Titanium-Enriched Slag Prepared by Atmospheric Hydrochloric Acid Leaching of Mechanically Activated Vanadium Titanomagnetite Concentrates
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussion
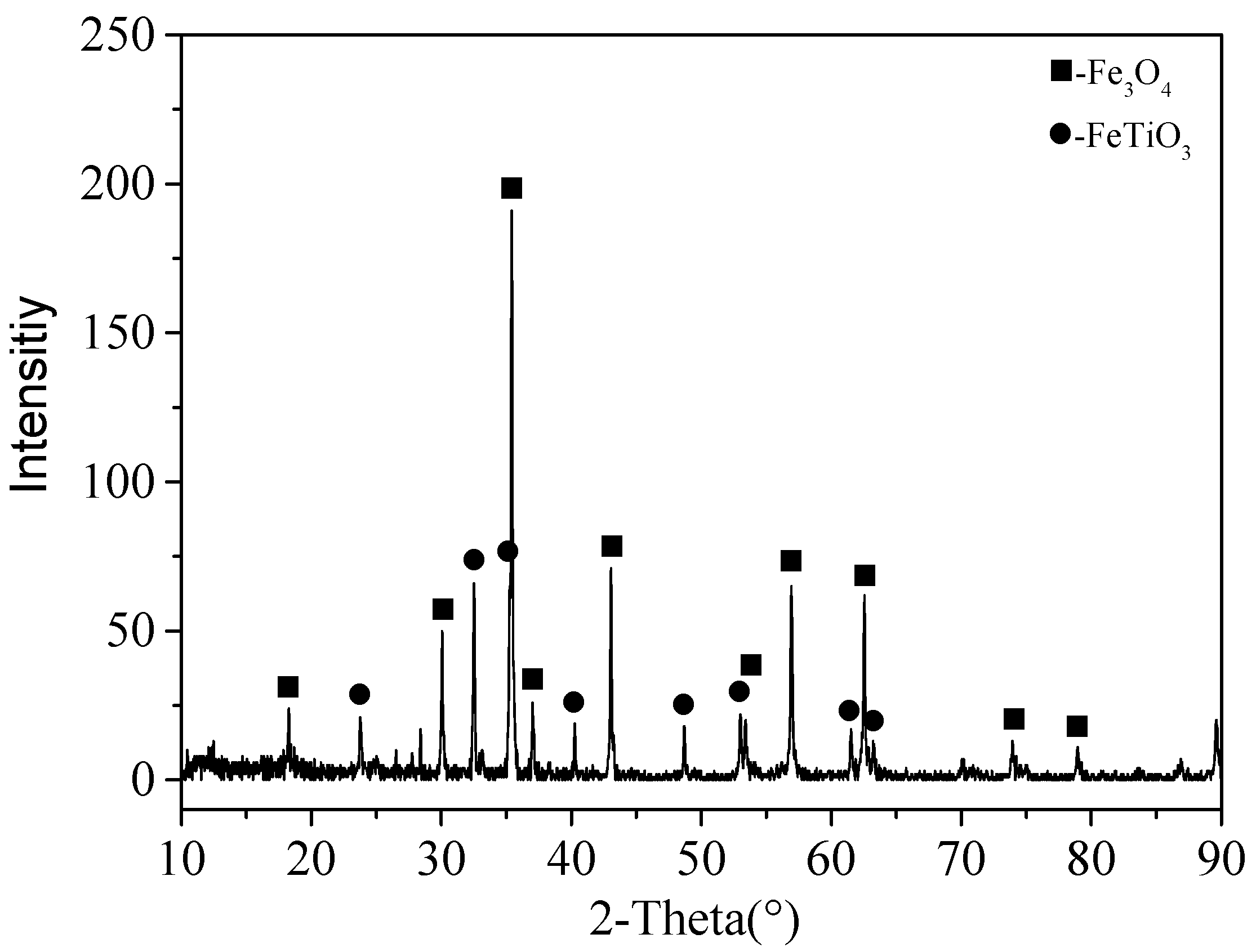
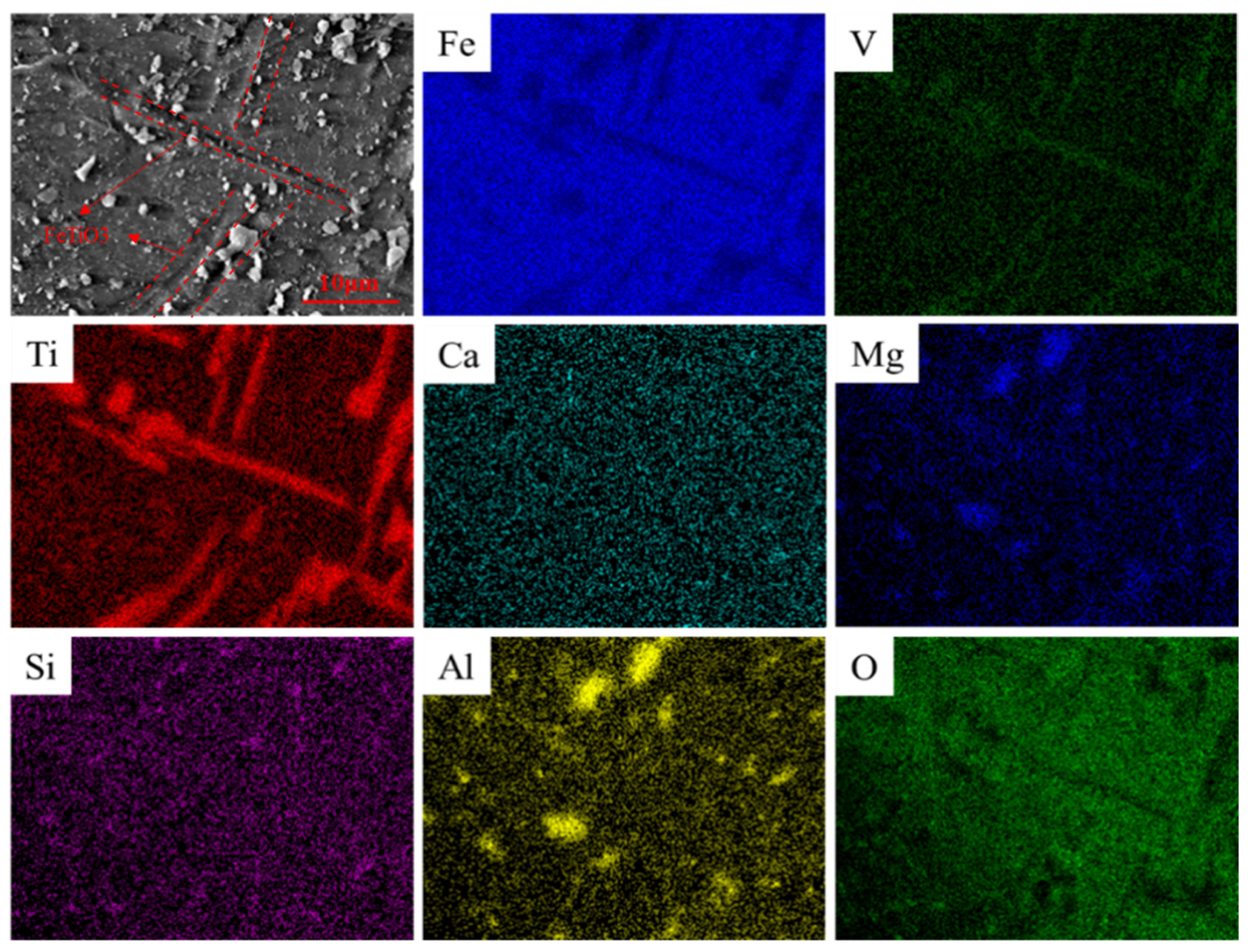
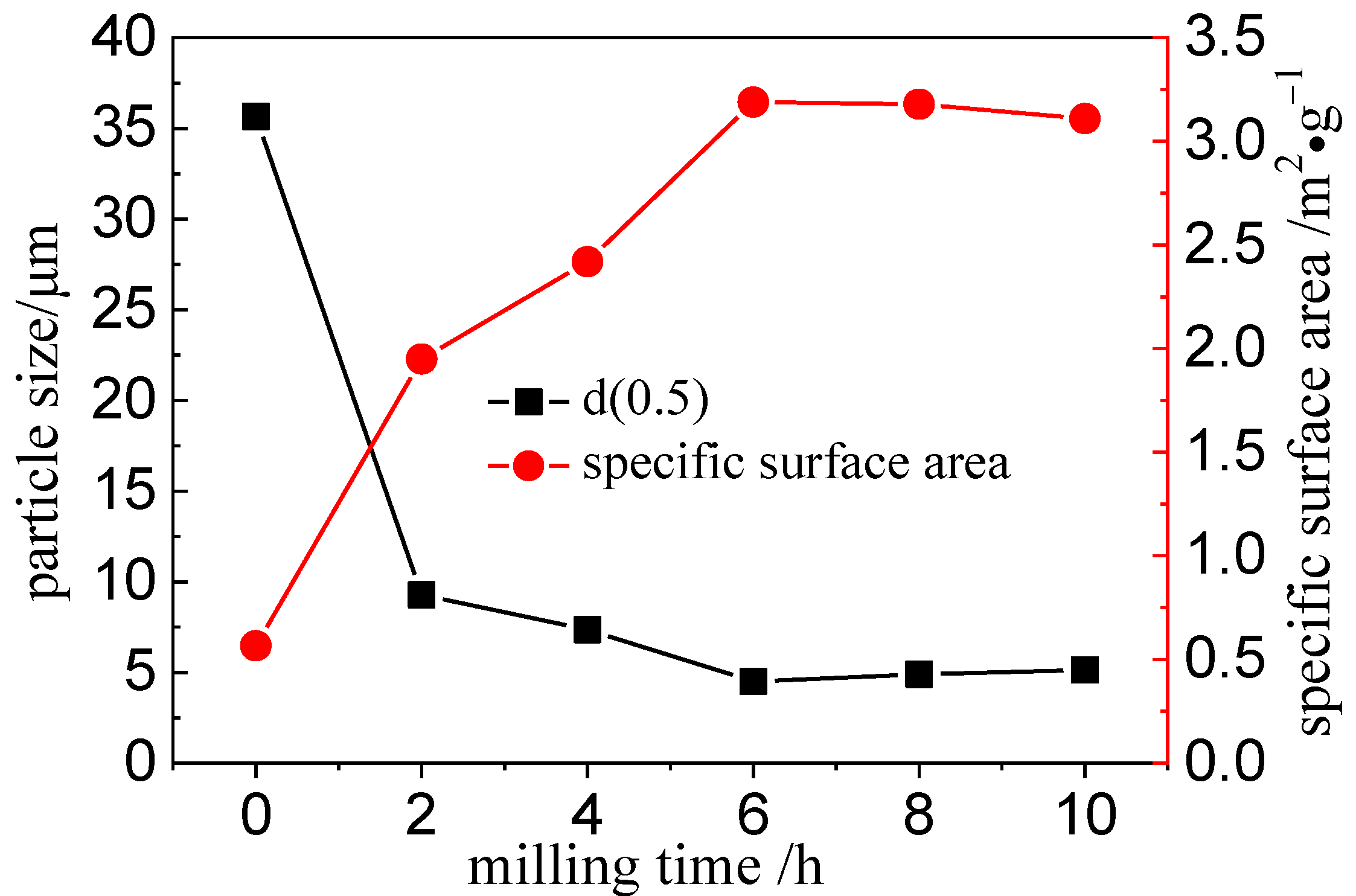
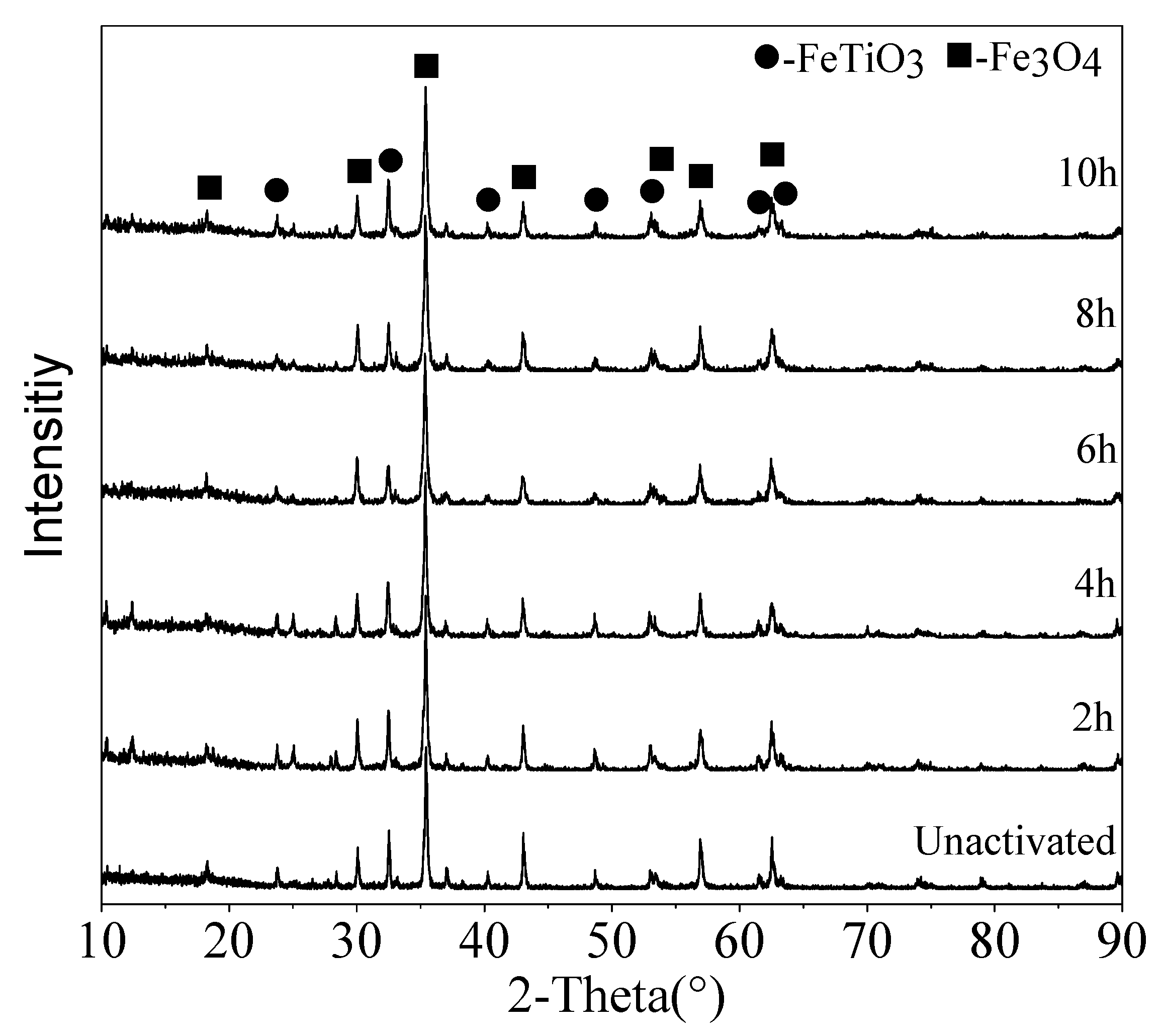
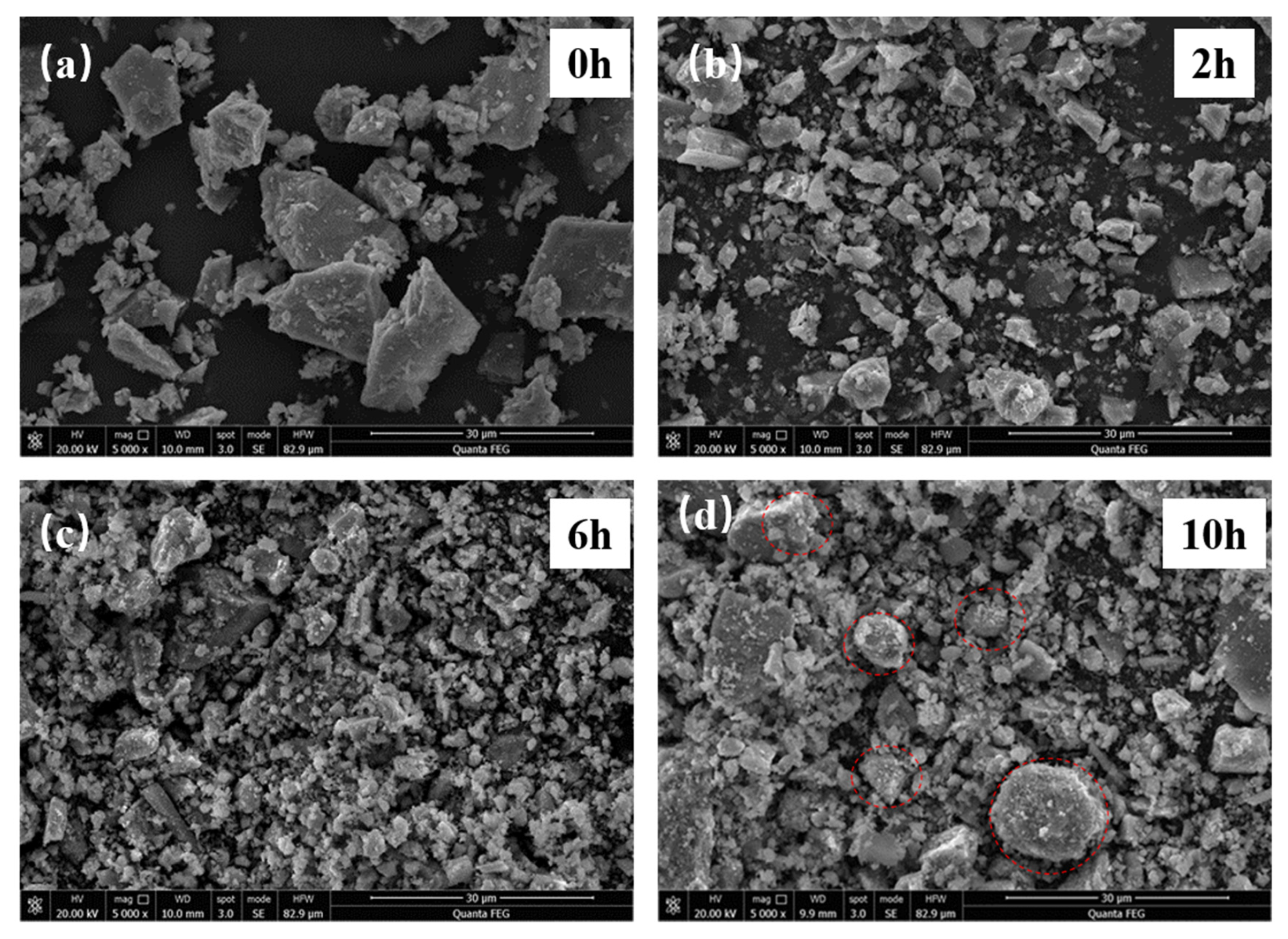
3.1. Structural Changes of the Mechanically Activated VTMCs
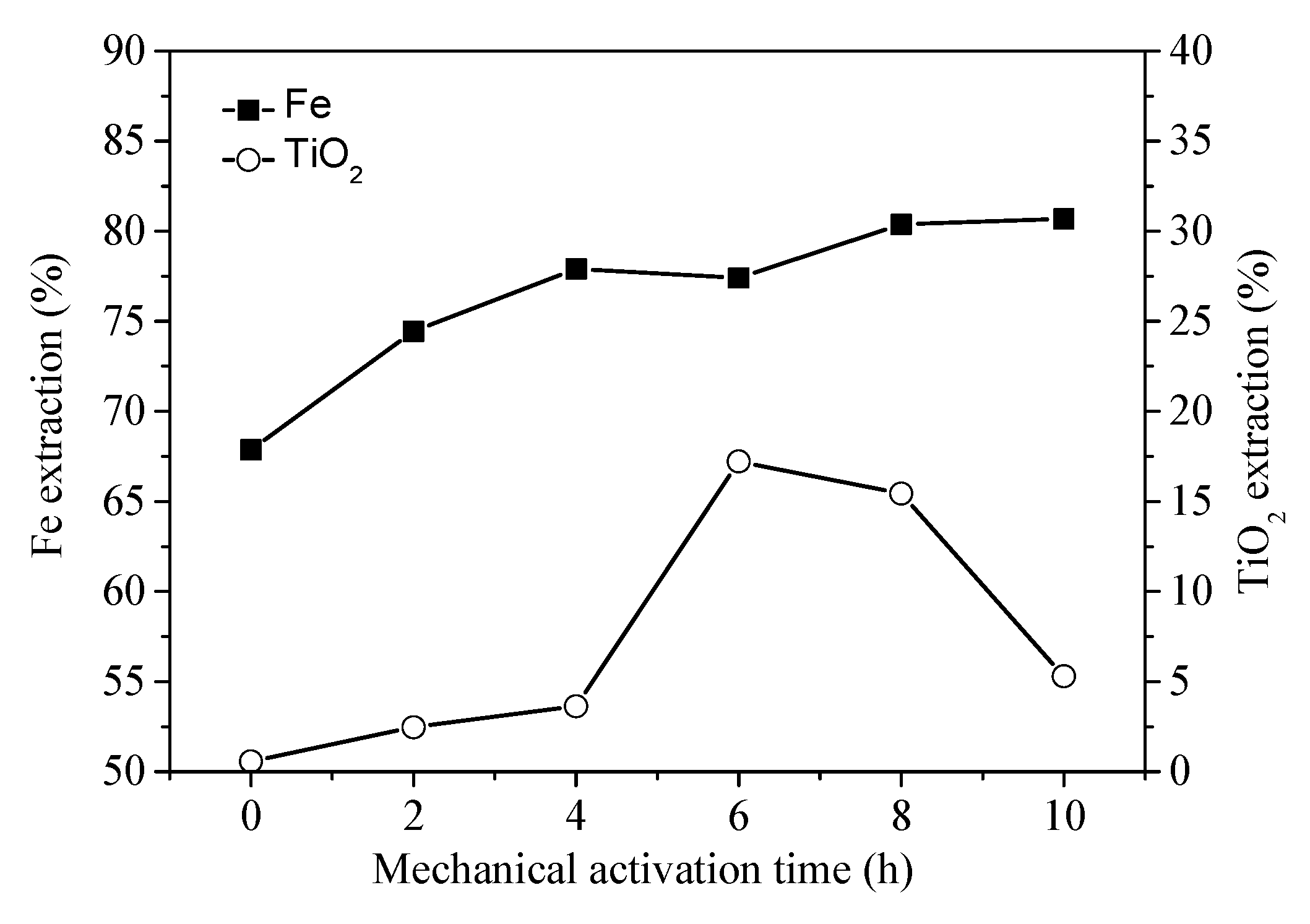
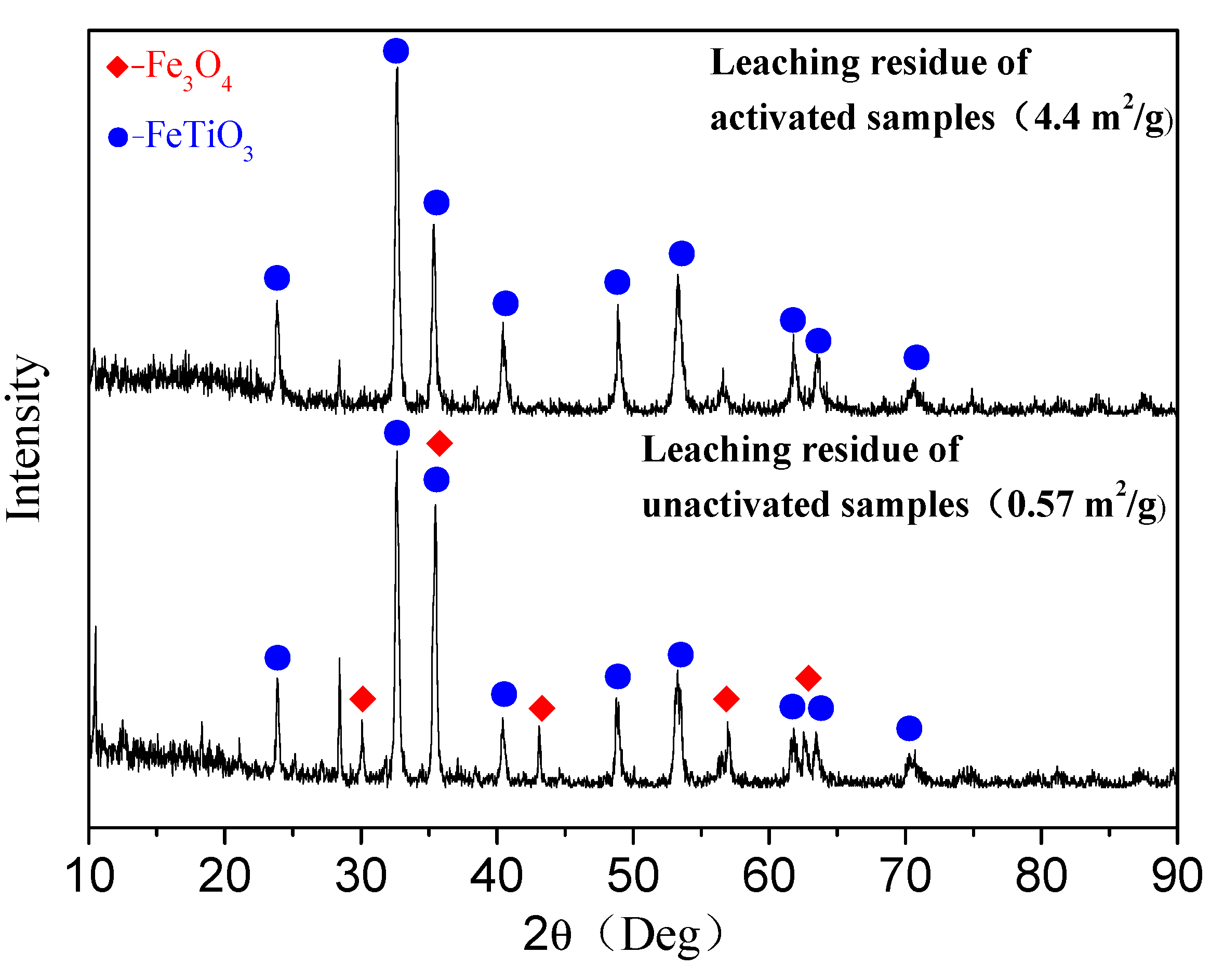
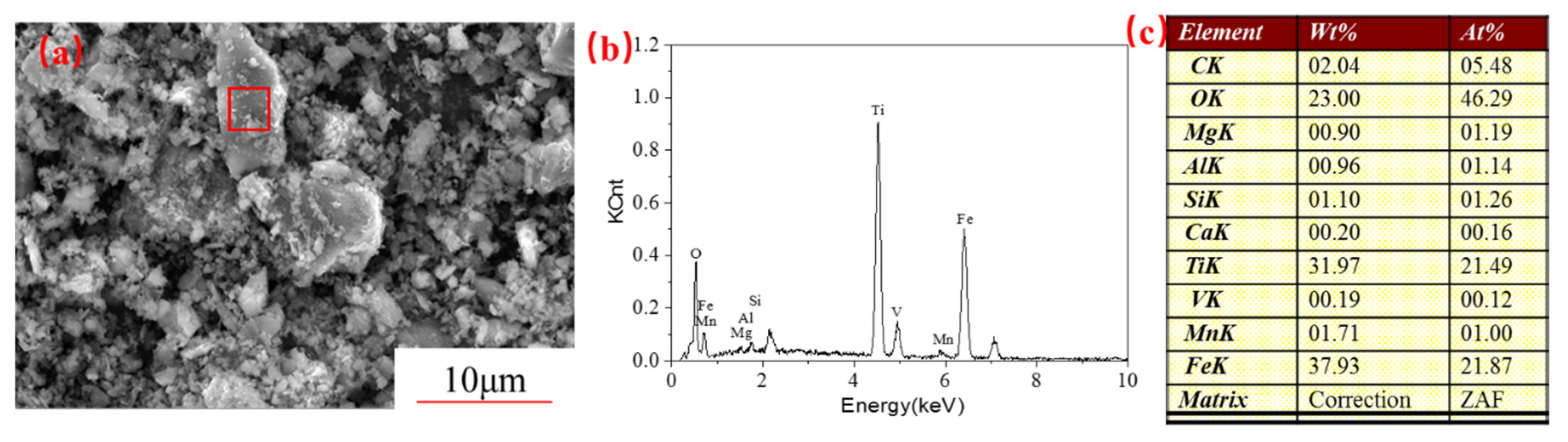
3.2. Hydrochloric Acid Leaching Behavior of Unactivated and Activated PTVMCs
4. Conclusions
- An effective technique to obtain Ti-enriched slag from mechanically activated VTMCs is atmospheric hydrochloric acid leaching.
- Mechanical activation causes a decrease in the median volume particle diameter (d50) and an increase in the specific surface area (SA) with an increase in the milling time. The XRD patterns and SEM were conducted, and they confirmed the effects of the mechanical activation.
- It was also demonstrated that mechanical activation could be employed to improve Fe recovery from VTMCs followed by hydrochloric acid leaching.
- Moreover, the content of the TiO2 in the Ti-enriched slag can reach 43.75%, and the XRD patterns, as well as EDS, confirmed that the major phase in the Ti-enriched slag is the ilmenite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, G.H.; Jiang, T.; Zhang, Y.B.; Huang, Y.F.; Li, G.H. High-Temperature Oxidation Behavior of Vanadium, Titanium-Bearing Magnetite Pellet. J. Iron Steel Res. Int. 2011, 18, 14–19. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Influence of TiO2 addition on the oxidation induration and reduction behavior of Hongge vanadium titanomagnetite pellets with simulated shaft furnace gases. Powder Technol. 2018, 326, 137–145. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Y.; Yang, K.; Rouff, A.A.; Elzinga, E.J.; Huang, J.H. Leaching characteristics of vanadium in mine tailings and soils near a vanadium titanomagnetite mining site. J. Hazard. Mater. 2014, 264, 498–504. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, T.; Chu, J.L.; Zhao, W. Removal of iron from ilmenite by KOH leaching-oxalate leaching method. Rare Metals. 15. [CrossRef]
- Gao, C.J.; Jiang, B.; Cao, Z.M.; Huang, K.; Zhu, H.M. Preparation of titanium oxycarbide from various titanium raw materials: Part, I. Carbothermal reduction. Rare Metals. 2010, 29, 547–551. [Google Scholar] [CrossRef]
- Bai, Y.Q.; Cheng, S.S.; Bai, Y.M. Analysis of Vanadium-Bearing Titanomagnetite Sintering Process by Dissection of Sintering Bed. J. Iron Steel Res. Int. 2011, 18, 8–15. [Google Scholar] [CrossRef]
- Fu, W.G.; Wen, Y.C.; Xie, H.E. Development of Intensified Technologies of Vanadium-Bearing Titanomagnetite Smelting. J. Iron Steel Res. Int. 2011, 18, 7–10. [Google Scholar] [CrossRef]
- Liu, J.X.; Cheng, G.J.; Liu, Z.G.; Chu, M.S.; Xue, X.X. Softening and melting properties of different burden structures containing high chromic vanadium titano-magnetite. Int. J. Miner. Process. 2015, 142, 113–118. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, M.S. Metalizing reduction and magnetic separation of vanadium titano-magnetite based on hot briquetting. Int. J. Min. Met. Mater. 2014, 21, 225–233. [Google Scholar] [CrossRef]
- Hu, T.; Lv, X.W.; Bai, C.G.; Lun, Z.G.; Qiu, G.B. Reduction Behavior of Panzhihua Titanomagnetite Concentrates with Coal. Metall Mater. Trans. B 2013, 44, 252–260. [Google Scholar] [CrossRef]
- Lv, X.W.; Lun, Z.G.; Yin, J.Q.; Bai, C.G. Carbothermic Reduction of Vanadium Titanomagnetite by Microwave Irradiation and Smelting Behavior. ISIJ. Int. 2013, 53, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Lv, X.W.; Bai, C.G.; Qiu, G.B. Isothermal reduction of titanomagnetite concentrates containing coal. Int. J. Min. Met. Mater. 2014, 21, 131–137. [Google Scholar] [CrossRef]
- Hu, T.; Lv, X.W.; Bai, C.G.; Lun, Z.G.; Qiu, G.B. Carbothermic Reduction of Titanomagnetite Concentrates with Ferrosilicon Ad dition. ISIJ. Int. 2013, 53, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zheng, H.Y.; Dong, Y.; Jiang, X.; Shen, Y.S.; Shen, F.M. Melting and separation behavior of slag and metal phases in metallized pellets obtained from the direct-reduction process of vanadium-bearing titanomagnetite. Int. J. Miner. Process. 2015, 142, 119–124. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Yi, L.Y.; Wang, L.N.; Chen, D.S.; Wang, W.J.; Liu, Y.H.; Zhao, H.X.; Qi, T. A novel process for the recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite: Sodium modification–direct reduction coupled process. Int. J. Min. Met. Mater. 2017, 24, 504–511. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, Y.F.; Qiu, G.Z.; Jiang, T.; Chen, F. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium–tita nium magnetite concentrate. Trans. Nonferrous Met. Soc. China 2014, 24, 3372–3377. [Google Scholar] [CrossRef]
- Zhao, L.S.; Wang, L.N.; Chen, D.S.; Zhao, H.X.; Liu, Y.H.; Qi, T. Behaviors of vanadium and chromium in coal-based direct reduction of high-chromium vanadium-bearing titanomagnetite concentrates followed by magnetic separation. Trans. Nonferrous Met. Soc. China 2015, 25, 1325–1333. [Google Scholar] [CrossRef]
- Chen, D.S.; Zhao, L.S.; Liu, Y.H.; Qi, T.; Wang, J.C.; Wang, L.N. A novel process for recovery of iron, titanium, and vanadium from titanomagnetite concentrates: NaOH molten salt roasting and water leaching processes. J. Hazard. Mater. 2013, 244–245, 588–595. [Google Scholar] [CrossRef]
- Zhao, L.S.; Wang, L.N.; Qi, T.; Chen, D.S.; Zhao, H.X.; Liu, Y.H. A novel method to extract iron, titanium, vanadium, and chro mium from high-chromium vanadium-bearing titanomagnetite concentrates. Hydrometallurgy 2014, 149, 106–109. [Google Scholar] [CrossRef]
- Chen, D.S.; Zhao, H.X.; Hu, G.P.; Qi, T.; Yu, H.D.; Zhang, G.Z.; Wang, L.N.; Wang, W.J. An extraction process to recover vana dium from low-grade vanadium-bearing titanomagnetite. J. Hazard. Mater. 2015, 294, 35–40. [Google Scholar] [CrossRef]
- Zhong, B.N.; Xue, T.Y.; Zhao, L.S.; Zhao, H.X.; Qi, T.; Chen, W.L. Preparation of Ti-enriched slag from V-bearing titanomagnetite by two-stage hydrochloric acid leaching route. Sep. Purif. Technol. 2014, 137, 59–65. [Google Scholar] [CrossRef]
- Bian, Z.Z.; Feng, Y.L.; Li, H.R.; Wu, H. Efficient separation of vanadium, titanium and iron from vanadium-bearing titanomag netite by pressurized pyrolysis of ammonium chloride-acid leaching solvent extraction process. Sep. Purif. Technol. 2021, 225, 117–169. [Google Scholar] [CrossRef]
- Basturkcu, H.; Acarkan, N.; Gock, E. The role of mechanical activation on atmospheric leaching of a lateritic nickel ore. Int. J. Miner. Process. 2017, 163, 1–8. [Google Scholar] [CrossRef]
- Song, G.; Yuan, W.; Zhu, X.; Wang, X.Y.; Zhang, C.l.; Li, J.H.; Bai, J.F.; Wang, J.W. Improvement in rare earth element recovery from waste trichromatic phosphors by mechanical activation. J. Clean Prod. 2017, 151, 361–370. [Google Scholar] [CrossRef]
- Vafaeian, S.; Ahmadian, M.; Rezaei, B. Sulphuric acid leaching of mechanically activated copper sulphidic concentrate. Miner. Eng. 2011, 24, 1713–1716. [Google Scholar] [CrossRef]
- Wu, E.H.; Hou, J.; Li, J.; Huang, P. Experimental Study on Separation of Iron and Titanium from Vanadium-titanium Iron Con centrate by Hydrochloric Acid Leaching at Atmospheric Pressure. Iron Steel Vanadium Titan. 2017, 38, 8–12. [Google Scholar] [CrossRef]
- Analysis of Mining and Metallurgy Research Institute Beijing Office. Handbook on Analysis of Minerals and Non-Ferrous Metals; Metallurgy Industry Press: Beijing, China, 1990. [Google Scholar]
- Zhang, L.; Hu, H.P.; Wei, L.P.; Chen, Q.Y.; Tan, J. Hydrochloric acid leaching behaviour of mechanically activated Panxi ilmenite (FeTiO3). Sep. Purif. Technol. 2010, 73, 173–178. [Google Scholar] [CrossRef]
- Tan, P.; Hu, H.P.; Zhang, L. Effects of mechanical activation and oxidation-reduction on hydrochloric acid leaching of Panxi il menite concentration. Trans. Nonferrous Met. Soc. China 2011, 21, 1414–1421. [Google Scholar] [CrossRef]


| TFe | TiO2 | V2O5 | Cr2O3 | MgO | CaO | Al2O3 | SiO2 | MnO | SO3 | K2O | NiO | ZnO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 49.96 | 13.96 | 0.87 | 0.45 | 1.12 | 1.04 | 3.00 | 7.36 | 0.45 | 0.08 | 0.10 | 0.05 | 0.03 |
| Chemical Composition | TFe | TiO2 | Cr2O3 | MgO | CaO | Al2O3 | SiO2 | MnO |
|---|---|---|---|---|---|---|---|---|
| leaching residues with unactivated (0.57 m2/g) | 35.5 | 32.19 | 0.25 | 0.54 | 1.33 | 1.57 | 11.92 | 1.08 |
| leaching residues for activated (4.4 m2/g) | 28.04 | 43.75 | 0.20 | 0.40 | 1.30 | 4.53 | 10.86 | 1.64 |
| Commercial product | 30.73 | 44.62 | - | 5.47 | 2.06 | 2.55 | 3.25 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, E.-H.; Lin, Y.-H.; Liu, J.; Wang, Z.; Liu, J.-C.; Yin, G.-L.; Li, J.-W.; Cheng, X.-K.; Jia, Y.-L. Titanium-Enriched Slag Prepared by Atmospheric Hydrochloric Acid Leaching of Mechanically Activated Vanadium Titanomagnetite Concentrates. Materials 2021, 14, 6736. https://doi.org/10.3390/ma14226736
Wu E-H, Lin Y-H, Liu J, Wang Z, Liu J-C, Yin G-L, Li J-W, Cheng X-K, Jia Y-L. Titanium-Enriched Slag Prepared by Atmospheric Hydrochloric Acid Leaching of Mechanically Activated Vanadium Titanomagnetite Concentrates. Materials. 2021; 14(22):6736. https://doi.org/10.3390/ma14226736
Chicago/Turabian StyleWu, En-Hui, Yin-He Lin, Jun Liu, Zhe Wang, Jin-Chuan Liu, Guo-Liang Yin, Jing-Wei Li, Xiang-Kui Cheng, and Yu-Long Jia. 2021. "Titanium-Enriched Slag Prepared by Atmospheric Hydrochloric Acid Leaching of Mechanically Activated Vanadium Titanomagnetite Concentrates" Materials 14, no. 22: 6736. https://doi.org/10.3390/ma14226736





