Formation of Silicide and Silicide-Aluminide Coatings on Molybdenum Alloy during Slurry Cementation Process: Influence of Slurry Volume
Abstract
:1. Introduction
2. Materials and Methods
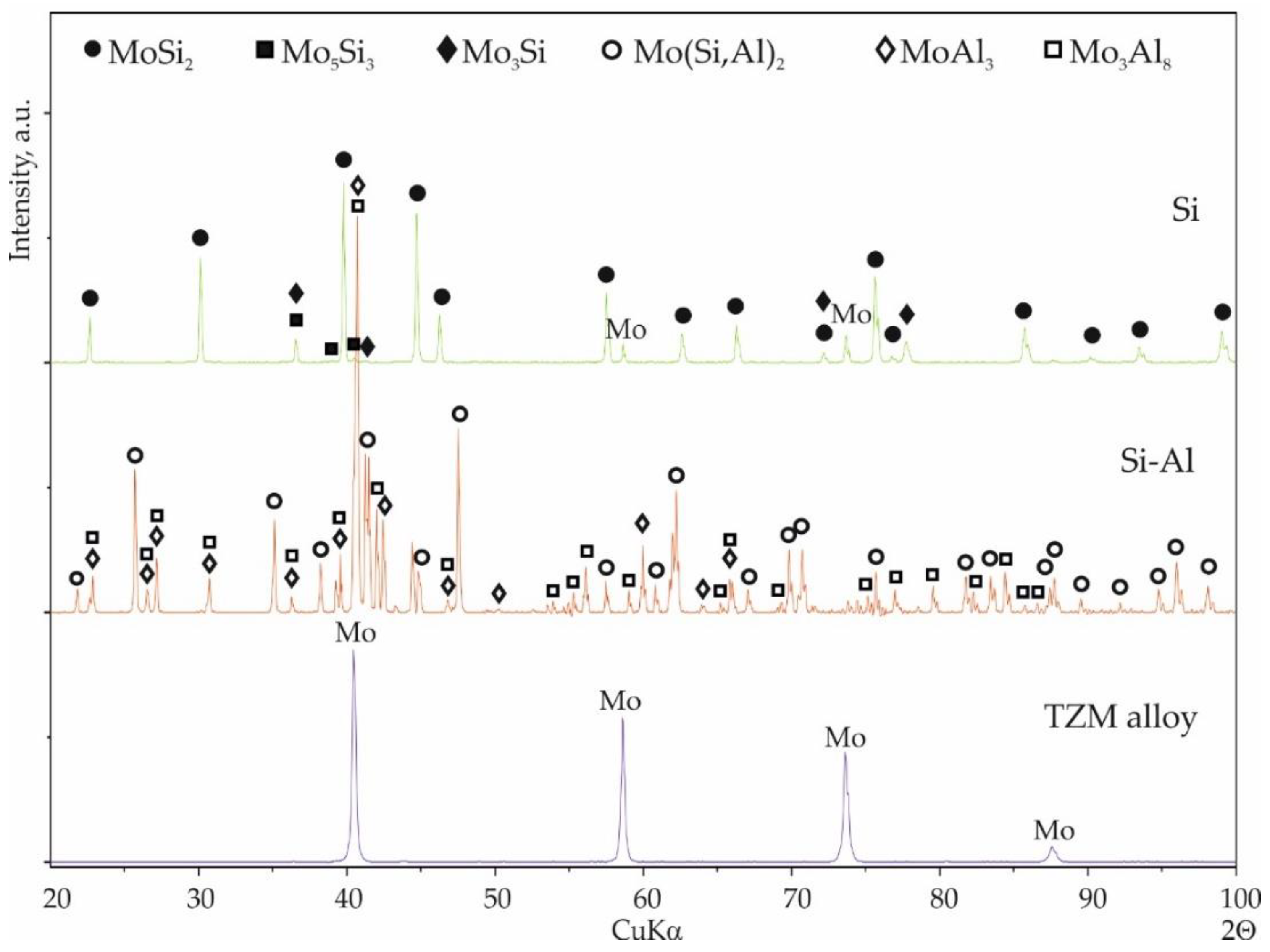
3. Results
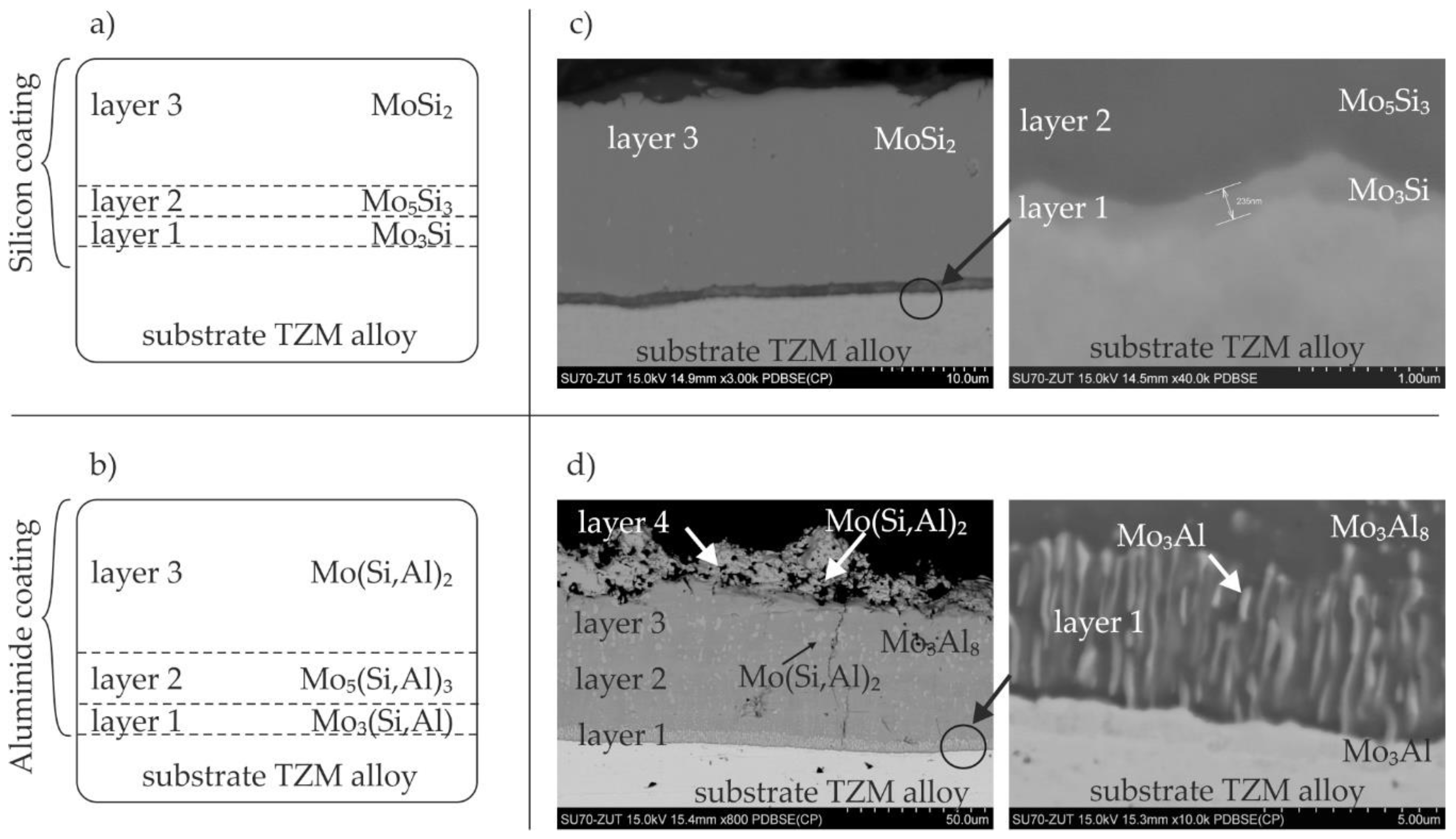
3.1. General Coating Morphology
3.2. Influence of Slurry Volume on Coating Microstructure
3.2.1. Silicide Coatings
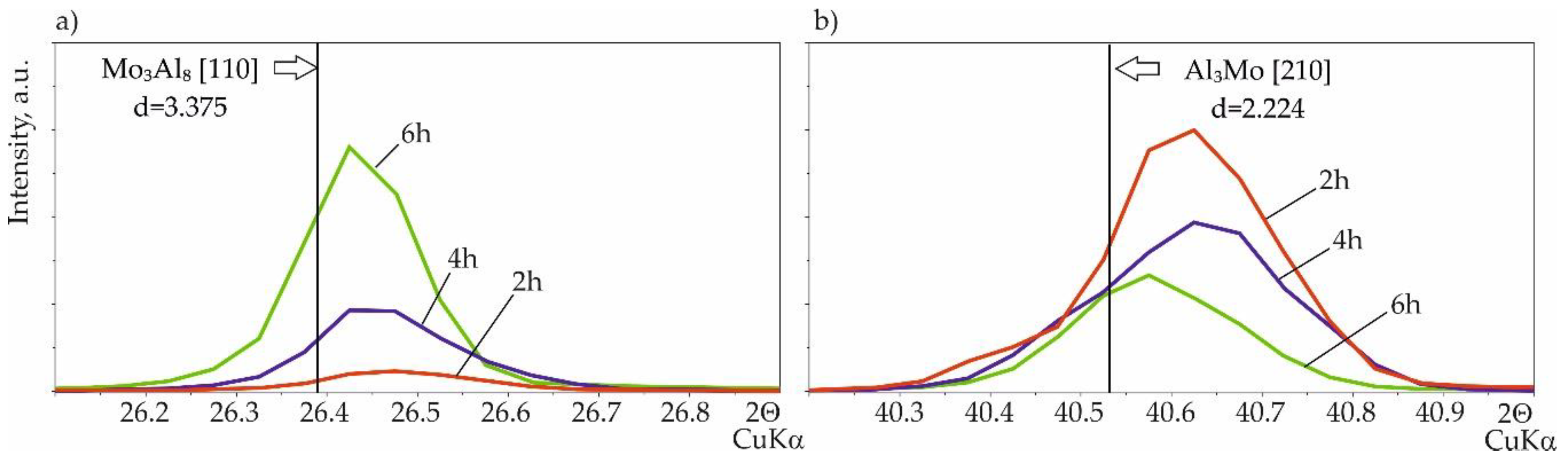
3.2.2. Silicide-Aluminide Coatings
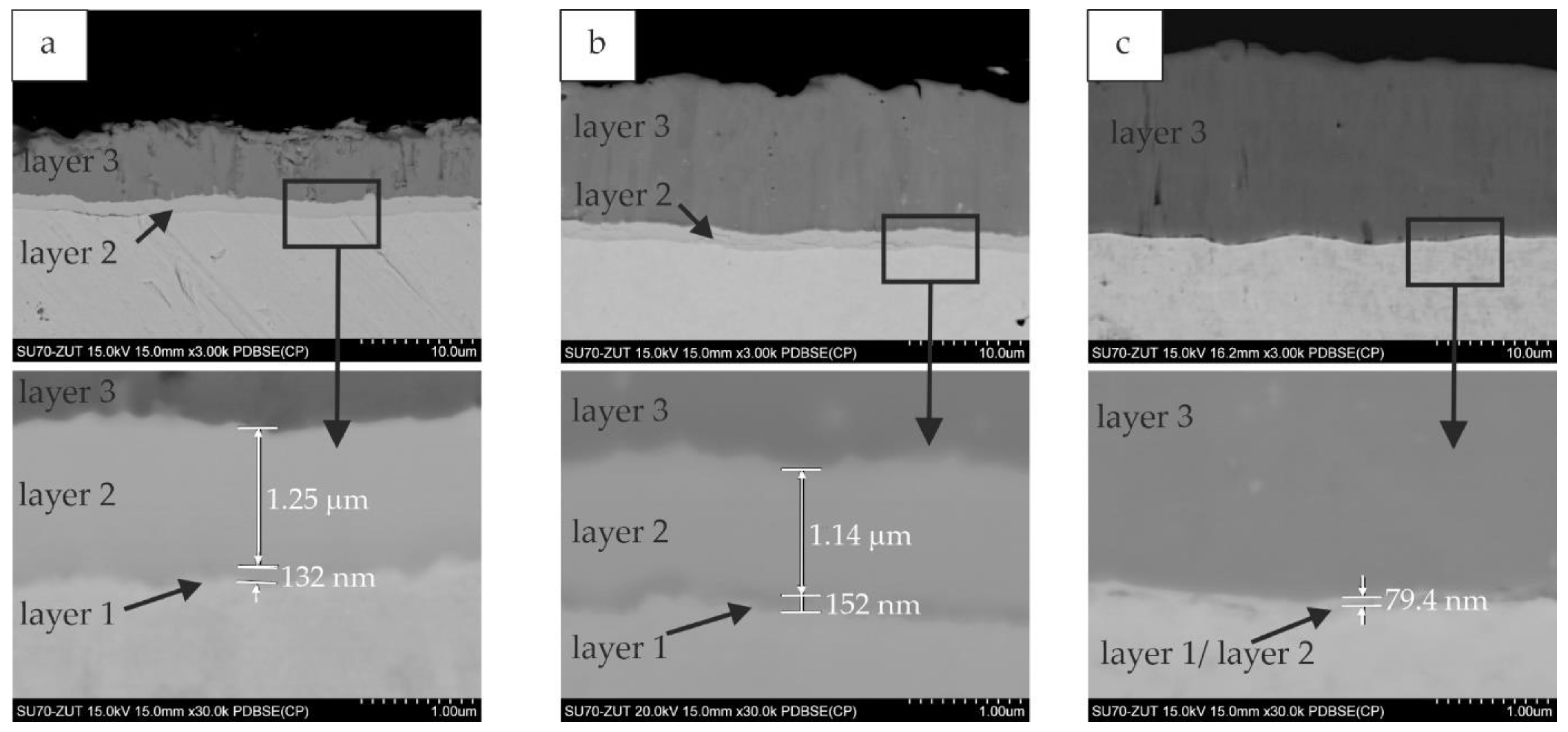
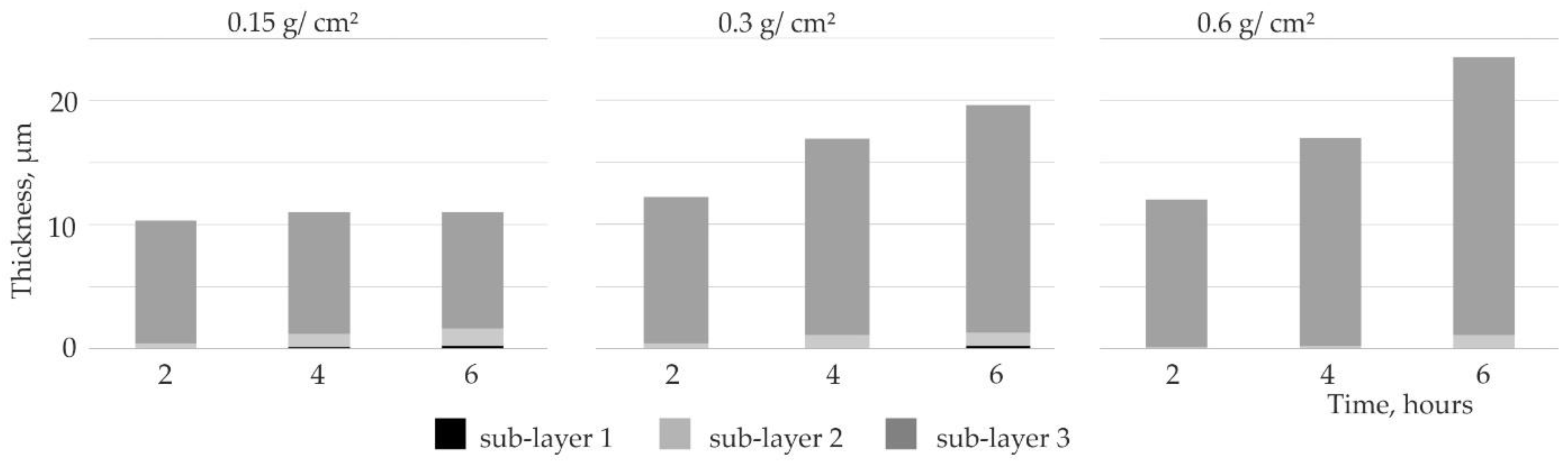
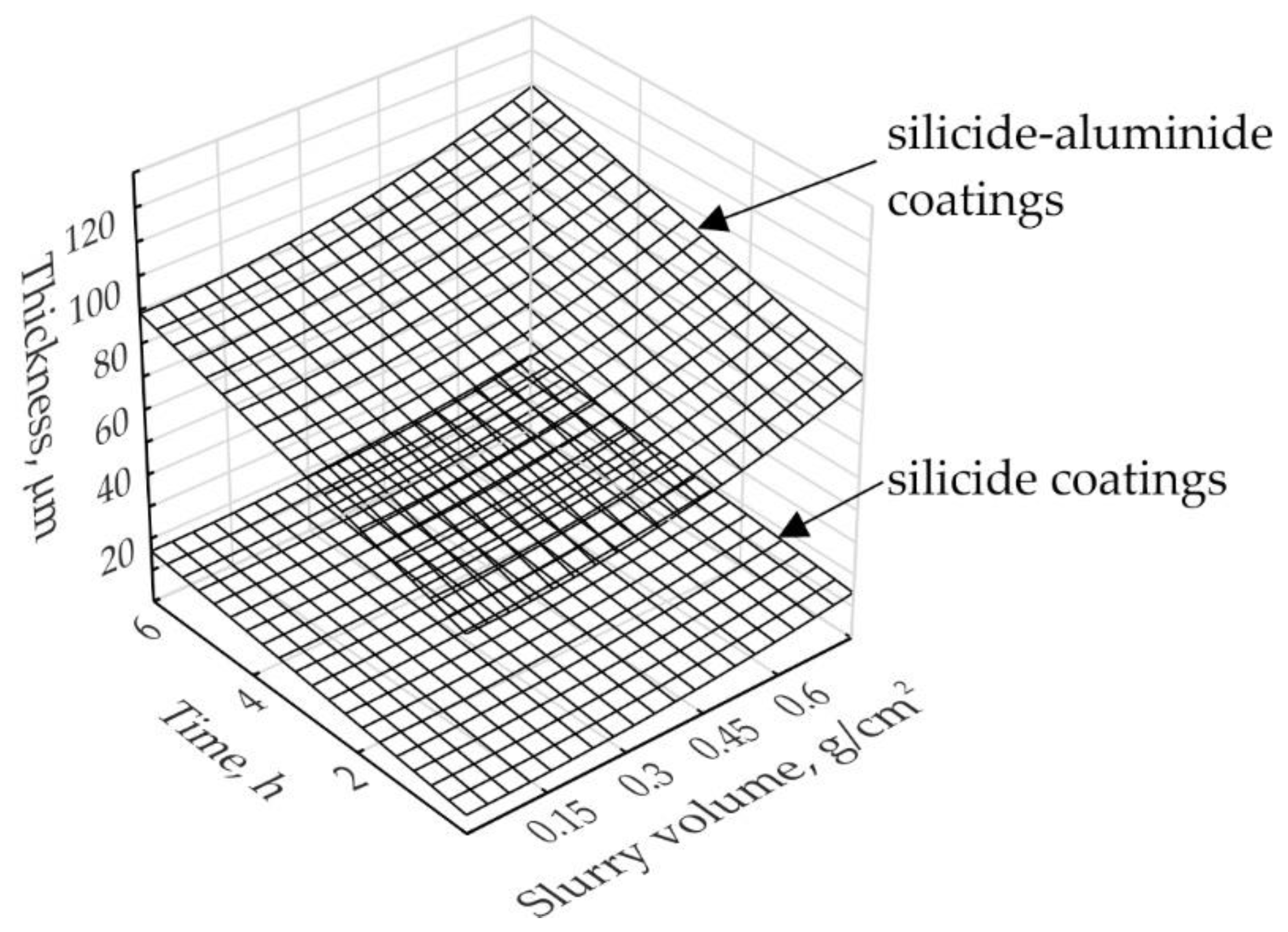
3.3. Influence of Slurry Volume on Coating Thickness
3.3.1. Silicide Coatings
3.3.2. Silicide-Aluminide Coatings
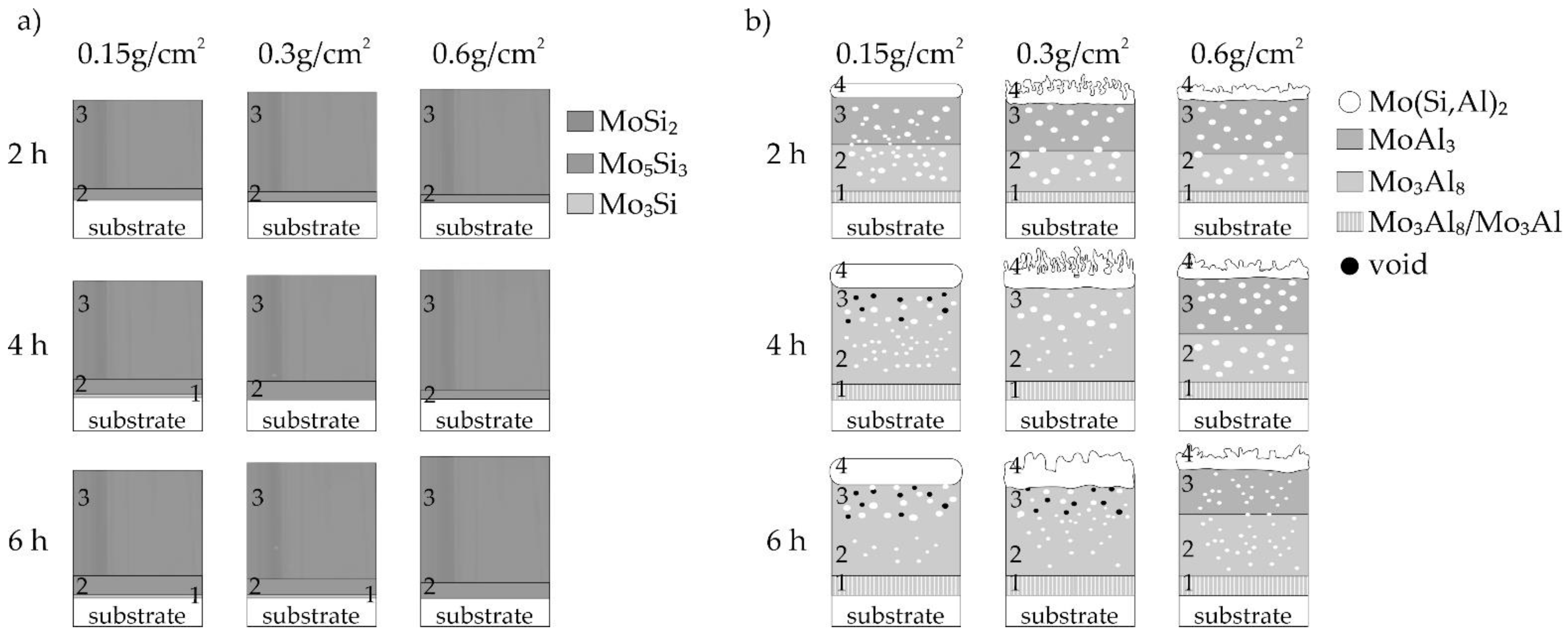
3.4. Structure and Growth Model of Coatings
3.5. Microhardness of Coatings
4. Conclusions
- the main part of the coating is the outer sublayer containing the MoSi2 phase,
- the use of a small volume of the slurry (0.15 g/cm2) during production and extension of the annealing time, did not significantly increase the thickness of the coatings, which was about 11 µm. In such conditions, increasing deficiency of the alloying elements slows down the kinetics of the Si-rich layer growth,
- the use of a larger volume of the slurry (0.3 and 0.6) during production and extension of the annealing time, significantly increased the thickness of the coatings, from about 12 µm in the case of 2 h to about 20 µm in the case of 6 h of annealing,
- the hardness of the coatings is similar, around 2100 HK0.01, since all the resulting coatings contain the MoSi2 phase.
- the main components of the coatings were the two phases MoAl3 and Mo3Al8, while the outer sublayer contained the Mo(Si,Al)2 phase, which should constitute a corrosion barrier,
- the use of a small volume of the slurry (0.15 g/cm2) during production and extension of the annealing time resulted in the formation of voids in the cross-section of the coatings, moreover, the formation of phases with lower aluminum content is observed.
- the coating growth kinetics for 0.15 and 0.3 slurry volumes were similar. The thickness of the coatings produced for 2, 4, and 6 h was on average 75, 82, 85 µm, respectively,
- the use of a larger volume of the slurry (0.6) during production and extension of the annealing time, significantly increased the thickness of the coatings, from about 75 µm in the case of 2 h to about 100 µm in the case of 6 h of annealing,
- the hardness of the sublayers increased with increasing aluminum content and ranged from about 1200 to 1700 HK0.01.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Genk, M.S.; Tournier, J.M. A review of refractory metal alloys and mechanically alloyed-oxide dispersion strengthened steels for space nuclear power systems. J. Nucl. Mater. 2005, 340, 93–112. [Google Scholar] [CrossRef]
- Smolik, G.R.; Petti, D.A.; Schuetz, S.T. Oxidation and volatilization of TZM alloy in air. J. Nucl. Mater. 2000, 283, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.Z.; Venkataraman, B.; Sarma, B.; Das, D.K. MoSi2 coating on Mo substrate for short-term oxidation protection in air. J. Alloy. Compd. 2009, 487, 335–340. [Google Scholar] [CrossRef]
- Yavas, B.; Goller, G. A novel approach to boriding of TZM by spark plasma sintering method. Int. J. Refract. Met. Hard Mater. 2019, 78, 273–281. [Google Scholar] [CrossRef]
- Majumdar, S.; Sharma, I.G. Oxidation behavior of MoSi2 and Mo(Si, Al)2 coated Mo-0.5Ti-0.1Zr-0.02C alloy. Intermetallics 2011, 19, 541–545. [Google Scholar] [CrossRef]
- Majumdar, S. Formation of MoSi2 and Al doped MoSi2 coatings on molybdenum base TZM (Mo-0.5Ti-0.1Zr-0.02C) alloy. Surf. Coat. Technol. 2012, 206, 3393–3398. [Google Scholar] [CrossRef]
- Choi, K.; Yang, W.; Baik, K.H.; Kim, Y.; Lee, S.; Lee, S.; Park, J.S. Growth kinetics and isothermal oxidation behavior of a Si pack cementation-coated Mo-Si-B alloy. Appl. Surf. Sci. 2019, 489, 668–676. [Google Scholar] [CrossRef]
- Pu, R.; Sun, Y.; Xu, J.; Zhou, X.; Li, S.; Zhang, B.; Cai, Z.; Liu, S.; Zhao, X.; Xiao, L. Microstructure and properties of Mo-based double-layer MoSi2 thick coating by a new two-step method. Surf. Coat. Technol. 2020, 394, 125840. [Google Scholar] [CrossRef]
- Yoon, J.K.; Byun, J.Y.; Kim, G.H.; Kim, J.S.; Choi, C.S. Growth kinetics of three Mo-silicide layers formed by chemical vapor deposition of Si on Mo substrate. Surf. Coat. Technol. 2002, 155, 85–95. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Zhai, L.L.; Li, J.D.; Zhang, J.W. In-situ synthesis of silicide coatings on molybdenum substrates by electrodeposition in chloride-fluoride molten salts. Int. J. Refract. Met. Hard Mater. 2019, 82, 340–348. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Fu, T.; Wang, J.; Shen, F.; Zhang, X.; Yu, L. Formation of MoSi2 and Si/MoSi2 coatings on TZM (Mo–0.5Ti–0.1Zr–0.02C) alloy by hot dip silicon-plating method. Ceram. Int. 2021, 47, 23053–23065. [Google Scholar] [CrossRef]
- Majumdar, S.; Sharma, I.G.; Raveendra, S.; Samajdar, I.; Bhargava, P. In situ chemical vapour co-deposition of Al and Si to form diffusion coatings on TZM. Mater. Sci. Eng. A 2008, 492, 211–217. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, G.; Tsakiropoulos, P. Thermodynamic reassessment of the Mo-Si and Al-Mo-Si systems. Intermetallics 2000, 8, 953–962. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.M.; Lee, S.; Park, J.S. Oxidation behaviors of the aluminide coated TZM alloy via pack cementation. Phys. Met. Metallogr. 2014, 115, 1351–1355. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Banerjee, S.; Singh, K.; Sharma, I.G.; Grover, A.K.; Suri, A.K. Studies on the development of protective coating on TZM alloy and its subsequent characterization. J. Mater. Process. Technol. 2008, 207, 240–247. [Google Scholar] [CrossRef]
- Ponweiser, N.; Paschinger, W.; Ritscher, A.; Schuster, J.C.; Richter, K.W. Phase equilibria in the Al-Mo-Si system. Intermetallics 2011, 19, 409–418. [Google Scholar] [CrossRef]
- Tamarin, Y. Protective Coatings for Turbine Blades; ASM Int.: Novelty, OH, USA, 2002; p. 300. [Google Scholar]
- Bermejo Sanz, J.; Roussel García, R.; Kolarik, V.; del Mar Juez Lorenzo, M. Influence of the Slurry Thickness and Heat Treatment Parameters on the Formation of Aluminium Diffusion Coating. Oxid. Met. 2017, 88, 179–190. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.C.; Schütze, M. A Novel Type of Environmentally Friendly Slurry Coatings. Jom 2015, 67, 77–86. [Google Scholar] [CrossRef]
- Grégoire, B.; Bonnet, G.; Pedraza, F. Mechanisms of formation of slurry aluminide coatings from Al and Cr microparticles. Surf. Coat. Technol. 2019, 359, 323–333. [Google Scholar] [CrossRef]
- Kochmańska, A.E. Microstructure of Al-Si Slurry Coatings on Austenitic High-Temperature Creep Resisting Cast Steel. Adv. Mater. Sci. Eng. 2018, 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Jarlaczyńska, A.; Kochmańska, A.; Woitas, A. The structure of silicon coatings obtained on TZM molybdenum alloy by slurry method. Mater. Eng.-Inz. Mater. 2017, 1, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Kochmańska, A.E. Microstructure of aluminide coatings on Ti6Al4V alloy produced by the slurry method with inorganic binder. Int. J. Mater. Res. 2018, 109, 735–742. [Google Scholar] [CrossRef]
- Kochmańska, A.E. Aluminide coatings on inconel 617 obtained by slurry method with inorganic binder. J. Achiev. Mater. Manuf. Eng. 2017, 85, 49–55. [Google Scholar] [CrossRef]
- Tortorici, P.C.; Dayananda, M.A. Growth of silicides and interdiffusion in the Mo-Si system. Metall. Mater. Trans. A 1999, 30, 545–550. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Banerjee, S.; Sharma, I.G.; Suri, A.K. Development of silicide coating over molybdenum based refractory alloy and its characterization. J. Nucl. Mater. 2010, 403, 152–159. [Google Scholar] [CrossRef]
- Kochmańska, A. Hot corrosion resistance properties of Al–Si coatings obtained by slurry method. In Defect and Diffusion Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2012; pp. 273–278. [Google Scholar]
- Prasad, S.; Paul, A. Growth mechanism of phases by interdiffusion and atomic mechanism of diffusion in the molybdenum-silicon system. Intermetallics 2011, 19, 1191–1200. [Google Scholar] [CrossRef]
- Tatemoto, K.; Ono, Y.; Suzuki, R.O. Silicide coating on refractory metals in molten salt. J. Phys. Chem. Solids 2005, 66, 526–529. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sprengel, W.; Staab, T.E.M.; Inui, H.; Schaefer, H.-E. Formation of Thermal Vacancies on the Si Sublattice of the Intermetallic Compound MoSi2. Phys. Rev. Lett. 2004, 92, 155502. [Google Scholar] [CrossRef] [Green Version]
- Salamon, M.; Strohm, A.; Voss, T.; Laitinen, P.; Riihimäki, I.; Divinski, S.; Frank, W.; Räisänen, J.; Mehrer, H. Self-diffusion of silicon in molybdenum disilicide. Philos. Mag. 2004, 84, 737–756. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997; ISBN 978-0-08-037941-8. [Google Scholar]

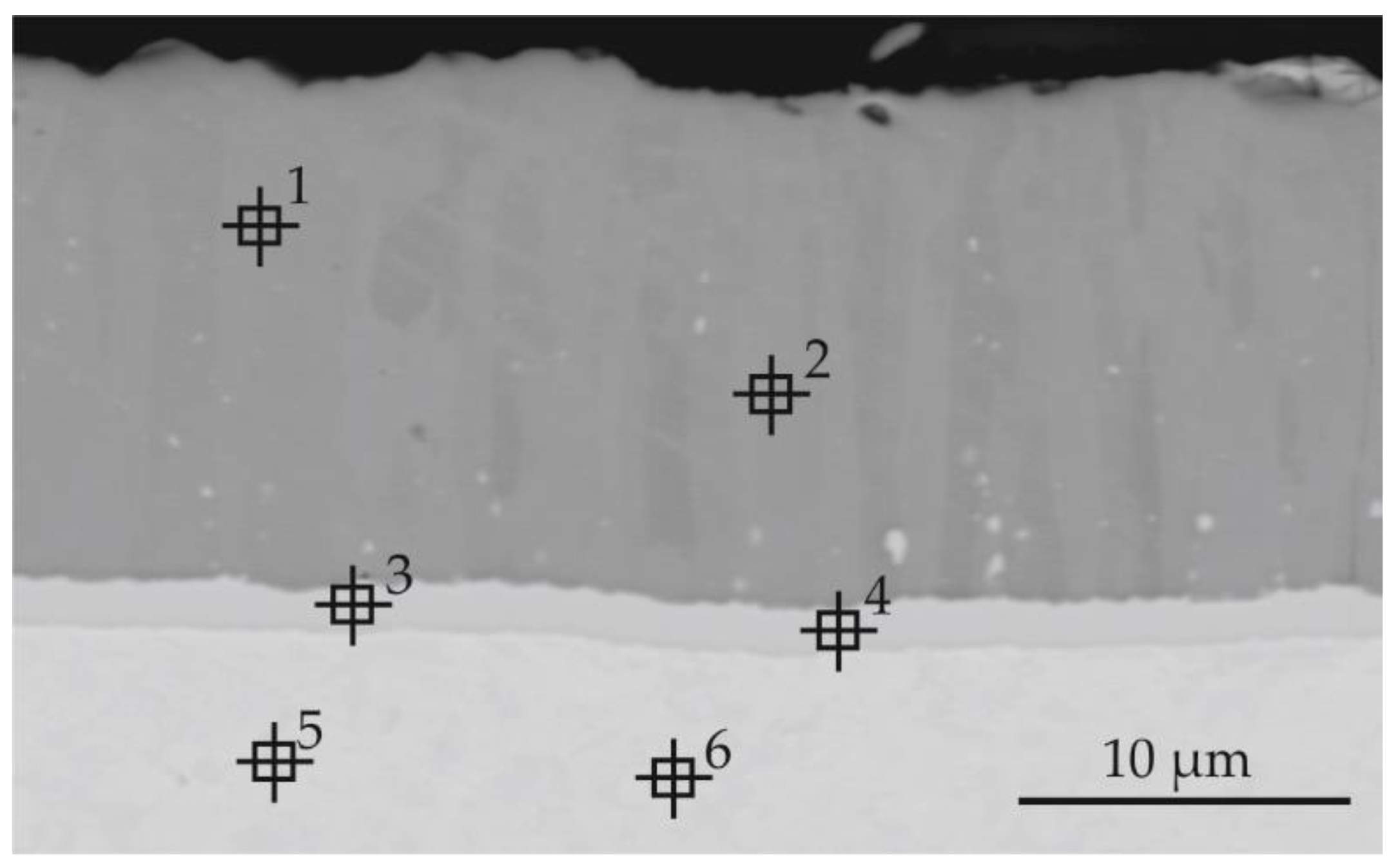





| Point of Analysis | Chemical Composition, % at | ||||
|---|---|---|---|---|---|
| Al | Si | Mo | Ti | Zr | |
| 1 | 70.3 | 0.7 | 29.0 | 0.0 | 0.0 |
| 2 | 43.5 | 14.1 | 42.4 | 0.0 | 0.0 |
| 3 | 19.7 | 6.5 | 73.8 | 0.0 | 0.0 |
| 4 | 0.7 | 0.0 | 99.1 | 0.2 | 0.0 |
| 5 | 3.1 | 0.1 | 0.3 | 94.9 | 1.6 |
| 6 | 0.9 | 0.3 | 0.8 | 95.8 | 2.2 |
| Point of Analysis | Chemical Composition, % at | |
|---|---|---|
| Si | Mo | |
| 1 | 65.2 | 34.8 |
| 2 | 65.1 | 34.9 |
| 3 | 45.5 | 54.5 |
| 4 | 45.5 | 54.5 |
| 5 | 0.0 | 100.0 |
| 6 | 0.0 | 100.0 |
| Point of Analysis | Chemical Composition, % at | ||
|---|---|---|---|
| Al | Si | Mo | |
| 1 | 23.1 | 42.4 | 34.5 |
| 2 | 77.0 | 2.4 | 20.6 |
| 3 | 70.3 | 1.1 | 28.6 |
| 4 | 56.6 | 7.3 | 36.1 |
| 5 | 0.8 | 0.0 | 99.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochmańska, A.E.; Jarlaczyńska, A.; Baranowska, J. Formation of Silicide and Silicide-Aluminide Coatings on Molybdenum Alloy during Slurry Cementation Process: Influence of Slurry Volume. Materials 2021, 14, 6940. https://doi.org/10.3390/ma14226940
Kochmańska AE, Jarlaczyńska A, Baranowska J. Formation of Silicide and Silicide-Aluminide Coatings on Molybdenum Alloy during Slurry Cementation Process: Influence of Slurry Volume. Materials. 2021; 14(22):6940. https://doi.org/10.3390/ma14226940
Chicago/Turabian StyleKochmańska, Agnieszka Elżbieta, Aneta Jarlaczyńska, and Jolanta Baranowska. 2021. "Formation of Silicide and Silicide-Aluminide Coatings on Molybdenum Alloy during Slurry Cementation Process: Influence of Slurry Volume" Materials 14, no. 22: 6940. https://doi.org/10.3390/ma14226940






