: 2,4-dichlorophenoxyacetic acid and 2-methyl-4-chlorophenoxy-acetic acid. Int. J. Environ. Sci. Technol. 2019, 16, 5623–5634. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ballesta, M.C.; Gil-Izquierdo, A.; Garcia-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, G.F.; Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of Incorporating Plant-Derived Bioactive Compounds into Films Made with Agro-Based Polymers for Application as Food Packaging: A Brief Review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef]
- Tchieno, F.M.M.; Tonle, I.K. p-Nitrophenol determination and remediation: An overview. Rev. Anal. Chem. 2018, 37, 20170019. [Google Scholar] [CrossRef]
- Harrison, M.A.J.; Barra, S.; Borghesi, D.; Vione, D.; Arsene, C.; Olariu, R.I. Nitrated phenols in the atmosphere: A review. Atmos. Environ. 2005, 39, 231–248. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, D.; Lv, X.; Xu, X.; Zhou, H.; Liu, P.; Cui, B.; Wang, L. Simultaneous electrochemical determination of nitrophenol isomers Based on spirofluorene-based microporous polymer film modified electrodes through one-step electropolymerization strategy. Sens. Actuators B Chem. 2021, 333, 129568. [Google Scholar] [CrossRef]
- Geissler, A.; Schoeler, H.F. Gas chromatographic determination of phenol, methylphenols, chlorophenols, nitrophenols and nitroquinones in water at 0.1 g l−1. Water Res. 1994, 28, 2047–2053. [Google Scholar]
- Zhang, P.-P.; Shi, Z.-G.; Feng, Y.-Q. Determination of phenols in environmental water samples by two-step liquid-phase microextraction coupled with high performance liquid chromatography. Talanta 2011, 85, 2581–2586. [Google Scholar] [CrossRef]
- Pastor-Belda, M.; Sanchez-Lopez, M.J.; Campillo, N.; Vinas, P.; Hernandez-Cordoba, M. Determination of nitrophenols in environmental samples using stir bar sorptive extraction coupled to thermal desorption gas chromatography-mass spectrometry. Talanta 2018, 189, 543–549. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, W.; Yang, Z.; Dai, Z.; Yang, Y. Spectrophotometric Determination of p-Nitrophenol under ENP Interference. J. Anal. Methods Chem. 2021, 2021, 6682722. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Schroeder, G. Adsorption studies of Cu(II) ions on dendrimer-grafted silica-based materials. J. Mol. Liq. 2019, 281, 176–185. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Schroeder, G. Dual-Polymeric Resin Based on Poly(methyl vinyl ether-alt-maleic anhydride) and PAMAM Dendrimer as a Versatile Supramolecular Adsorbent. ACS Appl. Polym. Mater. 2021, 3, 956–967. [Google Scholar] [CrossRef]
- Zhao, Z.; Pu, J.; Dai, J.; He, F.; Ren, B.B.; Zhang, C.; Duan, X. A mechanism study of positive ionization processes in flowing atmospheric-pressure afterglow (FAPA) ambient ion source with controlled plasma and ambient conditions. Talanta 2019, 205, 120090. [Google Scholar] [CrossRef]
- Bruggemann, M.; Karu, E.; Stelzer, T.; Hoffmann, T. Real-Time Analysis of Ambient Organic Aerosols Using Aerosol Flowing Atmospheric-Pressure Afterglow Mass Spectrometry (AeroFAPA-MS). Environ. Sci. Technol. 2015, 49, 5571–5578. [Google Scholar] [CrossRef]
- Guć, M.; Schroeder, G. Application of molecularly imprinted polymers (MIP) and magnetic molecularly imprinted polymers (mag-MIP) to selective analysis of quercetin in flowing atmospheric-pressure afterglow mass spectrometry (FAPA-MS) and in electrospray ionization mass spectrometry (ESI-MS). Molecules 2019, 24, 2364–2378. [Google Scholar]
- Guć, M.; Reszke, E.; Cegłowski, M.; Schroeder, G. Construction of Plasma Ion Sources to be Applied in Analysis of Small Organic Compounds Using Mass Spectrometry. Plasma Chem. Plasma Process. 2020, 40, 235–260. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cresswell, M. Inorganic Controlled Release Technology: Materials and Concepts for Advanced Drug Formulation; Elsevier Ltd.: Waltham, MA, USA, 2015; pp. 17–48. [Google Scholar]
- Cegłowski, M.; Kurczewska, J.; Smoluch, M.; Reszke, E.; Silberring, J.; Schroeder, G. Magnetic scavengers as carriers of analytes for flowing atmospheric pressure afterglow mass spectrometry (FAPA-MS). Analyst 2015, 140, 6138–6144. [Google Scholar] [CrossRef] [PubMed]

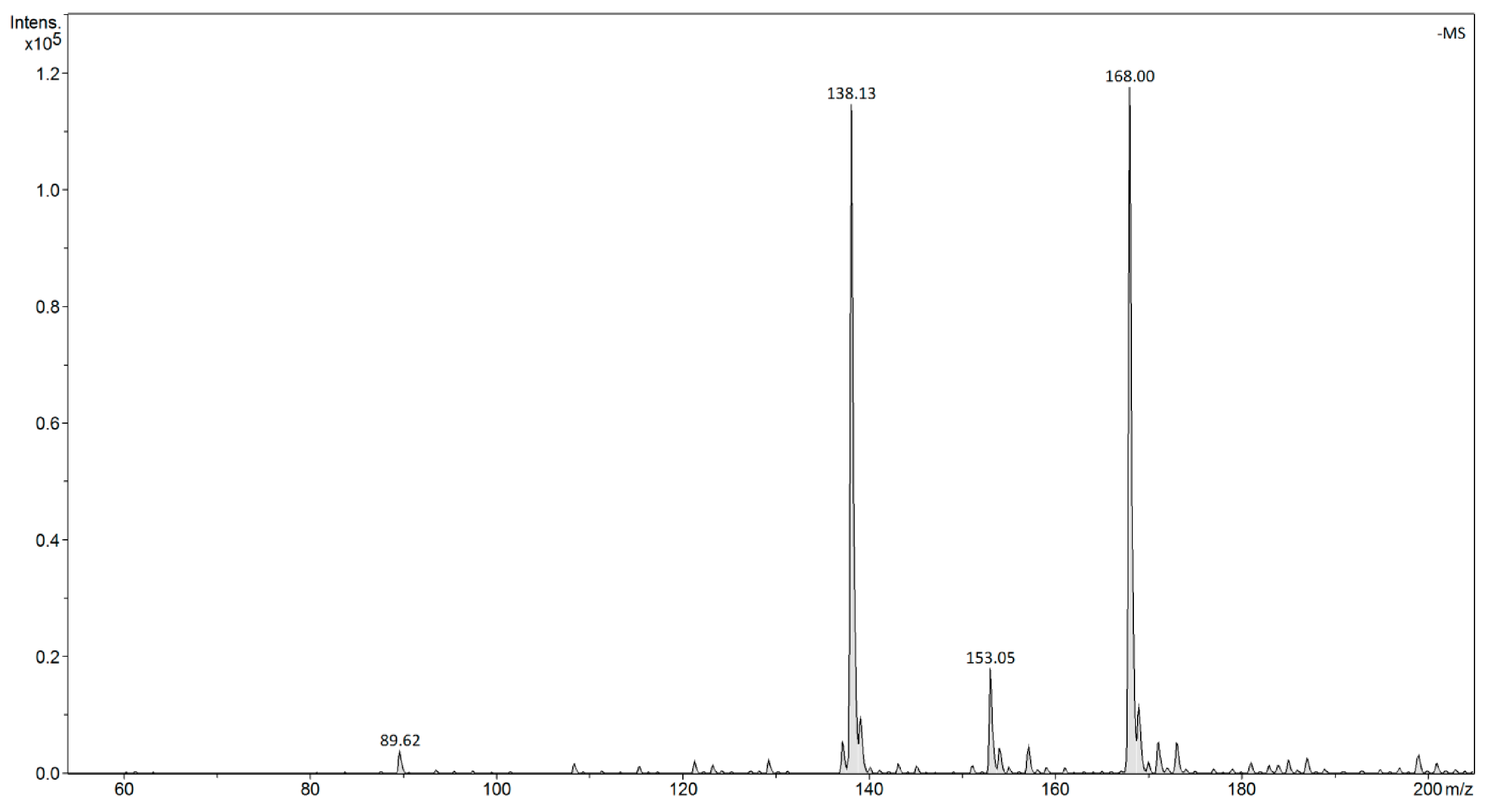
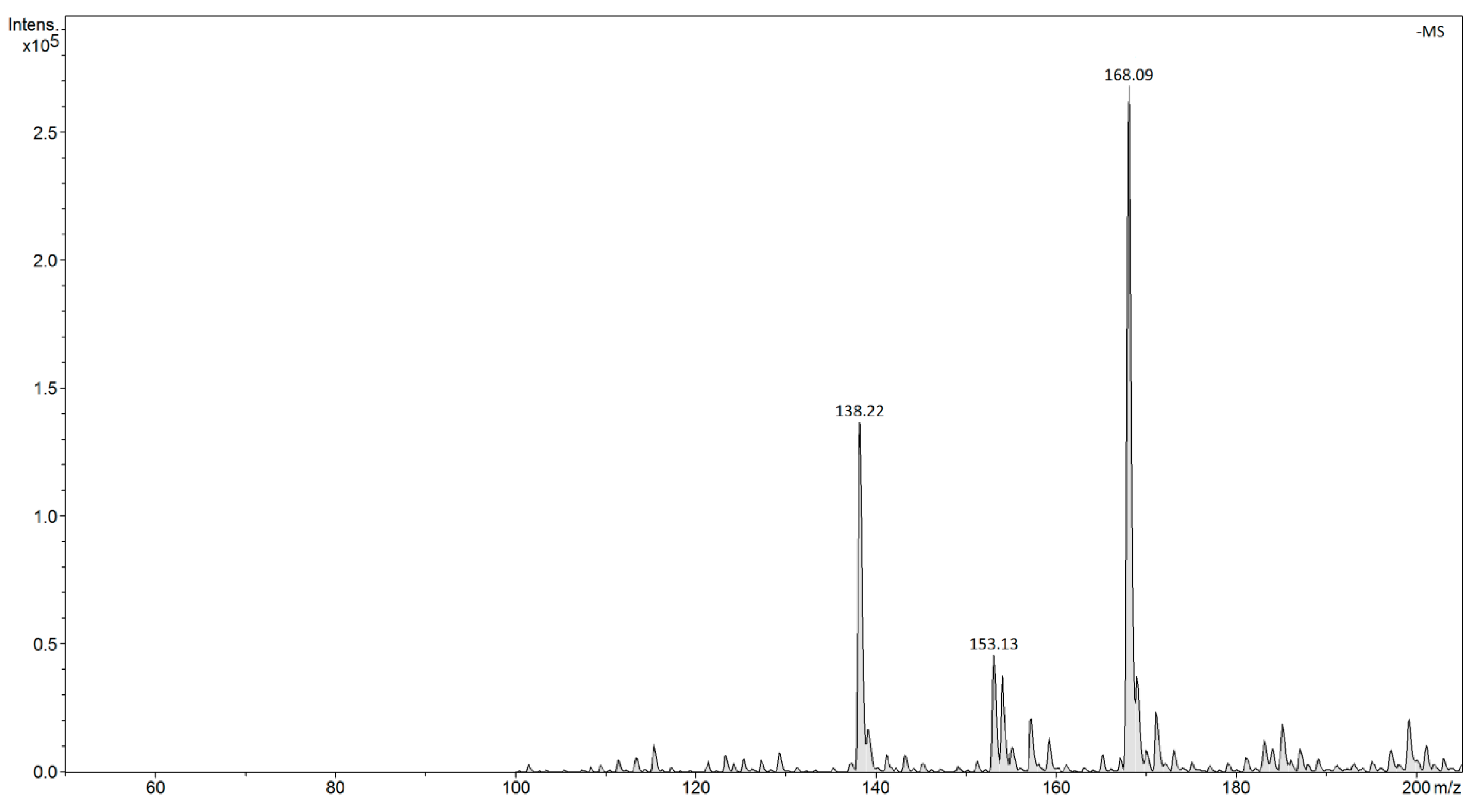


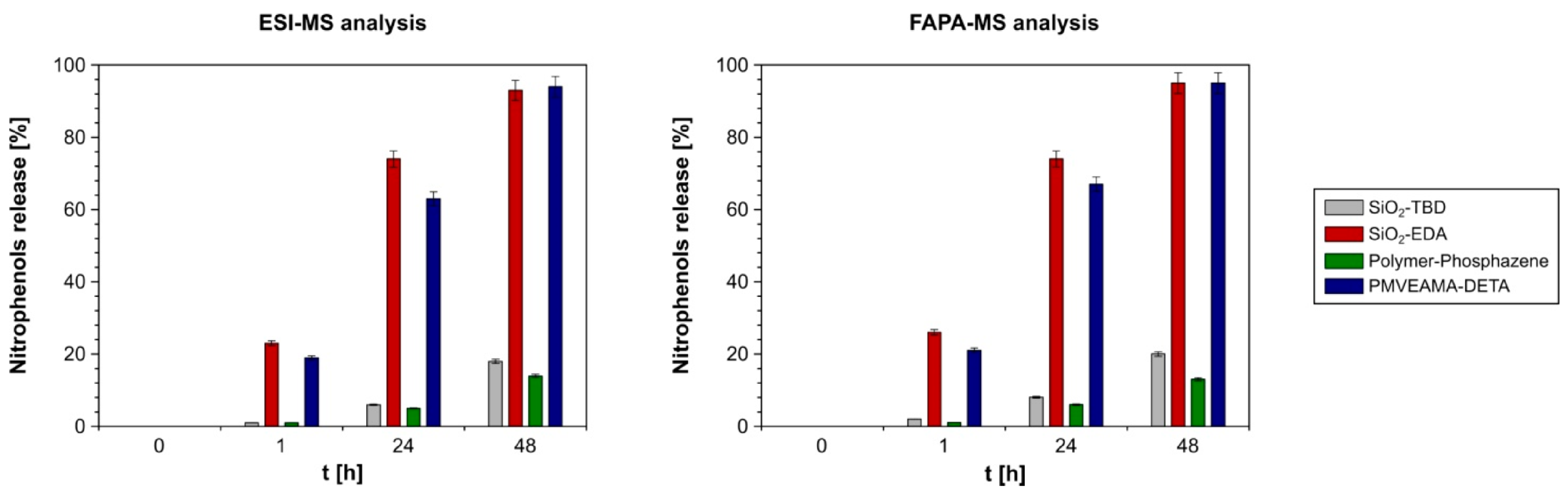
 | |
| SiO2-EDA | |
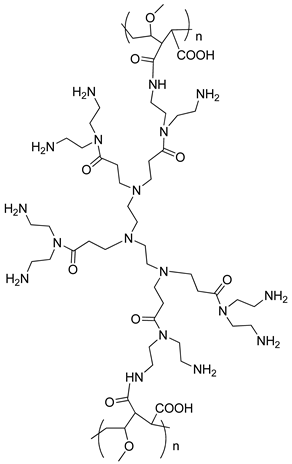 | 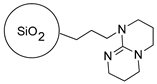 |
| SiO2-TBD | |
 | |
| PMVEAMA-DETA | Polymer-Phosphazene |
| Concentration of Nitrophenols in Solution after Adsorption [g L−1] | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2-TBD | SiO2-EDA | Polymer-Phosphazene | PMVEAMA-DETA | |||||
| c0 [g L−1] | p-NP | MNP | p-NP | MNP | p-NP | MNP | p-NP | MNP |
| 1.4 × 10−1 | 4.3 × 10−3 | 3.9 × 10−3 | 5.3 × 10−3 | 5.9 × 10−3 | 1.2 × 10−3 | 1.1 × 10−3 | 5.8 × 10−3 | 5.9 × 10−3 |
| 1.4 × 10−2 | 4.6 × 10−4 | 4.1 × 10−4 | 6.6 × 10−4 | 6.3 × 10−4 | 2.6 × 10−4 | 2.4 × 10−4 | 7.0 × 10−4 | 7.0 × 10−4 |
| 1.4 × 10−3 | 1.2 × 10−5 | 1.4 × 10−5 | 4.2 × 10−5 | 4.1 × 10−5 | 0.9 × 10−5 | 1.1 × 10−5 | 5.1 × 10−5 | 4.9 × 10−5 |
| 1.4 × 10−4 | 1.1 × 10−6 | 0.9 × 10−6 | 2.2 × 10−6 | 1.9 × 10−6 | 0.9 × 10−6 | 0.6 × 10−6 | 2.8 × 10−6 | 2.9 × 10−6 |
| 1.4 × 10−5 | 2.1 × 10−7 | 2.0 × 10−7 | 4.1 × 10−7 | 4.2 × 10−7 | nd | nd | 4.9 × 10−7 | 4.9 × 10−7 |
| The Amount of the Nitrophenols Adsorbed on the Functional Materials [g g−1] with Indicated Adsorption Percentages | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2-TBD | SiO2-EDA | Polymer-Phosphazene | PMVEAMA-DETA | |||||
| p-NP | MNP | p-NP | MNP | p-NP | MNP | p-NP | MNP | |
| (a) | 1.02 × 10−1 | 1.01 × 10−1 | 8.94 × 10−2 | 8.84 × 10−2 | 1.40 × 10−1 | 1.41 × 10−1 | 7.24 × 10−2 | 7.61 × 10−2 |
| (72.8%) | (72.1%) | (63.8%) | (63.1%) | (100.0%) | (100.7%) | (53.00%) | (54.4%) | |
| (b) | 1.28 × 10−3 | 1.34 × 10−3 | 9.97 × 10−4 | 9.23 × 10−4 | 1.39 × 10−3 | 1.36 × 10−3 | 8.42 × 10−4 | 8.91 × 10−3 |
| (91.4%) | (95.7%) | (71.2%) | (65.9%) | (99.2%) | (97.1%) | (60.1%) | (63.6%) | |
| (c) | 1.42 × 10−5 | 1.54 × 10−5 | 8.90 × 10−5 | 8.57 × 10−5 | 1.42 × 10−5 | 1.46 × 10−5 | 7.44 × 10−5 | 7.64 × 10−5 |
| (101%) | (110%) | (63.5%) | (40.7%) | (101.4%) | (104.3%) | (53.1%) | (54.6%) | |
| Concentration of Nitrophenols in Water Solution [g L−1] | The Calculated Concentrations of the Nitrophenols in Water Solutions with the Use of Adsorbents, Detected by the FAPA MS Technique | ||||
|---|---|---|---|---|---|
| SiO2-TBD | SiO2-EDA | Polymer-Phosphazene | PMVEAMA-DETA | ||
| p-NP MNP | 1 × 10−6 1 × 10−6 | (0.92–1.10) × 10−6 (0.91–1.14) × 10−6 | (0.89–1.12) × 10−6 (0.92–1.13) × 10−6 | (0.90–1.15) × 10−6 (0.88–1.14) × 10−6 | (0.97–1.12) × 10−6 (0.90–1.16) × 10−6 |
| p-NP MNP | 1.4 × 10−5 1.4 × 10−5 | (1.38–1.54) × 10−5 (1.28–1.58) × 10−5 | (1.32–1.54) × 10−5 (1.21–1.59) × 10−5 | (1.37–1.53) × 10−5 (1.31–1.58) × 10−5 | (1.31–1.56) × 10−5 (1.28–1.56) × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlaczyk, M.; Cegłowski, M.; Frański, R.; Kurczewska, J.; Schroeder, G. The Electrospray (ESI) and Flowing Atmosphere-Pressure Afterglow (FAPA) Mass Spectrometry Studies of Nitrophenols (Plant Growth Stimulants) Removed Using Strong Base-Functionalized Materials. Materials 2021, 14, 6388. https://doi.org/10.3390/ma14216388
Pawlaczyk M, Cegłowski M, Frański R, Kurczewska J, Schroeder G. The Electrospray (ESI) and Flowing Atmosphere-Pressure Afterglow (FAPA) Mass Spectrometry Studies of Nitrophenols (Plant Growth Stimulants) Removed Using Strong Base-Functionalized Materials. Materials. 2021; 14(21):6388. https://doi.org/10.3390/ma14216388
Chicago/Turabian StylePawlaczyk, Mateusz, Michał Cegłowski, Rafał Frański, Joanna Kurczewska, and Grzegorz Schroeder. 2021. "The Electrospray (ESI) and Flowing Atmosphere-Pressure Afterglow (FAPA) Mass Spectrometry Studies of Nitrophenols (Plant Growth Stimulants) Removed Using Strong Base-Functionalized Materials" Materials 14, no. 21: 6388. https://doi.org/10.3390/ma14216388






