Solketal Removal from Aqueous Solutions Using Activated Carbon and a Metal–Organic Framework as Adsorbents
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Characterization Techniques
2.2. Adsorption Procedure
3. Results and Discussion
3.1. Characterization of the Adsorbents
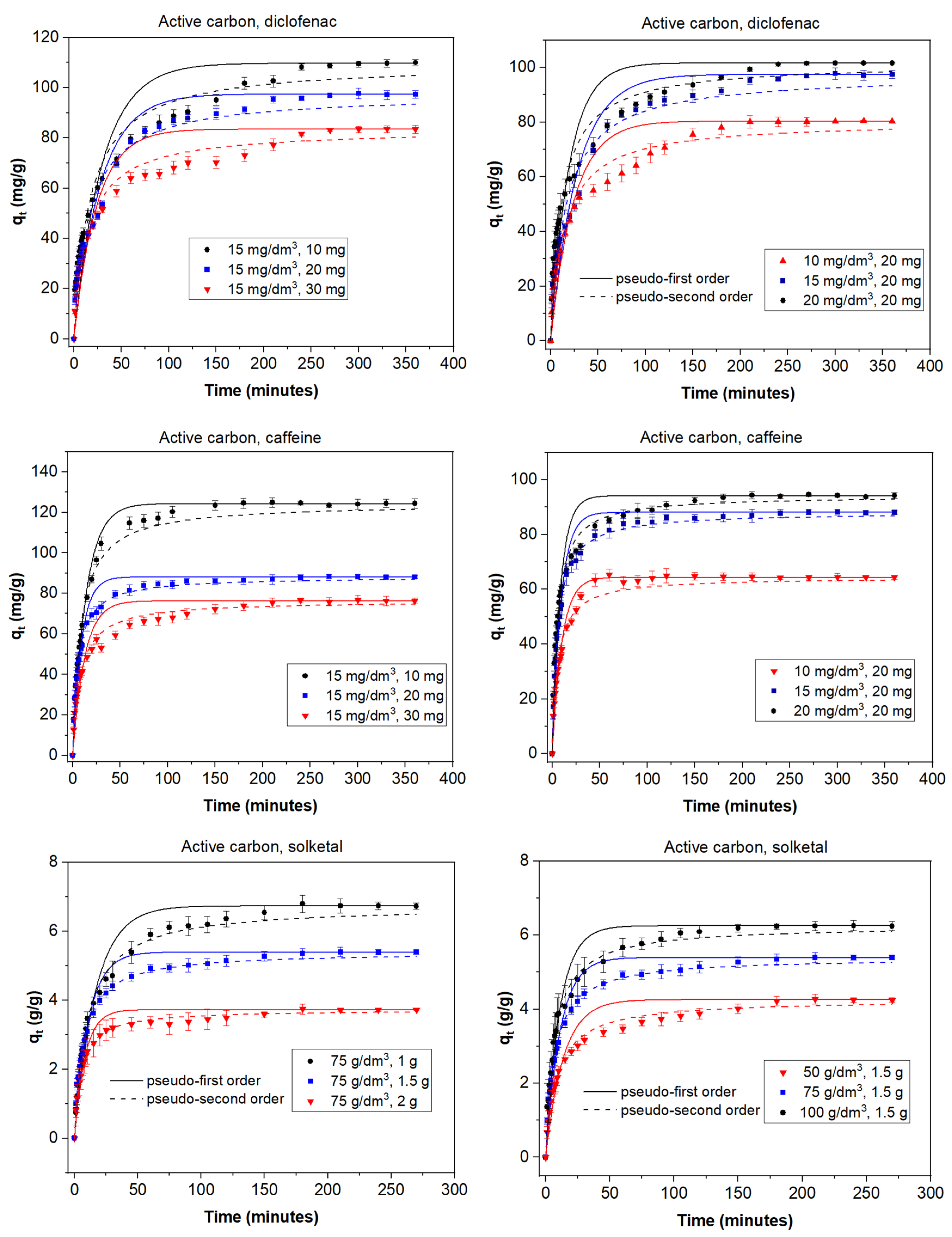
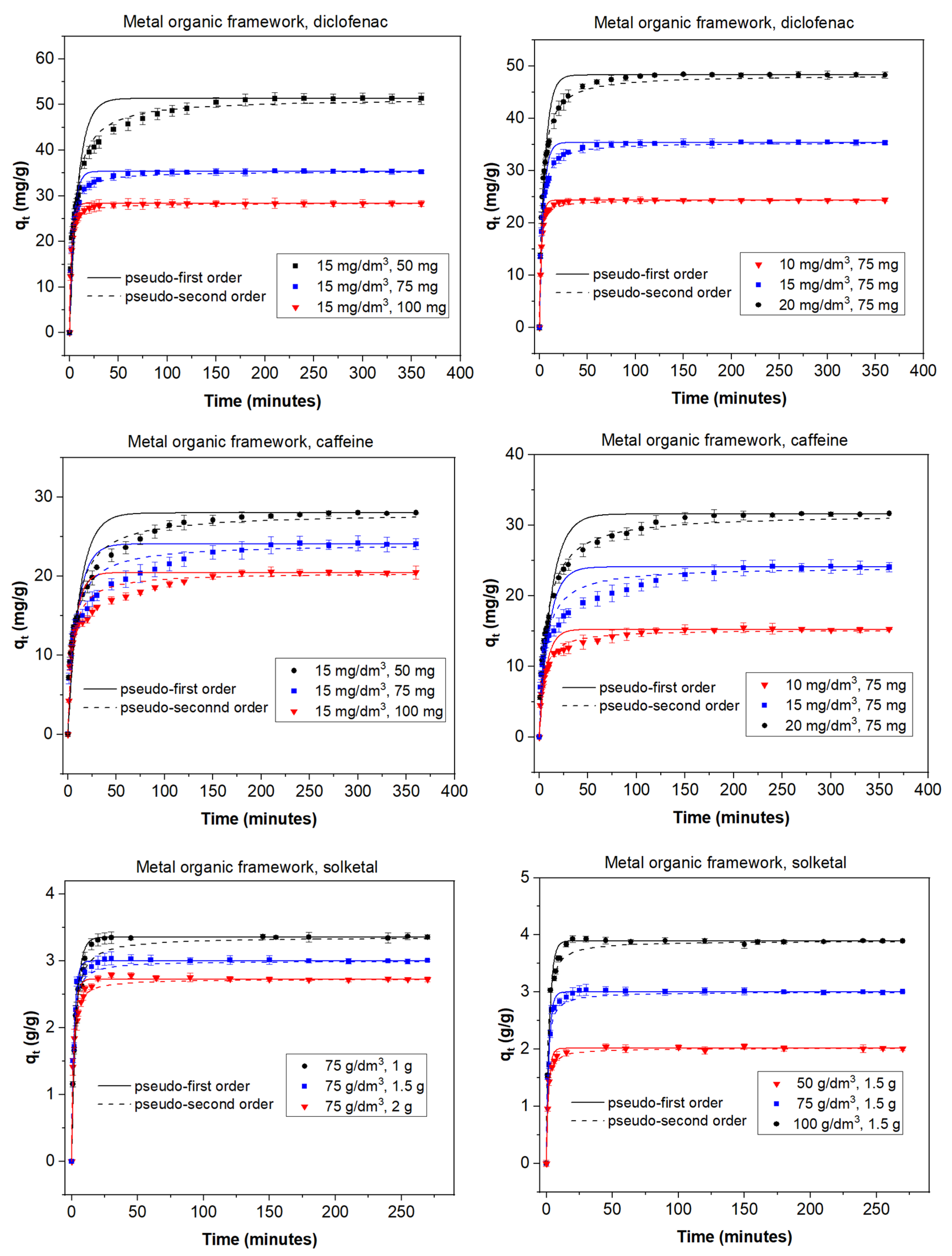
3.2. Adsorption Experiments
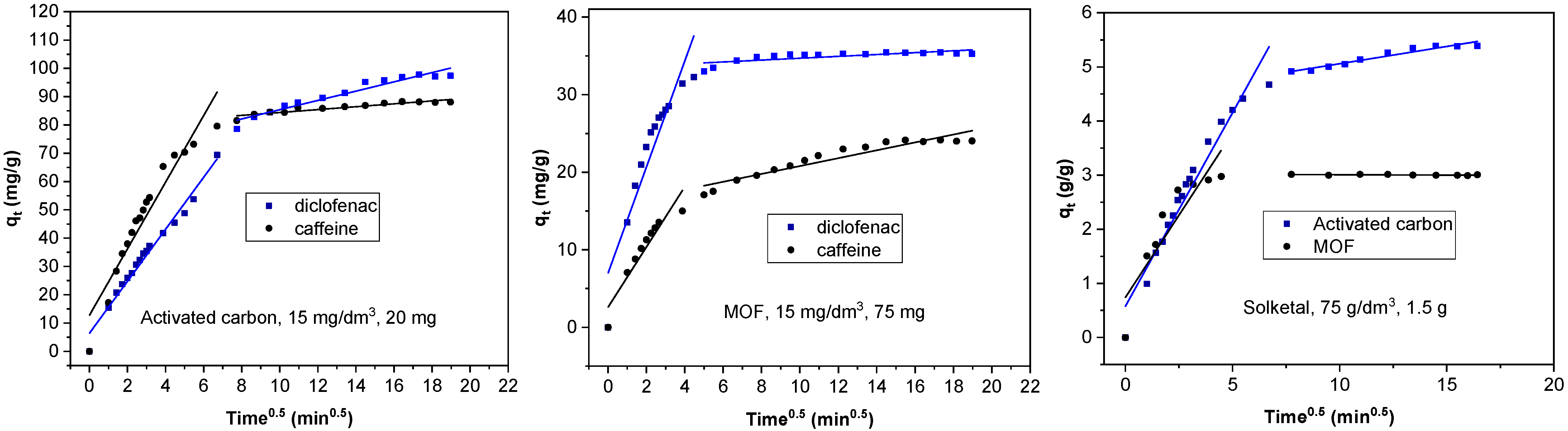
3.3. Kinetic Models
3.4. Adsorption Isotherms
3.5. Mechanism of Adsorption on the Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Najaafi, N.; Tarighi, S. A review on the catalytic acetalization of bio-renewable glycerol to fuel additives. Front. Chem. 2018, 6, 573. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Tat, M.E.; van Gerpen, J.H. The kinematic viscosity of biodiesel and its blends with diesel fuel. J. Am. Oil Chem. Soc. 1999, 76, 1511–1513. [Google Scholar] [CrossRef]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Yehye, W.A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy Rev. 2015, 41, 113–127. [Google Scholar] [CrossRef]
- Moreira, M.N.; Faria, R.P.V.; Ribeiro, A.M.; Rodrigues, A.E. Solketal production from glycerol ketalization with acetone: Catalyst selection and thermodynamic and kinetic reaction study. Ind. Eng. Chem. Res. 2019, 58, 17746–17759. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Krupskaya, V.V.; Gil, A.; Vicente, M.A. Effect of nitric acid modification of montmorillonite clay on synthesis of solketal from glycerol and acetone. Catal. Commun. 2017, 90, 65–69. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Khan, N.A.; Hasan, Z.; Prosvirin, I.P.; Tsybulya, S.V.; Jhung, S.H. Isostructural metal-carboxylates MIL-100(M) and MIL-53(M) (M: V, Al, Fe and Cr) as catalysts for condensation of glycerol with acetone. Appl. Catal. A Gen. 2017, 529, 167–174. [Google Scholar] [CrossRef]
- Zahid, I.; Ayoub, M.; Abdullah, B.B.; Mukhtar, A.; Saqib, S.; Rafiq, S.; Ullah, S.; Al-Sehemi, A.G.; Farrukh, S.; Danish, M. Glycerol conversion to solketal: Catalyst and reactor design, and factors affecting the yield. ChemBioEng Rev. 2021, 8, 227–238. [Google Scholar] [CrossRef]
- Fatimah, I.; Sahroni, I.; Fadillah, G.; Musawwa, M.M.; Mahlia, T.M.; Muraza, O. Glycerol to solketal for fuel additive: Recent progress in heterogeneous catalysts. Energies 2019, 12, 2872. [Google Scholar] [CrossRef] [Green Version]
- Trifoi, A.R.; Agachi, P.Ş.; Pap, T. Glycerol acetals and ketals as possible diesel additives. A review of their synthesis protocols. Renew. Sustain. Energy Rev. 2016, 62, 804–814. [Google Scholar] [CrossRef]
- Mota, C.J.A.; Da Silva, C.X.A.; Rosenbach, N.; Costa, J.; Da Silva, F. Glycerin derivatives as fuel additives the addition of glycerol/acetone ketal (solketal) in gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.A.; Xu, C.C. A new continuous-flow process for catalytic conversion of glycerol to oxygenated fuel additive: Catalyst screening. Appl. Energy 2014, 123, 75–81. [Google Scholar] [CrossRef]
- Maes, J.; Verlooy, L.; Buenafe, O.E.; de Witte, P.A.M.; Esguerra, C.V.; Crawford, A.D. Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae. PLoS ONE 2012, 7, e43850. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Santamaria, L.; Korili, S.A. Removal of caffeine and diclofenac from aqueous solution by adsorption on multiwalled carbon nanotubes. Coll. Interface Sci. Commun. 2018, 22, 25–28. [Google Scholar] [CrossRef]
- Marçal, L.; de Faria, E.H.; Nassar, E.J.; Trujillano, R.; Martín, N.; Vicente, M.A.; Rives, V.; Gil, A.; Korili, S.A.; Ciuffi, K.J. Organically modified saponites: SAXS study of swelling and application in caffeine removal. ACS Appl. Mater. Interfaces 2015, 7, 10853–10862. [Google Scholar] [CrossRef]
- Santamaria, L.; Vicente, M.A.; Korili, S.A.; Gil, A. Progress in the removal of pharmaceutical compounds from aqueous solution using layered double hydroxides as adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104577. [Google Scholar] [CrossRef]
- Santamaria, L.; Devred, F.; Gaigneaux, E.M.; Vicente, M.A.; Korili, S.A.; Gil, A. Effect of the surface properties of Me2+/Al layered double hydroxides synthesized from aluminum saline slag wastes on the adsorption removal of drugs. Micropor. Mesopor. Mater. 2020, 209, 110560. [Google Scholar] [CrossRef]
- Santamaria, L.; López-Aizpún, M.; García-Padial, M.; Vicente, M.A.; Korili, S.A.; Gil, A. Zn-Ti-Al layered double hydroxides synthesized from aluminum saline slag wastes as efficient drug adsorbents. Appl. Clay Sci. 2020, 187, 105486. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, Y.; Yun, Y.S. Highly effective removal of nonsteroidal anti-inflammatory pharmaceuticals from water by Zr(IV)-based metal-organic framework: Adsorption performance and mechanisms. ACS Appl. Mater. Interfaces 2018, 10, 28076–28085. [Google Scholar] [CrossRef]
- Luo, Z.; Fan, S.; Liu, J.; Liu, W.; Shen, X.; Wu, C.; Huang, Y.; Huang, G.; Huang, H.; Zheng, M. A 3D stable metal-organic framework for highly efficient adsorption and removal of drug contaminants from water. Polymers 2018, 10, 209. [Google Scholar] [CrossRef] [Green Version]
- van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Nguyen, O.T.K.; Nguyen, V.H.; Nguyen, T.T.; Bach, L.G.; Nguyen, T.D. A hollow mesoporous carbon from metal-organic framework for robust adsorbability of ibuprofen drug in water. R. Soc. Open Sci. 2019, 6, 190058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, T.C.; Cabrera-Codony, A.; Barceló, D.; Rodriguez-Mozaz, S.; Pinheiro, A.; Gonzalez-Olmos, R. Influencing factors on the removal of pharmaceuticals from water with micro-grain activated carbon. Water Res. 2018, 144, 402–412. [Google Scholar] [CrossRef]
- Bahamon, D.; Vega, L.F. Pharmaceuticals removal from water effluents by adsorption in activated carbons using Monte Carlo simulations. Comput. Aided Chem. Eng. 2017, 40, 2695–2700. [Google Scholar] [CrossRef]
- Delgado, N.; Capparelli, A.; Navarro, A.; Marino, D. Pharmaceutical emerging pollutants removal from water using powdered activated carbon: Study of kinetics and adsorption equilibrium. J. Environ. Manage. 2019, 236, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Taoufik, N.; García, A.M.; Korili, S.A. Comparative removal of emerging conaminants from aqueous solution by adsorption on an activated carbon. Environ. Technol. 2019, 40, 3017–3030. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; ISBN 978-0-12-300956-2. [Google Scholar]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption at the Liquid–Solid Interface: Thermodynamics and Methodology; Academic Press: London, UK, 1999; Chapter 5; pp. 117–163. ISBN 978-0-08-097035-6. [Google Scholar]
- Plazinski, W.; Rudzinski, W. Kinetics of adsorption at solid/Solution interfaces controlled by intraparticle diffusion: A theoretical analysis. J. Phys. Chem. C 2009, 113, 12495–12501. [Google Scholar] [CrossRef]
- Khraisheh, M.A.M.; Al-Degs, Y.S.; Allen, S.J.; Ahmad, M.N. Elucidation of controlling steps of reactive dye adsorption on activated carbon. Ind. Eng. Chem. Res. 2002, 41, 1651–1657. [Google Scholar] [CrossRef]
- Gil, A.; Assis, F.C.C.; Albeniz, S.; Korili, S.A. Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. J. 2011, 168, 1032–1040. [Google Scholar] [CrossRef]
- Valderrama, C.; Gamisans, X.; de las Heras, X.; Farrán, A.; Cortina, J.L. Sorption kinetics of polycyclic aromatic hydrocarbons removal using granular activated carbon: Intraparticle diffusion coefficients. J. Hazard. Mater. 2008, 157, 386–396. [Google Scholar] [CrossRef]
- Urano, K.; Tachikawa, H. Process development for removal and recovery of phosphorus from wastewater by a new adsorbent. II. Adsorption rates and breakthrough curves. Ind. Eng. Chem. Res. 1991, 30, 1897–1899. [Google Scholar] [CrossRef]
- Da̧browski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Ramadoss, R.; Subramania, D. Adsorption of chromium using blue green algae-modeling and application of various isotherms. Int. J. Chem. Technol. 2018, 10, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhao, X.-H.; Yang, H.; Chen, X.-H.; Yang, Q.; Yu, L.-Y.; Jiang, J.-H.; Chen, X.-Q. Aqueous adsorption and removal of organic contaminants by carbon nanotubes. Sci. Total Environ. 2014, 482–483, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef] [PubMed]
| Name | Structure and Characteristics | |
|---|---|---|
| Caffeine | 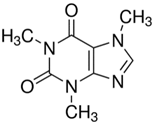 | C8H10N4O2 |
| m = 194.19 g/mol | ||
| pka = 10.4 | ||
| Diclofenac sodium | 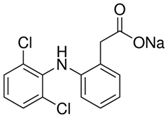 | C14H10Cl2NNaO2 |
| m = 296.15 g/mol | ||
| pka = 4.15 | ||
| Solketal | 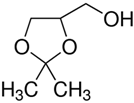 | C6H12O3 |
| m = 132.16 g/mol | ||
| pka = 14.20 | ||
| Solketal * | Diclofenac | Caffeine | |
|---|---|---|---|
| Freundlich | |||
| kF (dm3/mg)mF | 3.7 | 4.6 | 12.6 |
| mF | 1.82 | 2.05 | 2.63 |
| χ2 | 726 | 449 | 3581 |
| R | 0.93 | 0.95 | 0.76 |
| Langmuir | |||
| qL (mg/g) | 83.7 | 68.4 | 82.8 |
| kL (dm3/mg) | 0.013 | 0.021 | 0.058 |
| χ2 | 331 | 112 | 2128 |
| R | 0.97 | 0.99 | 0.86 |
| Toth | |||
| qT (mg/g) | 90.3 | 2122 | 79.6 |
| kT (dm3/mg) | 0.013 | 0.27 | 0.37 |
| mT | 0.66 | 0.13 | 0.61 |
| χ2 | 121 | 244 | 496 |
| R | 0.99 | 0.998 | 0.991 |
| Solketal * | Diclofenac | Caffeine | |
|---|---|---|---|
| Freundlich | |||
| kF (dm3/mg)mF | 2.57 | 6.13 | 7.87 |
| mF | 1.76 | 2.60 | 2.41 |
| χ2 | 377 | 410 | 1428 |
| R | 0.91 | 0.91 | 0.78 |
| Langmuir | |||
| qL (mg/g) | 56.9 | 43.0 | 55.5 |
| kL (dm3/mg) | 0.016 | 0.050 | 0.063 |
| χ2 | 189 | 109 | 833 |
| R | 0.96 | 0.98 | 0.88 |
| Toth | |||
| qT (mg/g) | 124.4 | 78.91 | 71.4 |
| kT (dm3/mg) | 0.017 | 0.26 | 0.51 |
| mT | 0.46 | 0.35 | 0.42 |
| χ2 | 219 | 170 | 401 |
| R | 0.99 | 0.998 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamaría, L.; Korili, S.A.; Gil, A. Solketal Removal from Aqueous Solutions Using Activated Carbon and a Metal–Organic Framework as Adsorbents. Materials 2021, 14, 6852. https://doi.org/10.3390/ma14226852
Santamaría L, Korili SA, Gil A. Solketal Removal from Aqueous Solutions Using Activated Carbon and a Metal–Organic Framework as Adsorbents. Materials. 2021; 14(22):6852. https://doi.org/10.3390/ma14226852
Chicago/Turabian StyleSantamaría, Leticia, Sophia A. Korili, and Antonio Gil. 2021. "Solketal Removal from Aqueous Solutions Using Activated Carbon and a Metal–Organic Framework as Adsorbents" Materials 14, no. 22: 6852. https://doi.org/10.3390/ma14226852






