Tin Whiskers’ Behavior under Stress Load and the Mitigation Method for Immersion Tin Surface Finish
Abstract
:1. Introduction
2. Materials and Methods
- Grinding with silicon carbide paper (800 grit) and rinsing with distilled water to remove the oxide layer.
- Immersing in 2-Butoxyethanol solution at 70–90 °C for 5 min and rinsing with distilled water to remove the dirt.
- Soaking in a NaOH solution at 60 °C for 3 min and rinsing with distilled water for surface degreasing.
- Etching in a 10% H2SO4 solution for 1 min and rinsing with distilled water to deoxidize the surface.
- Immersing in palladium chloride solution for 3 min and rinsing with distilled water to activate the surface.
3. Results and Discussion
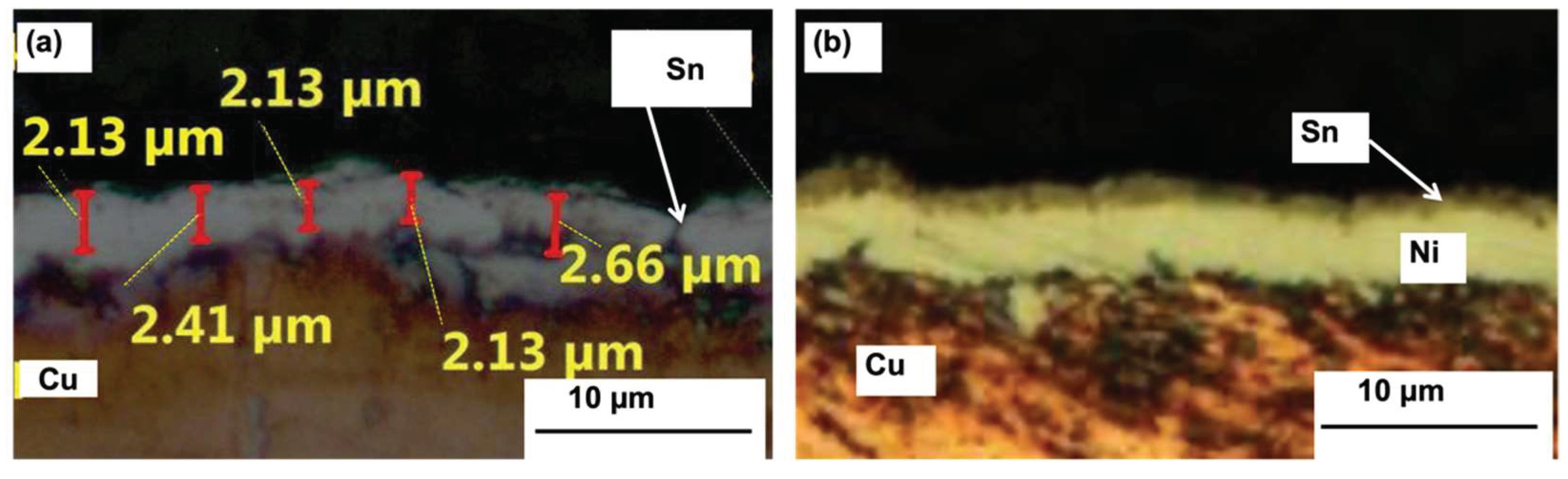
3.1. Characterization of Immersion Tin Coating
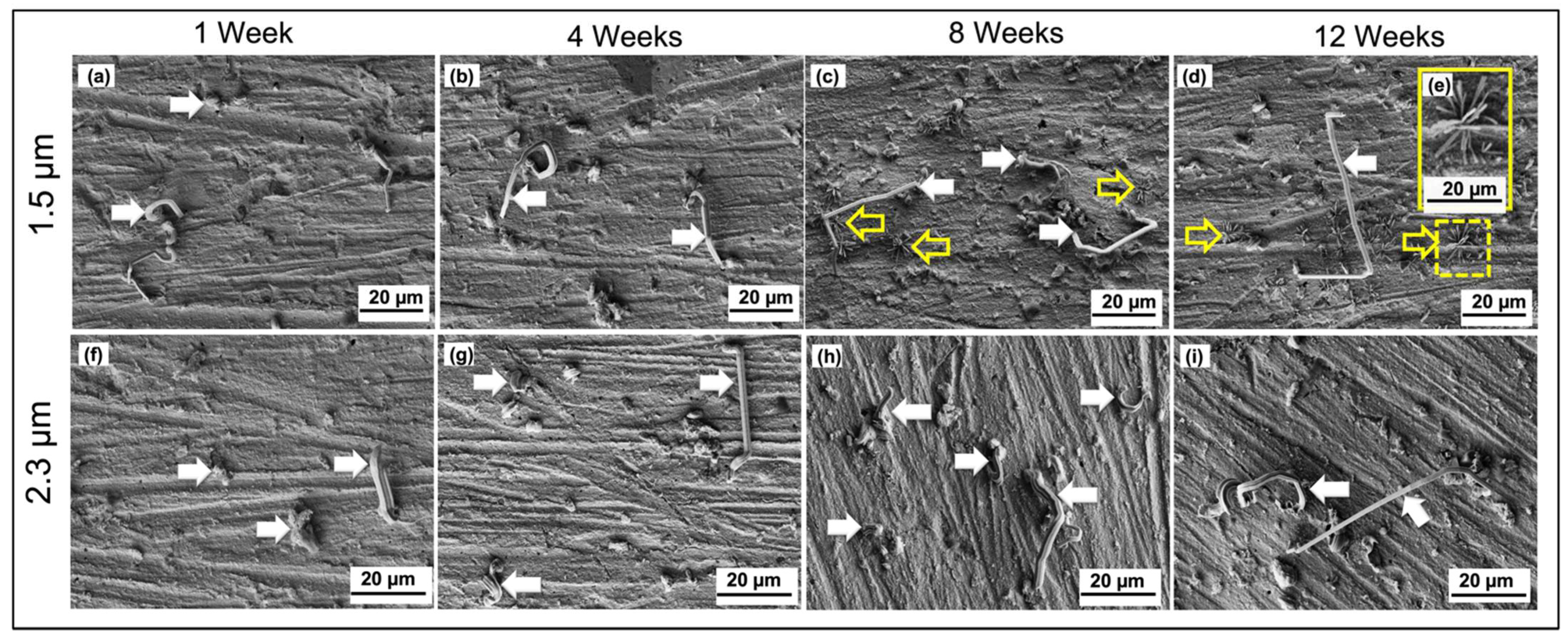
3.2. Whiskers’ Behavior on Non-Indented Tin Coating
3.3. Whiskers’ Behavior on Indented Tin Coating
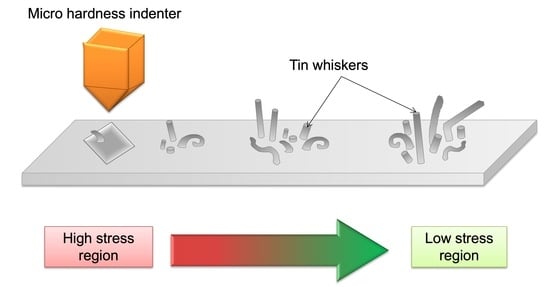
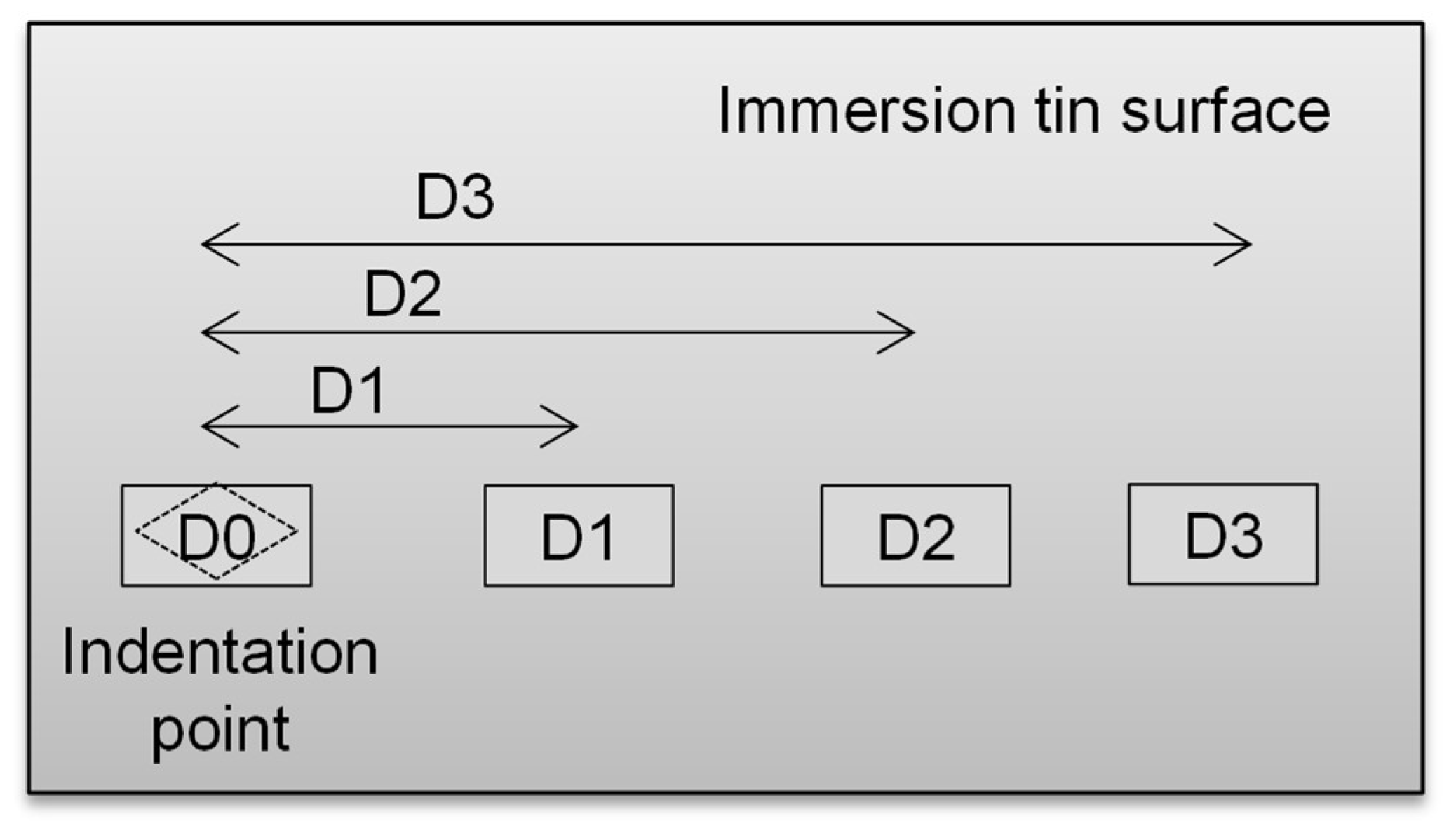
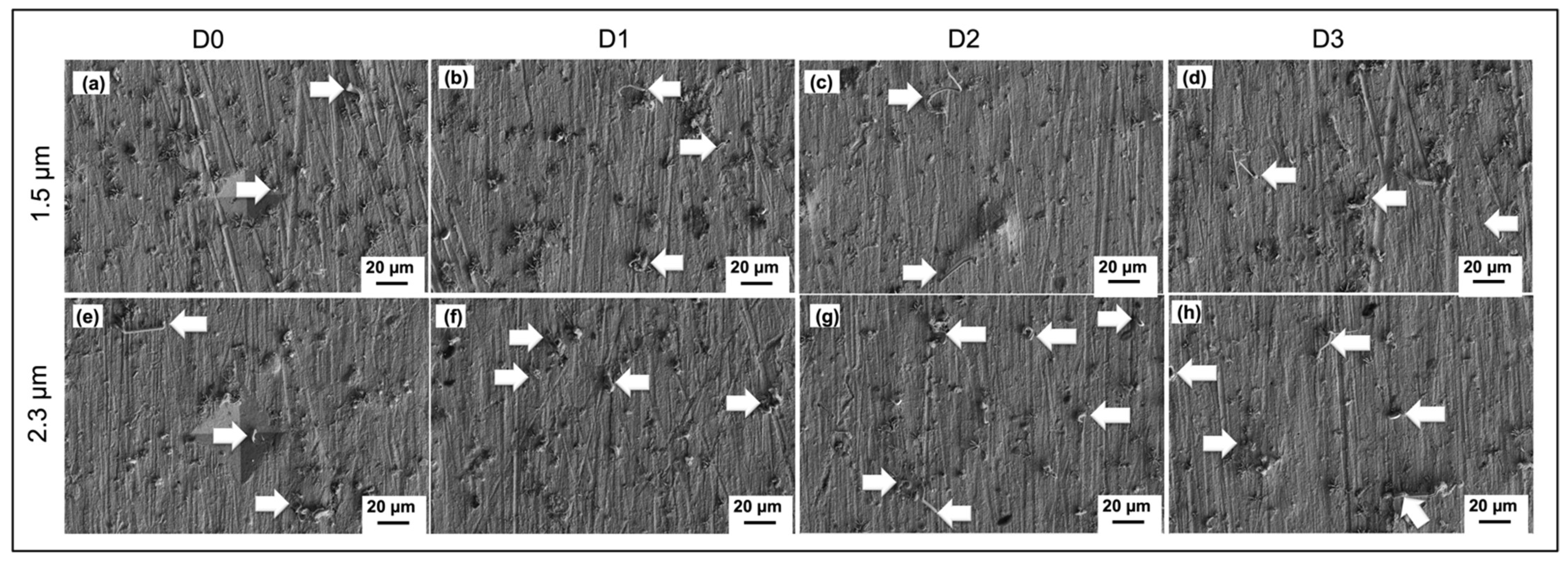
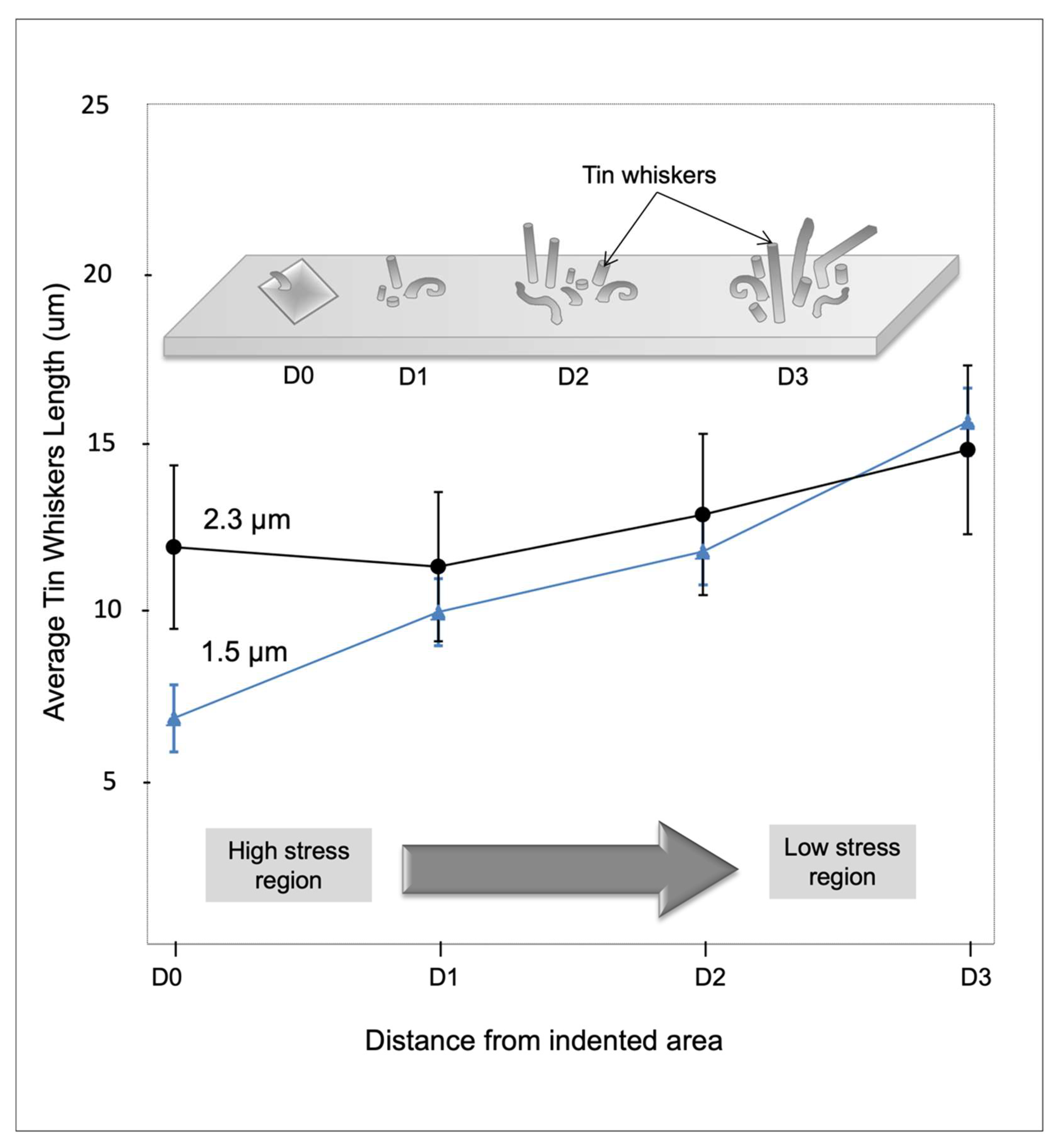
3.4. Whiskers Behavior as a Function of Load Distance
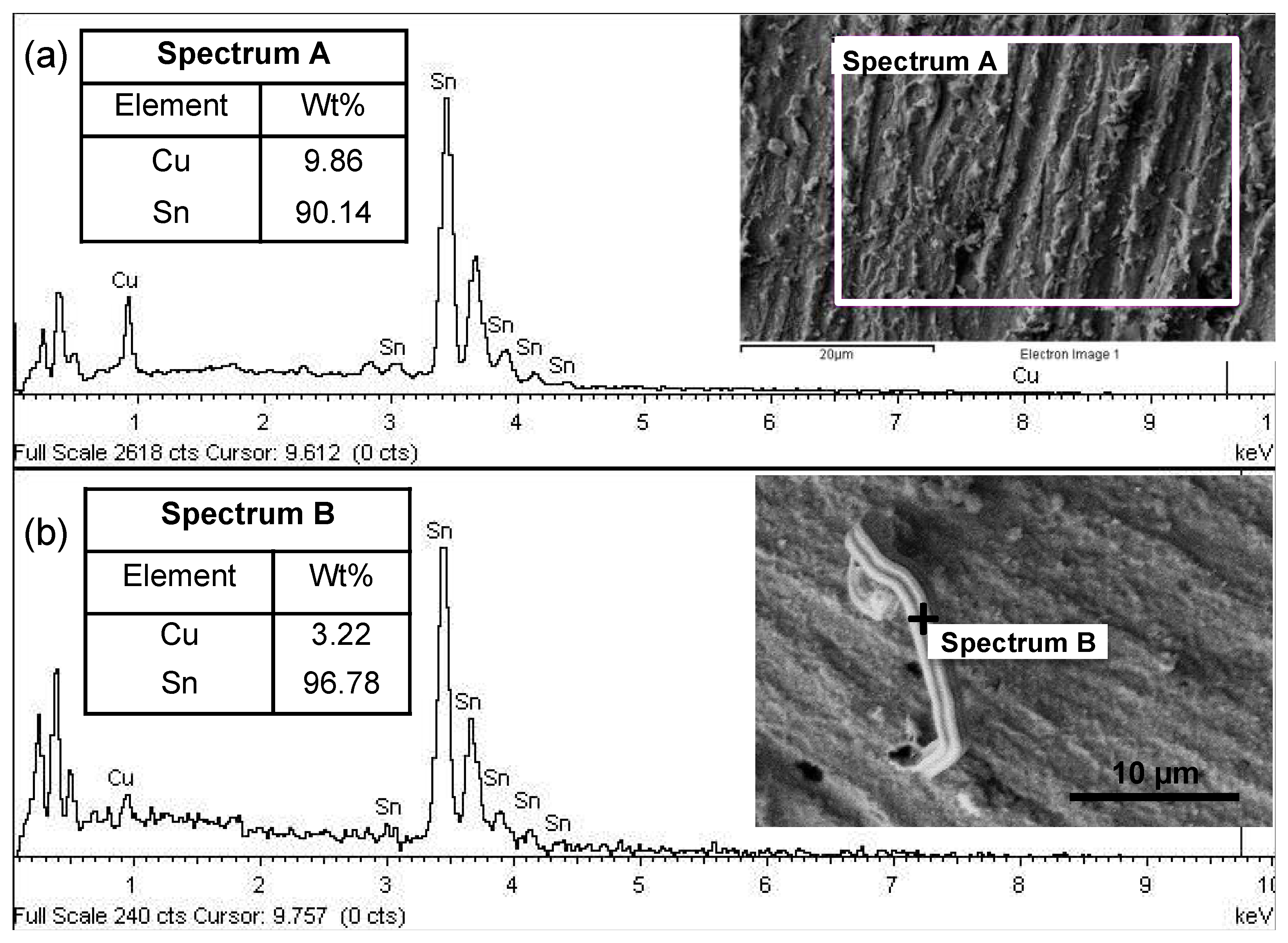
3.5. Influence of Inter-Diffusion
4. Conclusions
- Increasing the tin plating thickness up to 2.3 µm causes an increase in the tin whiskers’ length.
- The tin whiskers’ length is increased directly proportional to the storage time.
- As for the effects of externally applied stress, tin whiskers formed inside and outside the indented area except for the center tip of the indented area, since the tip has a high stress concentration while the other parts of the area have a lower stress concentration. The whiskers’ length increased with the increasing distance from the indented area.
- The formation and growth of the CuO flowers on the indented surface suppressed the growth of the tin whiskers.
- The whiskers formed as various types, including straight, bent, kinked, and spiral with striations along its circumference.
- The Ni underlayer acted as a barrier layer, which effectively mitigated the formation of tin whiskers since no whiskers were observed on the tin surfaces up to week 12 by slowing down the inter-diffusion between the Cu and Sn atoms.
- The identified IMC that influenced the formation and growth of the tin whiskers that formed at the interface between the immersion tin surface finish and the Cu substrate was Cu5Sn6.
5. Recommendation for Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puttlitz, K.J.; Galyon, G.T. Impact of the ROHS Directive on high-performance electronic systems. Part II: Key realibilty issues preventing the implementation of lead-free solders. J. Mater. Sci. Mater. Electron. 2007, 18, 347–365. [Google Scholar] [CrossRef]
- Shepherd, J.; Handwerker, C.; Moon, K.W.; Kattner, U.; Sethuraman, S.; Faure, C.; Bath, J.; Pan, J.; Willie, D.; Zhou, X.; et al. Lead-Free Soldering; Bath, J., Ed.; Springer Science+Business Media, LLC: New York, NY, USA, 2007. [Google Scholar]
- Mokhtar, N.Z.M.; Salleh, M.A.A.M.; Sandu, A.V.; Ramli, M.M.; Chaiprapa, J.; Vizureanu, P.; Ramli, M.I.I. Effect of Electromigration and Thermal Ageing on the Tin Whiskers’ Formation in Thin Sn–0.7Cu–0.05Ga Lead (Pb)-Free Solder Joints. Coatings 2021, 11, 935. [Google Scholar] [CrossRef]
- Shen, C.; Hai, Z.; Zhao, C.; Zhang, J.; Evans, J.L.; Bozack, M.J.; Suhling, J.C. Packaging Reliability Effect of ENIG and ENEPIG Surface Finishes in Board Level Thermal Test under Long-Term Aging and Cycling. Materials 2017, 10, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, P.; Xiao, K.; Ding, K.; Dong, C.; Li, X. Electrochemical Migration Behavior of Copper-Clad Laminate and Electroless Nickel/Immersion Gold Printed Circuit Boards under Thin Electrolyte Layers. Materials 2017, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadzadeh, A.; Fox-Robichaud, A.; Selvaganapathy, P.R. Electroplating of Multiple Materials in Parallel Using Patterned Gels with Applications in Electrochemical Sensing. Sensors 2020, 20, 886. [Google Scholar] [CrossRef] [Green Version]
- Idris, S.R.A. Interfacial Reactions During Soldering of Sn-Ag-Cu Lead Free Solders on Immersion Silver and Electroless Nickel/ Immersion Gold Surface Finishes; Universiti Teknologi Malaysia: Johor Bahru, Malaysia, 2008; p. 168. [Google Scholar]
- Liu, T.; Wang, Y.; Ding, D.; Galuschki, K.P.; Hu, Y.; Gong, Y.; Li, M.; Mao, D. Tin Whisker Formation on Electroless Tin Films Deposited on Lead-frame Alloys. In Proceedings of the International Conference on Electronic Packaging Technology & High Density Packaging, Shanghai, China, 8–11 August 2011; pp. 810–814. [Google Scholar]
- Chen, Y.H.; Wang, Y.Y.; Wan, C.C. Microstructural characteristics of immersion tin coatings on copper circuitries in circuit boards. Surfac Coat. Technol. 2007, 202, 417–424. [Google Scholar] [CrossRef]
- Courey, K.J.; Asfour, S.S.; Bayliss, J.A.; Ludwig, L.L.; Zapata, M.C. Tin Whisker Electrical Short Circuit Characteristics-Part I. IEEE Trans. Electron. Packag. Manuf. 2008, 31, 32–40. [Google Scholar] [CrossRef]
- Evans, R. Analysis of the Effects of Tin Whiskers at High Frequencies. IEEE Trans. Electron. Packag. Manuf. 2006, 29, 274–279. [Google Scholar] [CrossRef]
- Zaimi, N.S.M.; Salleh, M.A.A.M.; Sandu, A.V.; Abdullah, M.M.A.B.; Saud, N.; Rahim, S.Z.A.; Vizureanu, P.; Said, R.M.; Ramli, M.I.I. Performance of Sn-3.0Ag-0.5Cu Composite Solder with Kaolin Geopolymer Ceramic Reinforcement on Microstructure and Mechanical Properties under Isothermal Ageing. Materials 2021, 14, 776. [Google Scholar] [CrossRef]
- Asrar, N.; Vancauwenberghe, O.; Prangere, S. Tin Whiskers Formation on an Electronic Product: A Case Study. J Fail. Anal. Preven. 2007, 7, 179–182. [Google Scholar] [CrossRef]
- Pinol, L.A.; Melngailis, J.; Charles, H.K.; Lee, D.M.; Deacon, R.; Coles, G.; Clatterbaugh, G. Infuence of Tin Deposition Methods on Tin Whisker Formation. IEEE Trans. Compon. Packag. Manuf. Technol. 2011, 1, 2028–2032. [Google Scholar] [CrossRef]
- Han, S.; Osterman, M.; Pecht, M.G. Electrical Shorting Propensity of Tin Whiskers. IEEE Trans. Electron. Packag. Manuf. 2010, 33, 205–211. [Google Scholar] [CrossRef]
- Huang, C.-M.; Nunez, D.; Coburn, J.; Pecht, M. Risk of tin whiskers in the nuclear industry. Microelectron. Reliab. 2018, 81, 22–30. [Google Scholar] [CrossRef]
- Tseng, T.-H.; Wu, A.T. Corrosion on automobile printed circuit broad. Microelectron. Reliab. 2019, 98, 19–23. [Google Scholar] [CrossRef]
- Bakhshi, R.; Sandborn, P.; Lillie, E. Assessing the Value of Corrosion Mitigation in Electronic Systems Using Cost-Based FMEA—Tin Whisker Mitigation. In Corrosion Processes: Sensing, Monitoring, Data Analytics, Prevention/Protection, Diagnosis/Prognosis and Maintenance Strategies; Vachtsevanos, G., Natarajan, K.A., Rajamani, R., Sandborn, P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 313–339. [Google Scholar] [CrossRef]
- Russo, S.G.; Henderson, M.J.; Hinton, B.R.W. Corrosion of an aircraft radar antenna waveguide. Eng. Fail. Anal. 2002, 9, 423–434. [Google Scholar] [CrossRef]
- Daddona, P. Reactor Shutdown: Dominion Learns Big Lesson From a Tiny 'Tin Whisker'. In The Day; The Day Publishing Co.: New London, CT, USA, 2005. [Google Scholar]
- Leidecker, H.; Panashchenko, L.; Brusse, J. Electrical Failure of an Accelerator Pedal Position Sensor Caused by a Tin Whisker and Discussion of Investigative Techniques Used for Whisker Detection. In Proceedings of the 5th International Tin Whisker Symposium, College Park, MD, USA, 14–15 September 2011. [Google Scholar]
- Sood, B.; Osterman, M.; Pecht, M. Tin Whisker Analysis of Throttle Controls. Circ. World 2011, 37, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Illés, B.; Hurtony, T.; Krammer, O.; Medgyes, B.; Dušek, K.; Bušek, D. Effect of Cu Substrate Roughness and Sn Layer Thickness on Whisker Development from Sn Thin-Films. Materials 2019, 12, 3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gedney, R.; Smetana, J.; Vo, N.; Galyon, G. NEMI Tin Whisker Projects. In Proceedings of the Second International Conference on Lead Free Electronics, Amsterdam, The Netherlands, 21–23 June 2004. [Google Scholar]
- Dudek, M.A.; Chawla, N. Mechanisms for Sn whisker growth in rare earth-containing Pb-free solders. Acta Mater. 2009, 57, 4588–4599. [Google Scholar] [CrossRef]
- Miller, S.M.; Sahaym, U.; Norton, M.G. Effect of Substrate Composition on Sn Whisker Growth in Pure Sn Films. Metall. Mater. Trans. A 2010, 41A, 3386–3395. [Google Scholar] [CrossRef]
- Hektor, J.; Hall, S.A.; Henningsson, N.A.; Engqvist, J.; Ristinmaa, M.; Lenrick, F.; Wright, J.P. Scanning 3DXRD Measurement of Grain Growth, Stress, and Formation of Cu6Sn5 around a Tin Whisker during Heat Treatment. Materials 2019, 12, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, P.; Ding, M.; Chopin, S. Effects of Reflow on the Microstructure and Whisker Growth Propensity of Sn Finish. In Proceedings of the 55th Elelctronic Components and Technology Conference, Lake Buena Vista, FL, USA, 31 May–4 June 2005; pp. 1–7. [Google Scholar]
- Hilty, R.D. NEMI Sn Whisker Project Status. In Proceedings of the NEMI Lead free Summit Meeting, Louisville, CO, USA, 18–20 October 2004. [Google Scholar]
- Illes, B.; Horvath, B.; Harsanyi, G. Effect of strongly oxidizing environment on whisker growth form tin coating. Surf. Coat. Technol. 2010, 205, 2262–2266. [Google Scholar] [CrossRef]
- Kato, T.; Akahoshi, H.; Nakamura, M.; Hashimoto, T.; Nishimura, A. Effects of Minor Elelments in Cu Leadframe on Whisker Initiation from Electrodeposited Sn/Cu Coating. IEEE Trans. Electron. Packag. Manuf. 2007, 30, 258–269. [Google Scholar] [CrossRef]
- Moriuchi, H.; Tadokoro, Y.; Sato, M.; Furusawa, T.; Suzuki, N. Microstructure of External Stress Whiskers and Mechanical Indentation Test Method. J. Electron. Mater. 2007, 36, 220–225. [Google Scholar] [CrossRef]
- Cao, X.; Xu, L.; Wang, C.; Li, S.; Wu, D.; Shi, Y.; Liu, F.; Xue, X. Electrochemical Behavior and Electrodeposition of Sn Coating from Choline Chloride–Urea Deep Eutectic Solvents. Coatings 2020, 10, 1154. [Google Scholar] [CrossRef]
- Wahi, A. Tin Whisker Formation and Intermetallic Compound between Tin-Silver-Copper solders and Immersion Tin Finish; Universiti Teknologi Malaysia: Johor Bahru, Malaysia, 2009. [Google Scholar]
- Boo, N.S. Interfacial Reactions between Lead-free Solders and Electroless Nickel/Immersion Gold (ENIG) Surface Finishes; Universiti Teknologi Malaysia: Johor Bahru, Malaysia, 2010. [Google Scholar]
- JEDEC. Test Method for Measuring Whisker Growth on Tin and Tin Alloy Surface Finishes; JEDEC Standard JESD22–A121A; JEDEC: Arlington, VA, USA, 2008. [Google Scholar]
- Liang, J.; Xu, Z.H.; Li, X. Whisker nucleation in indentation residual stress field on tin plated component leads. J. Mater. Sci. Mater. Electron. 2007, 18, 599–604. [Google Scholar] [CrossRef]
- Shibutani, T.; Yu, Q. Pressure Induced Tin Whisker Formation on SnCu Finish by Nanoindentation Creep. In Proceedings of the 10th Electronic Packaging Technology Conference, Singapore, 9–12 December 2008; pp. 1442–1447. [Google Scholar]
- Takeuchi, M.; Kamiyama, K.; Suganuma, K. Suppression of Tin Whisker Formation on Fine Pitch Connectors by Surface Roughening. J. Electron. Mater. 2006, 35, 1918–1925. [Google Scholar] [CrossRef]
- Boguslavsky, I.; Bush, P. Recrystallization principles applied to whisker growth in tin. In Proceedings of the IPC SMEMA Council APEX, Anaheim, CA, USA, 3 March–2 April 2003; pp. 1–10. [Google Scholar]
- Dittes, M.; Oberndoff, P.; Petit, L. Tin Whisker Formation: Results, Test Methods and Countermeasures. In Proceeding of the 53 IEEE Electronic Components and Technology Conference, New Orleans, LA, USA, 27–30 May 2003; pp. 822–826. [Google Scholar]
- Susan, D.; Michael, J.; Grant, R.P.; McKenzie, B.; Yelton, W.G. Morphology and Growth Kinetics of Straight and Kinked Tin Whiskers. Metall. Mater. Trans. A 2013, 44, 1485–1496. [Google Scholar] [CrossRef]
- Muraki, N.; Katagiri, G.; Sergo, V.; Pezzotti, G.; Nishida, T. Mapping of residual stresses around an indentation in β-Si3N4 using Raman spectroscopy. J. Mater. Sci. 1997, 32, 5419–5423. [Google Scholar] [CrossRef]
- Ho, C.E.; Yang, S.C.; Kao, C.R. Interfacial reaction issues for lead-free electronic solders. Lead-Free Electron. Solder. 2006, 2016, 155–174. [Google Scholar] [CrossRef]
- Hashim, A.N.; Salleh, M.A.; Sandu, A.V.; Ramli, M.M.; Yee, K.C.; Mohd Mokhtar, N.Z.; Chaiprapa, J. Effect of Ni on the Suppression of Sn Whisker Formation in Sn-0.7Cu Solder Joint. Materials 2021, 14, 738. [Google Scholar] [CrossRef] [PubMed]
- Laurila, T.; Vuorinen, V.; Kivilahti, J.K. Interfacial reactions between lead-free solders and common base materials. Mater. Sci. Eng. R 2005, 49, 1–60. [Google Scholar] [CrossRef]
- Horvath, B.; Illes, B.; Harsanyi, G. Investigation of Tin Whisker Growth: The Effect of Ni and Ag Underplates. In Proceedings of the Electronics Technology, ISSE 2009, 32nd International Spring Seminar, Brno, Czech Republic, 13–17 May 2009; pp. 1–5. [Google Scholar]
- Galyon, G.T.; Xu, C.; Lal, S.; Notohardjono, B.; Palmer, L. The Integrated Theory of Whisker Formation-A Stress Analysis. In Proceedings of the Electronic Components and Technology Conference, Lake Buena Vista, FL, USA, 3–31 May 2005; pp. 421–428. [Google Scholar]
- Sun, Q.; Selvaduray, G. Understanding and Minimizing Tin Whiskers; San Jose State University: San Jose, CA, USA, 2003. [Google Scholar]
- Tu, K.N.; Suh, J.O.; Wu, A.T.Z.; Tamura, N.; Tung, C.H. Mechanism and Prevention of Spontaneous Tin Whisker Growth. Mater. Trans. 2005, 46, 2300–2308. [Google Scholar] [CrossRef]





| Chemical | Function | Composition |
|---|---|---|
| Stannous chloride | Source of Sn ion | 20 g/L |
| Hydrochloric acid (37%) | Base bath | 37 mL/L |
| Sulfuric acid (50%) | Base bath | 50 mL/L |
| Sodium hypophosphite | Reducing agent | 16 g/L |
| Thiourea | Complexing agent | 200 g/L |
| Phenolsulfonic acid | Bath stabilizer | 5 mL/L |
| Temperature | 75 ± 1 °C | |
| Duration | 12, 20 min | |
| Chemical | Function | Quantity |
|---|---|---|
| Nickel sulphate | Source of Ni ion | 28 g/L |
| Sodium acetate | Complexing agent | 17 g/L |
| Sodium hypophosphite | Reducing agent | 24 g/L |
| Lead acetate | Bath stabilizer | 0.0015 g/L |
| Parameters | ||
| pH | 4.4–4.6 | |
| Temperature | 85 ± 1 °C | |
| Time | 10 and 20 min | |
| No. | Coating | Plating Time (min) | Coating Thickness (µm) | Indentation | Storage Time (Weeks) |
|---|---|---|---|---|---|
| 1 | Sn | 2 | 1.5 | No | 2, 4, 8, and 12 |
| 2 | Sn | 20 | 2.3 | No | 2, 4, 8, and 12 |
| 3 | Sn | 12 | 1.5 | Yes | 2, 4, 8, 12, and 52 |
| 4 | Sn | 20 | 2.3 | Yes | 2, 4, 8, 12, and 52 |
| 5 | Sn/Ni | 12/10 | 1.5/2.4 | No | 2, 4, 8, and 12 |
| 6 | Sn/Ni | 12/20 | 1.5/2.7 | No | 2, 4, 8, and 12 |
| 7 | Sn/Ni | 12/10 | 1.5/2.4 | Yes | 2, 4, 8, and 12 |
| 8 | Sn/Ni | 12/20 | 1.5/2.7 | Yes | 2, 4, 8, and 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadil, N.A.; Yusof, S.Z.; Abu Bakar, T.A.; Ghazali, H.; Mat Yajid, M.A.; Osman, S.A.; Ourdjini, A. Tin Whiskers’ Behavior under Stress Load and the Mitigation Method for Immersion Tin Surface Finish. Materials 2021, 14, 6817. https://doi.org/10.3390/ma14226817
Fadil NA, Yusof SZ, Abu Bakar TA, Ghazali H, Mat Yajid MA, Osman SA, Ourdjini A. Tin Whiskers’ Behavior under Stress Load and the Mitigation Method for Immersion Tin Surface Finish. Materials. 2021; 14(22):6817. https://doi.org/10.3390/ma14226817
Chicago/Turabian StyleFadil, Nor Akmal, Siti Zahira Yusof, Tuty Asma Abu Bakar, Habibah Ghazali, Muhamad Azizi Mat Yajid, Saliza Azlina Osman, and Ali Ourdjini. 2021. "Tin Whiskers’ Behavior under Stress Load and the Mitigation Method for Immersion Tin Surface Finish" Materials 14, no. 22: 6817. https://doi.org/10.3390/ma14226817