Enabling Intelligent Recovery of Critical Materials from Li-Ion Battery through Direct Recycling Process with Internet-of-Things
Abstract
:1. Introduction
2. Challenges in the Material Supply Chain of LIBs
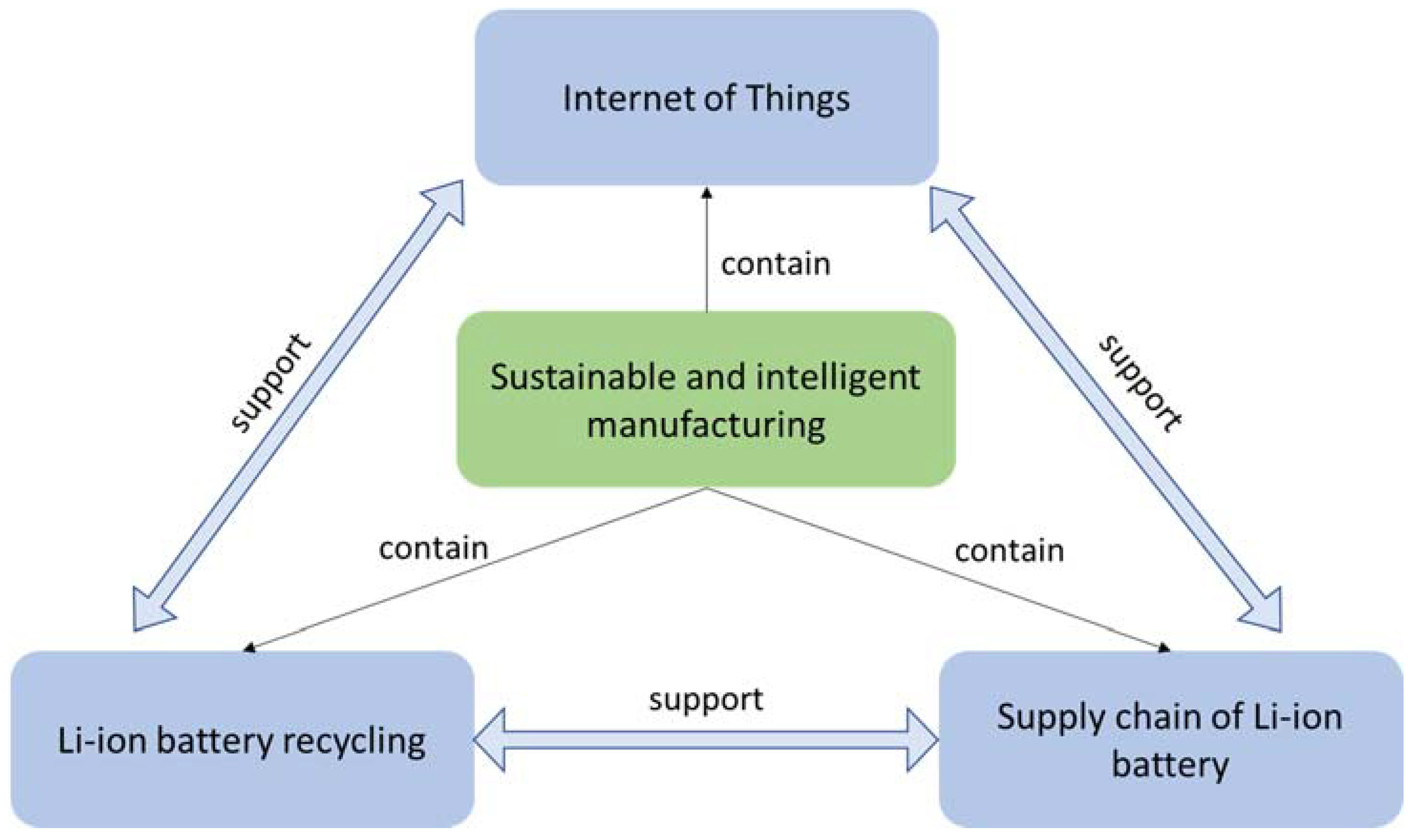
3. Direct Recycling of the LIBs and Its Impact on Supply Chain
4. The Development and Application of the Internet-of-Things
4.1. Overview of the IoT
4.2. The Application of IoT in Industry
4.3. Blockchain and Its Integration with IoT (BIoT)
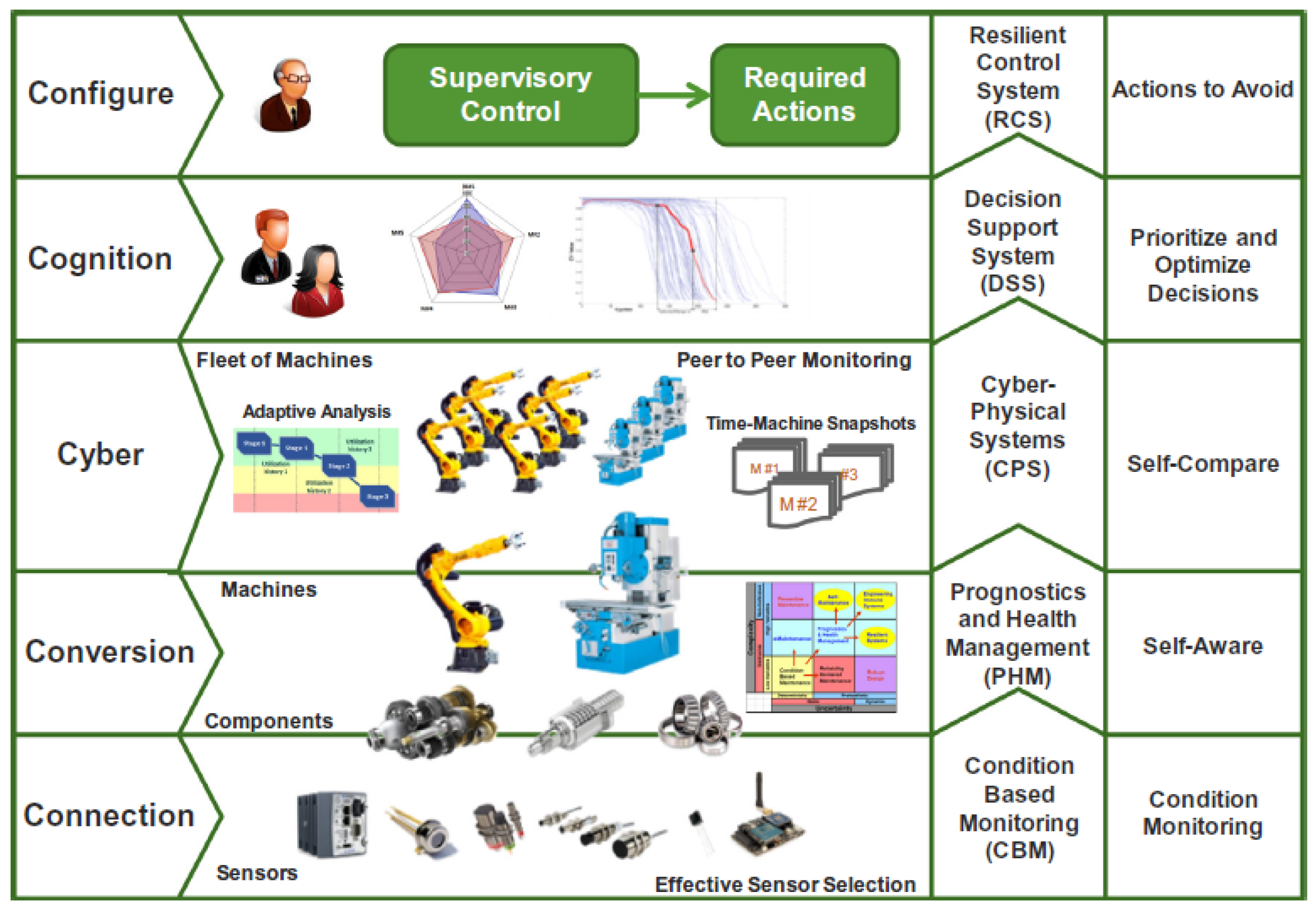
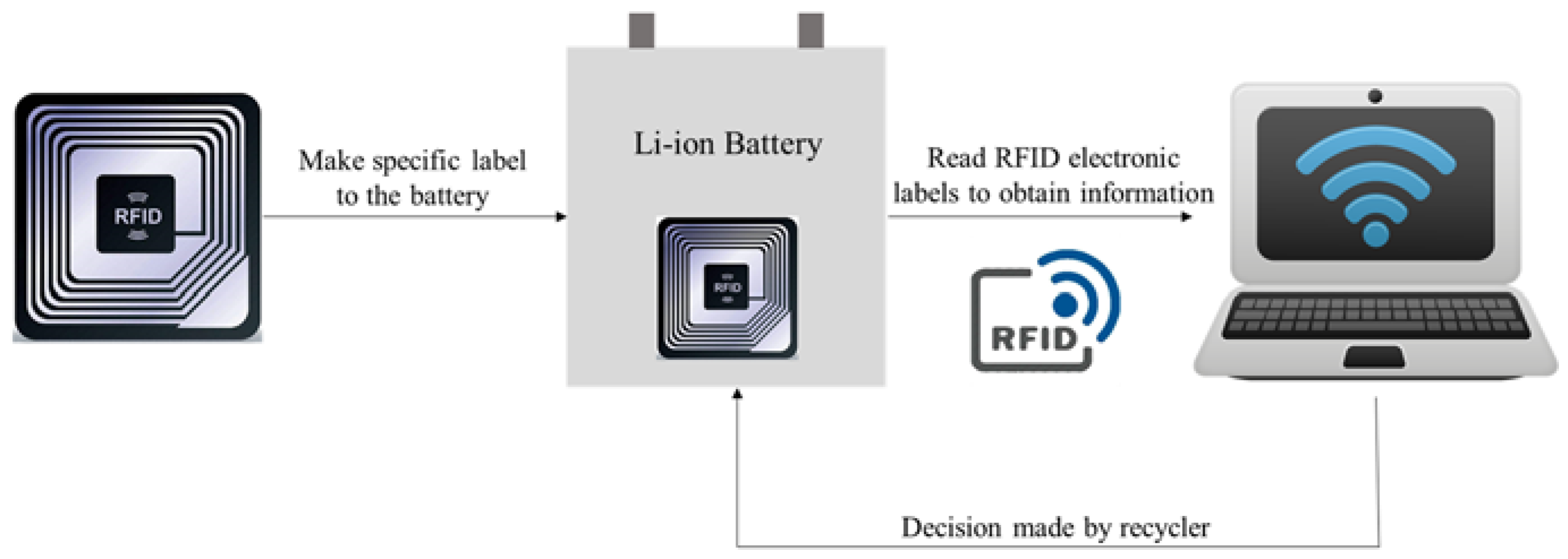
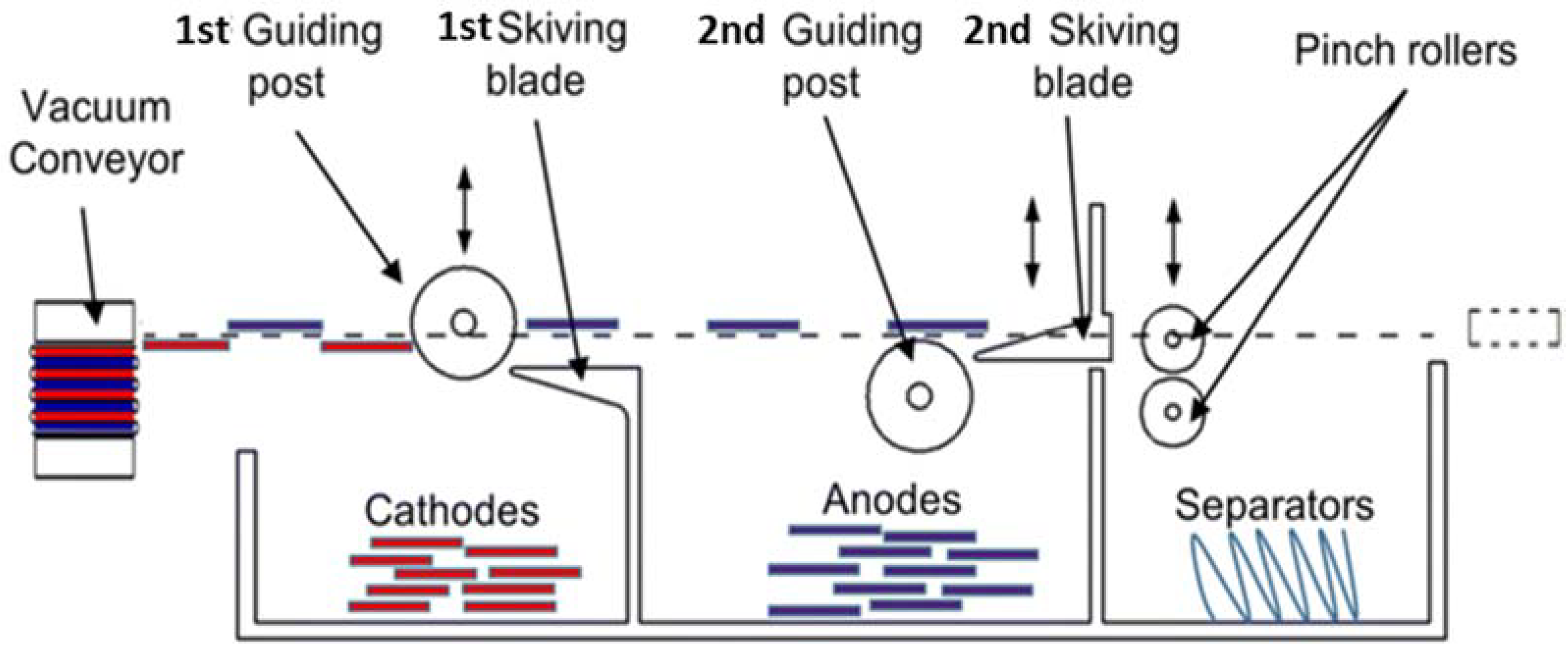
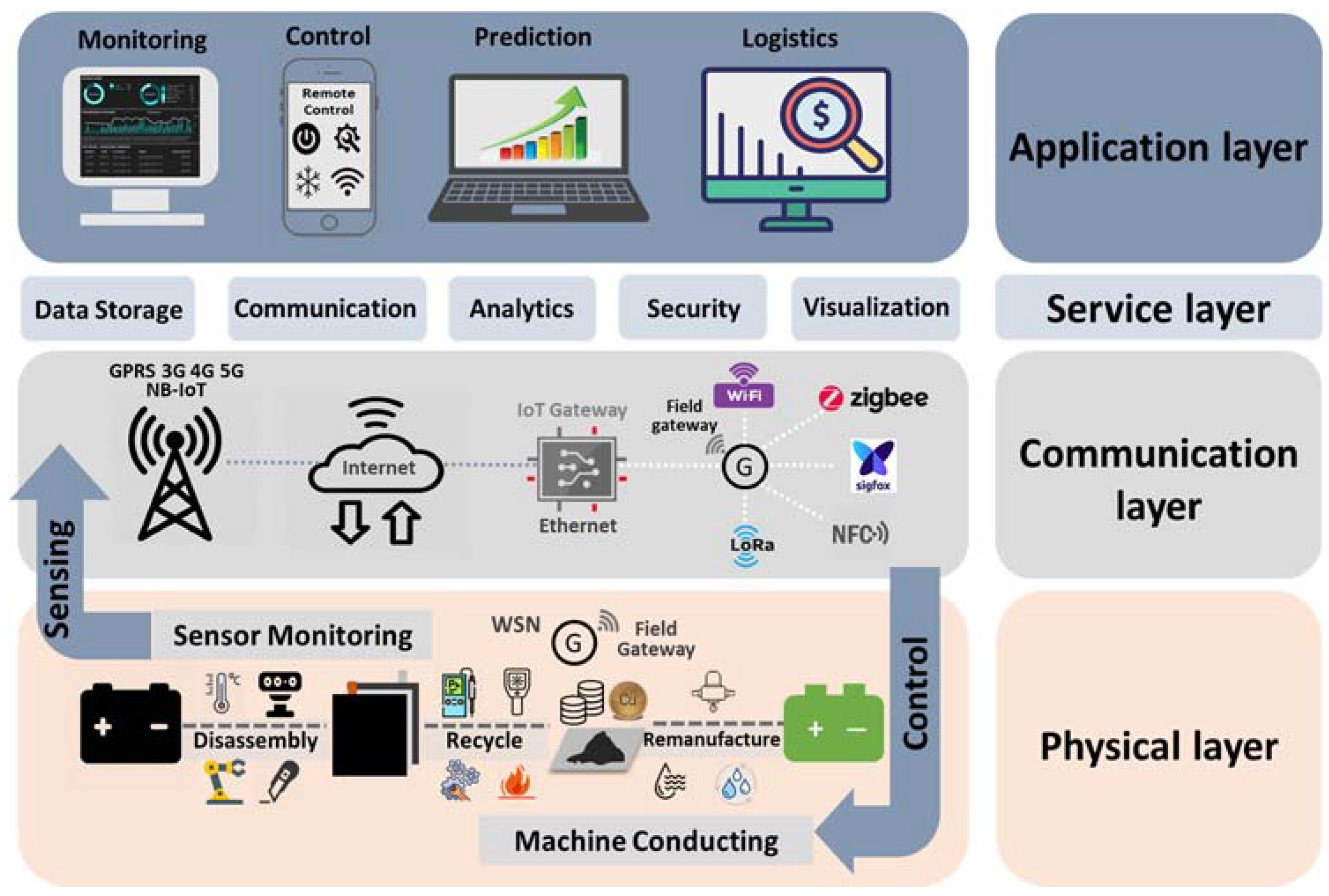
5. The IoT Enhanced Direct Recycling Process for LIB
5.1. The Application of IoT Devices in Different LIB Direct Recycling Processes
5.2. The Application of Artificial Intelligence and Machine Learning in Direct Recycling of LIBs
5.3. Proposed Paradigm
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffe, S. Vulnerable links in the lithium-ion battery supply chain. Joule 2017, 1, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Richa, K.; Babbitt, C.W.; Gaustad, G.; Wang, X. A future perspective on lithium-ion battery waste flows from electric vehicles. Resour. Conserv. Recycl. 2014, 83, 63–76. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Q.; Li, Y.; Wang, G.; Li, Y. Recycling mechanisms and policy suggestions for spent electric vehicles’ power battery-A case of Beijing. J. Clean. Prod. 2018, 186, 388–406. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.-K.; Passerini, S.; Whittingham, M.S.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Zhan, R.; Payne, T.; Leftwich, T.; Perrine, K.; Pan, L. De-agglomeration of cathode composites for direct recycling of Li-ion batteries. Waste Manag. 2020, 105, 39–48. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Yu, L.; Miao, C.; Nie, S.; Wen, M.; Wang, J.; Tan, Y.; Xiao, W. Feasible preparation of Cu6Sn5 alloy thin-film anode materials for lithium-ion batteries from waste printed circuit boards by electrodeposition. Solid State Ion. 2021, 364, 115625. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Lithium battery reusing and recycling: A circular economy insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef] [Green Version]
- Dias, P.A.; Blagoeva, D.; Pavel, C.; Arvanitidis, N. Cobalt: Demand-supply balances in the transition to electric mobility. Rep. EUR 2018, 10, 97710. [Google Scholar]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lu, Y.; Yang, T.; Ge, D.; Wood III, D.L.; Li, Z. Water-Based Electrode Manufacturing and Direct Recycling of Lithium-Ion Battery Electrodes—A Green and Sustainable Manufacturing System. iScience 2020, 23, 101081. [Google Scholar] [CrossRef]
- Li, L.; Dababneh, F.; Zhao, J. Cost-effective supply chain for electric vehicle battery remanufacturing. Appl. Energy 2018, 226, 277–286. [Google Scholar] [CrossRef]
- Mayyas, A.; Steward, D.; Mann, M. The case for recycling: Overview and challenges in the material supply chain for automotive li-ion batteries. Sustain. Mater. Technol. 2019, 19, e00087. [Google Scholar] [CrossRef]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Kelly, J.C.; Gaines, L.; Wang, M. Life Cycle Analysis of Lithium-Ion Batteries for Automotive Applications. Batteries 2019, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Dunn, J.B.; Gaines, L.; Kelly, J.C.; James, C.; Gallagher, K.G. The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. Energy Environ. Sci. 2015, 8, 158–168. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of recycling on cradle-to-gate energy consumption and greenhouse gas emissions of automotive lithium-ion batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Wallington, T.J.; Arsenault, R.; Bae, C.; Ahn, S.; Lee, J. Cradle-to-gate emissions from a commercial electric vehicle Li-ion battery: A comparative analysis. Environ. Sci. Technol. 2016, 50, 7715–7722. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, W.; Yuan, X.; Tang, H.; Tang, Y.; Wang, M.; Zuo, J.; Song, Z.; Sun, J. Environmental impact analysis and process optimization of batteries based on life cycle assessment. J. Clean. Prod. 2018, 174, 1262–1273. [Google Scholar] [CrossRef]
- Heymans, C.; Walker, S.B.; Young, S.B.; Fowler, M. Economic analysis of second use electric vehicle batteries for residential energy storage and load-levelling. Energy Policy 2014, 71, 22–30. [Google Scholar] [CrossRef]
- Saville, R.; Hatanaka, K.; Wada, M. ICT application of real-time monitoring and estimation system for set-net fishery. In Proceedings of the OCEANS 2015-MTS/IEEE Washington, Washington, DC, USA, 19–22 October 2015; pp. 1–5. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, Q.; Han, W.; Zheng, L. Value-centric design of the internet-of-things solution for food supply chain: Value creation, sensor portfolio and information fusion. Inf. Syst. Front. 2015, 17, 289–319. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.; Ni, Y.; Gu, L.; Zhu, H. Blockchain for the IoT and industrial IoT: A review. Internet Things 2020, 10, 100081. [Google Scholar] [CrossRef]
- Pillot, C. The rechargeable battery market and main trends 2014–2025. In Proceedings of the 31st International Battery Seminar & Exhibit, Ft. Lauderdale, FL, USA, 10–13 March 2014. [Google Scholar]
- Nkulu, C.B.L.; Casas, L.; Haufroid, V.; De Putter, T.; Saenen, N.D.; Kayembe-Kitenge, T.; Obadia, P.M.; Mukoma, D.K.W.; Ilunga, J.-M.L.; Nawrot, T.S.; et al. Sustainability of artisanal mining of cobalt in DR Congo. Nat. Sustain. 2018, 1, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Weimer, L.; Braun, T.; vom Hemdt, A. Design of a systematic value chain for lithium-ion batteries from the raw material perspective. Resour. Policy 2019, 64, 101473. [Google Scholar] [CrossRef]
- Shedd, K.B.; McCullough, E.; Bleiwas, D.I. Global trends affecting the supply security of cobalt. Min. Eng. 2017, 69, 37–42. [Google Scholar]
- Sun, X.; Hao, H.; Hartmann, P.; Liu, Z.; Zhao, F. Supply risks of lithium-ion battery materials: An entire supply chain estimation. Mater. Today Energy 2019, 14, 100347. [Google Scholar] [CrossRef]
- van den Brink, S.; Kleijn, R.; Sprecher, B.; Tukker, A. Identifying supply risks by mapping the cobalt supply chain. Resources, Conserv. Recycl. 2020, 156, 104743. [Google Scholar] [CrossRef]
- Crundwell, F.; Moats, M.; Ramachandran, V.; Robinson, T.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Sun, X.; Hao, H.; Zhao, F.; Liu, Z. Tracing global lithium flow: A trade-linked material flow analysis. Resour. Conserv. Recycl. 2017, 124, 50–61. [Google Scholar] [CrossRef]
- Schulz, K.J.; DeYoung, J.H.; Seal, R.R.; Bradley, D.C. Critical Mineral Resources of the United States: Economic and Environmental Geology and Prospects for Future Supply; Geological Survey; United States Government Publishing Office: Washington, DC, USA, 2018.
- Ewert, J.W.; Diefenbach, A.K.; Ramsey, D.W. 2018 Update to the U.S. Geological Survey National Volcanic Threat Assessment; No. 2018-5140; U.S. Geological Survey: Reston, VA, USA, 2018; p. 50.
- Kushnir, D.; Sandén, B.A. The time dimension and lithium resource constraints for electric vehicles. Resour. Policy 2012, 37, 93–103. [Google Scholar] [CrossRef]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global lithium availability: A constraint for electric vehicles? J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- Tran, T.; Luong, V.T. Lithium production processes. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 81–124. [Google Scholar]
- Merchant, E. Lithium-ion Battery Production Is Surging, but at What Cost? Greentech Media, 20 September 2017. [Google Scholar]
- Jiang, S.; Zhang, L.; Li, F.; Hua, H.; Liu, X.; Yuan, Z.; Wu, H. Environmental impacts of lithium production showing the importance of primary data of upstream process in life-cycle assessment. J. Environ. Manag. 2020, 262, 110253. [Google Scholar] [CrossRef]
- Xiao, W.; Nie, Y.; Miao, C.; Wang, J.; Tan, Y.; Wen, M. Structural design of high-performance Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode materials enhanced by Mg2+ doping and Li3PO4 coating for lithium ion battery. J. Colloid Interface Sci. 2022, 607, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-T.; Maglia, F.; Park, K.-J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Umeno, T.; Abe, T.; Ogumi, Z. Improvement of natural graphite as a lithium-ion battery anode material, from raw flake to carbon-coated sphere. J. Mater. Chem. 2004, 14, 1754–1758. [Google Scholar] [CrossRef]
- Nelson, P.; Gallagher, K.; Bloom, I.; Dees, D.; Ahmed, S. BatPac version 3.1. (Battery Performance and Cost) Software (Argonne National Lab, 2017). Available online: http://www.cse.anl.gov/batpac (accessed on 17 March 2017).
- Genovese, A.; Acquaye, A.A.; Figueroa, A.; Koh, S.C.L. Sustainable supply chain management and the transition towards a circular economy: Evidence and some applications. Omega 2017, 66, 344–357. [Google Scholar] [CrossRef]
- Gaines, L. The future of automotive lithium-ion battery recycling: Charting a sustainable course. Sustain. Mater. Technol. 2014, 1, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Gaines, L. Lithium-ion battery recycling processes: Research towards a sustainable course. Sustain. Mater. Technol. 2018, 17, e00068. [Google Scholar] [CrossRef]
- Li, J.; Shi, P.; Wang, Z.; Chen, Y.; Chang, C.-C. A combined recovery process of metals in spent lithium-ion batteries. Chemosphere 2009, 77, 1132–1136. [Google Scholar] [CrossRef]
- Takacova, Z.; Havlik, T.; Kukurugya, F.; Orac, D. Cobalt and lithium recovery from active mass of spent Li-ion batteries: Theoretical and experimental approach. Hydrometallurgy 2016, 163, 9–17. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.; Mankhand, T. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Sun, R.; Xu, J.; Chen, Z.; Kang, M. Recovery of cobalt from spent lithium ion batteries using sulphuric acid leaching followed by solid–liquid separation and solvent extraction. RSC Adv. 2016, 6, 85303–85311. [Google Scholar] [CrossRef]
- Barbieri, E.; Lima, E.; Cantarino, S.; Lelis, M.; Freitas, M. Recycling of spent ion-lithium batteries as cobalt hydroxide, and cobalt oxide films formed under a conductive glass substrate, and their electrochemical properties. J. Power Sources 2014, 269, 158–163. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 2003, 68, 5–10. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Qiu, K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]
- Gaines, L. Profitable Recycling of Low-Cobalt Lithium-Ion Batteries Will Depend on New Process Developments. One Earth 2019, 1, 413–415. [Google Scholar] [CrossRef]
- Sonoc, A.; Jeswiet, J.; Soo, V.K. Opportunities to improve recycling of automotive lithium ion batteries. Procedia cIRP 2015, 29, 752–757. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and status of hydrometallurgical and direct recycling of Li-ion batteries and beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [Green Version]
- Sloop, S.; Crandon, L.; Allen, M.; Koetje, K.; Reed, L.; Gaines, L.; Sirisaksoontorn, W.; Lerner, M. A direct recycling case study from a lithium-ion battery recall. Sustain. Mater. Technol. 2020, 25, e00152. [Google Scholar] [CrossRef]
- Li, X.; Dogan, F.; Lu, Y.; Antunes, C.; Shi, Y.; Burrell, A.; Ban, C. Fast Determination of Lithium Content in Spent Cathodes for Direct Battery Recycling. Adv. Sustain. Syst. 2020, 4, 2000073. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of end-of-life lithium ion batteries, Part I: Commercial processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Miorandi, D.; Sicari, S.; De Pellegrini, F.; Chlamtac, I. Internet of things: Vision, applications and research challenges. Ad Hoc Netw. 2012, 10, 1497–1516. [Google Scholar] [CrossRef] [Green Version]
- Perwej, Y.; Ahmed, M.; Kerim, B.; Ali, H. An Extended Review on Internet of Things (IoT) and its Promising Applications. Commun. Appl. Electron. 2019, 7, 8–22. [Google Scholar] [CrossRef]
- He, W.; Da Xu, L. Integration of distributed enterprise applications: A survey. IEEE Trans. Ind. Inform. 2012, 10, 35–42. [Google Scholar] [CrossRef]
- Gazis, V. A Survey of Standards for Machine-to-Machine and the Internet of Things. IEEE Commun. Surv. Tutor. 2016, 19, 482–511. [Google Scholar] [CrossRef]
- Verdouw, C.N.; Beulens, A.J.M.; van der Vorst, J.G.A.J. Virtualisation of floricultural supply chains: A review from an Internet of Things perspective. Comput. Electron. Agric. 2013, 99, 160–175. [Google Scholar] [CrossRef] [Green Version]
- Monostori, L.; Kádár, B.; Bauernhansl, T.; Kondoh, S.; Kumara, S.; Reinhart, G.; Sauer, O.; Schuh, G.; Sihn, W.; Ueda, K. Cyber-physical systems in manufacturing. CIRP Ann. 2016, 65, 621–641. [Google Scholar] [CrossRef]
- Lee, J.; Bagheri, B.; Kao, H.-A. A cyber-physical systems architecture for industry 4.0-based manufacturing systems. Manuf. Lett. 2015, 3, 18–23. [Google Scholar] [CrossRef]
- Asghari, P.; Rahmani, A.M.; Javadi, H.H.S. Internet of Things applications: A systematic review. Comput. Netw. 2019, 148, 241–261. [Google Scholar] [CrossRef]
- Čolaković, A.; Hadžialić, M. Internet of Things (IoT): A review of enabling technologies, challenges, and open research issues. Comput. Netw. 2018, 144, 17–39. [Google Scholar] [CrossRef]
- Vijayakumar, N.; Ramya, R. The real time monitoring of water quality in IoT environment. In Proceedings of the 2015 International Conference on Circuits, Power and Computing Technologies [ICCPCT-2015], Coimbatore, India, 19–20 March 2015; pp. 1–4. [Google Scholar]
- Lazarescu, M.T. Design of a WSN Platform for Long-Term Environmental Monitoring for IoT Applications. IEEE J. Emerg. Sel. Top. Circuits Syst. 2013, 3, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Bhattacharya, S. Value Creation with IoT Driven Supply Chain Management for Indian Automobile Battery Industry. J. Eng. Comput. Archit. 2019, 9, 4719–4742. [Google Scholar]
- Tjahjono, B.; Esplugues, C.; Ares, E.; Pelaez, G. What does industry 4.0 mean to supply chain? Procedia Manuf. 2017, 13, 1175–1182. [Google Scholar] [CrossRef]
- Dekker, R.; Fleischmann, M.; Inderfurth, K.; van Wassenhove, L.N. Reverse Logistics: Quantitative Models For Closed-Loop Supply Chains; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Khan, M.A.; Salah, K. IoT security: Review, blockchain solutions, and open challenges. Future Gener. Comput. Syst. 2018, 82, 395–411. [Google Scholar] [CrossRef]
- Florea, B.C.; Taralunga, D.D. Blockchain IoT for Smart Electric Vehicles Battery Management. Sustainability 2020, 12, 3984. [Google Scholar] [CrossRef]
- Aitzhan, N.Z.; Svetinovic, D. Security and privacy in decentralized energy trading through multi-signatures, blockchain and anonymous messaging streams. IEEE Trans. Dependable Secur. Comput. 2016, 15, 840–852. [Google Scholar] [CrossRef]
- Bazilian, M.D. The mineral foundation of the energy transition. Extr. Ind. Soc. 2018, 5, 93–97. [Google Scholar] [CrossRef]
- Raza, U.; Kulkarni, P.; Sooriyabandara, M. Low power wide area networks: An overview. IEEE Commun. Surv. Tutor. 2017, 19, 855–873. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xu, Z.; Tan, X.; Ju, M.; Yang, J. Evaluation and recovery of power batteries based on trusted blockchain traceability. IOP Conf. Ser. Mater. Sci. Eng. 2019, 612, 042012. [Google Scholar] [CrossRef]
- Sun, H.; Hua, S.; Zhou, E.; Pi, B.; Sun, J.; Yamashita, K. Using Ethereum Blockchain in Internet of Things: A Solution for Electric Vehicle Battery Refueling; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–17. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Kim, K.W.; Hong, H.G.; Nam, G.P.; Park, K.R. A study of deep CNN-based classification of open and closed eyes using a visible light camera sensor. Sensors 2017, 17, 1534. [Google Scholar] [CrossRef] [Green Version]
- Badmos, O.; Kopp, A.; Bernthaler, T.; Schneider, G. Image-based defect detection in lithium-ion battery electrode using convolutional neural networks. J. Intell. Manuf. 2020, 31, 885–897. [Google Scholar] [CrossRef]
- Sommerville, R.; Shaw-Stewart, J.; Goodship, V.; Rowson, N.; Kendrick, E. A review of physical processes used in the safe recycling of lithium ion batteries. Sustain. Mater. Technol. 2020, 25, e00197. [Google Scholar] [CrossRef]
- Myung, S.-T.; Hitoshi, Y.; Sun, Y.-K. Electrochemical behavior and passivation of current collectors in lithium-ion batteries. J. Mater. Chem. 2011, 21, 9891–9911. [Google Scholar] [CrossRef]
- Ruiz, V.; Pfrang, A.; Kriston, A.; Omar, N.; Van den Bossche, P.; Boon-Brett, L. A review of international abuse testing standards and regulations for lithium ion batteries in electric and hybrid electric vehicles. Renew. Sustain. Energy Rev. 2018, 81, 1427–1452. [Google Scholar] [CrossRef]
- Dorella, G.; Mansur, M.B. A study of the separation of cobalt from spent Li-ion battery residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Hu, T.; Bai, X.; Wang, S.; Xie, W.; Hao, J.; He, Y. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by cryogenic grinding and froth flotation. Miner. Eng. 2020, 148, 106223. [Google Scholar] [CrossRef]
- Li, L.; Zheng, P.; Yang, T.; Sturges, R.; Ellis, M.W.; Li, Z. Disassembly Automation for Recycling End-of-Life Lithium-Ion Pouch Cells. JOM 2019, 71, 4457–4464. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Gaye, N.; Gueye, R.S.; Ledauphin, J.; Balde, M.; Seck, M.; Wele, A.; Diaw, M. Alkaline Leaching of Metals from Cathodic Materials of Spent Lithium-Ion Batteries. Asian J. Appl. Chem. Res. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Zhan, R.; Oldenburg, Z.; Pan, L. Recovery of active cathode materials from lithium-ion batteries using froth flotation. Sustain. Mater. Technol. 2018, 17, e00062. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, N.; Yao, Q.; Wu, Z.; Jin, W. Real-time moisture control in sintering process using offline–online NARX neural networks. Neurocomputing 2020, 396, 209–215. [Google Scholar] [CrossRef]
- Meng, X.; Hao, J.; Cao, H.; Lin, X.; Ning, P.; Zheng, X.; Chang, J.; Zhang, X.; Wang, B.; Sun, Z. Recycling of LiNi1/3Co1/3Mn1/3O2 cathode materials from spent lithium-ion batteries using mechanochemical activation and solid-state sintering. Waste Manag. 2019, 84, 54–63. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Chen, Z. Effective regeneration of LiCoO2 from spent lithium-ion batteries: A direct approach towards high-performance active particles. Green Chem. 2018, 20, 851–862. [Google Scholar] [CrossRef]
- Hazama, H.; Murai, D.; Nagasako, N.; Hasegawa, M.; Ogihara, N. Optimization of Material Composition of Li-Intercalated Metal–Organic Framework Electrodes Using a Combination of Experiments and Machine Learning of X-Ray Diffraction Patterns. Adv. Mater. Technol. 2020, 5, 2000254. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Liu, F.; Yue, X.; Chen, Z. Resolving the Compositional and Structural Defects of Degraded LiNixCoyMnzO2 Particles to Directly Regenerate High-Performance Lithium-Ion Battery Cathodes. ACS Energy Lett. 2018, 3, 1683–1692. [Google Scholar] [CrossRef]
- Yang, T.; Lu, Y.; Li, L.; Ge, D.; Yang, H.; Leng, W.; Zhou, H.; Han, X.; Schmidt, N.; Ellis, M. An Effective Relithiation Process for Recycling Lithium-Ion Battery Cathode Materials. Adv. Sustain. Syst. 2020, 4, 1900088. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhang, M.; Meng, Y.S.; Chen, Z. Ambient-Pressure Relithiation of Degraded LixNi0.5Co0.2Mn0.3O2 (0 < x < 1) via Eutectic Solutions for Direct Regeneration of Lithium-Ion Battery Cathodes. Adv. Energy Mater. 2019, 9, 1900454. [Google Scholar]
- Garrido-Hidalgo, C.; Ramirez, F.J.; Olivares, T.; Roda-Sanchez, L. The adoption of internet of things in a circular supply chain framework for the recovery of WEEE: The case of lithium-ion electric vehicle battery packs. Waste Manag. 2020, 103, 32–44. [Google Scholar] [CrossRef]
- Talavera, J.M.; Tobón, L.E.; Gómez, J.A.; Culman, M.A.; Aranda, J.M.; Parra, D.T.; Quiroz, L.A.; Hoyos, A.; Garreta, L.E. Review of IoT applications in agro-industrial and environmental fields. Comput. Electron. Agric. 2017, 142, 283–297. [Google Scholar] [CrossRef]
- He, B.; Bai, K.-J. Digital twin-based sustainable intelligent manufacturing: A review. Adv. Manuf. 2020, 9, 1–21. [Google Scholar] [CrossRef]


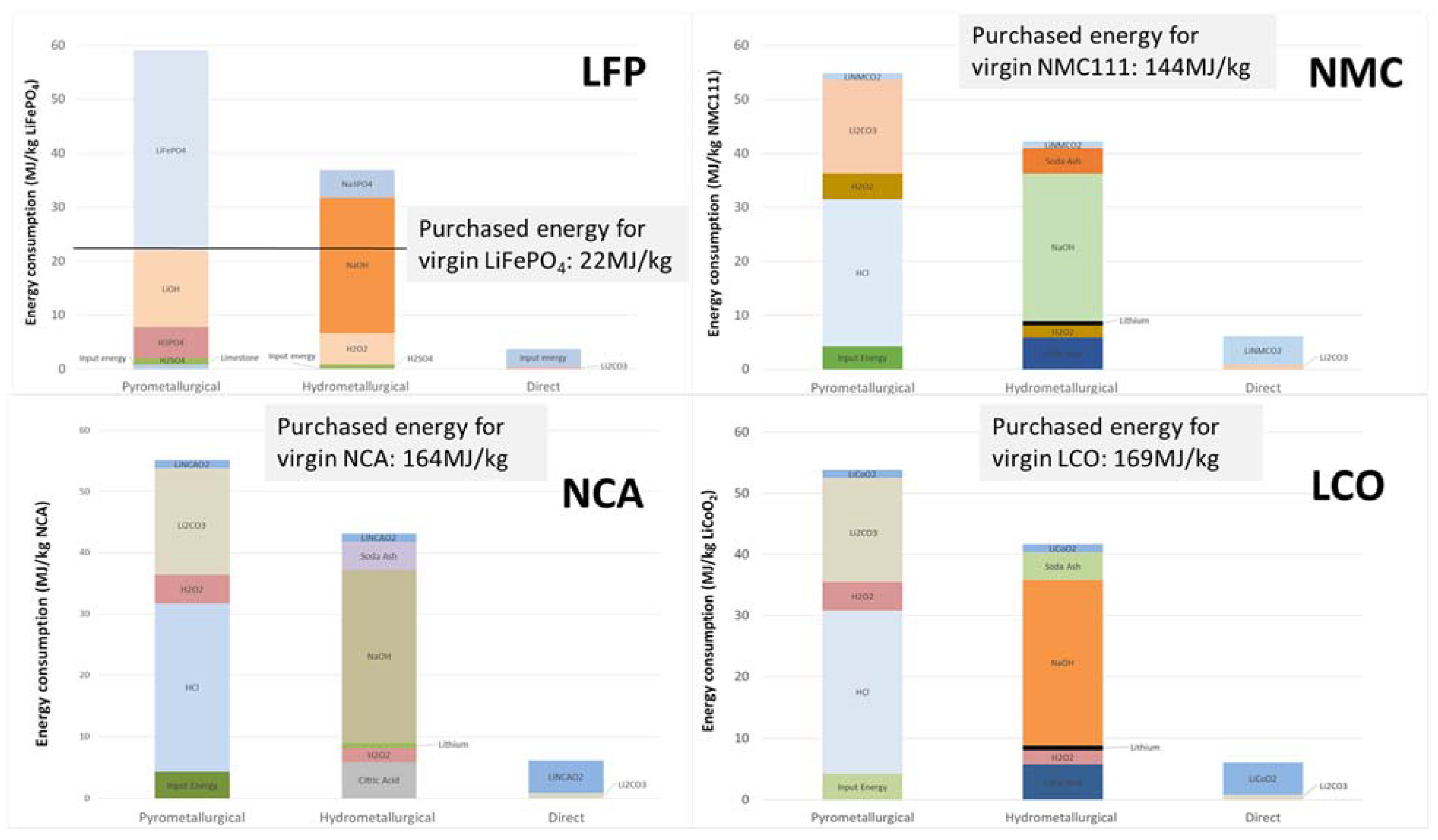

| Electrode | Element | Weight Percent (kg/kWh) | Cost Percent (USD/kWh) | Mine Production and Supply Information Worldwide |
|---|---|---|---|---|
| Cathode | Cobalt | 0.22 | 40.77 | 14,800 tons: 60% Congo (Kinshasa) |
| Nickel | 0.55 | 2,400,000 tons: 25% Indonesia, 15% Philippines | ||
| Manganese | 0.31 | 18,900 tons: 31% South Africa, 18% Australia | ||
| Lithium | 0.13 | 95,000 tons: 62% Australia, 18% Chile | ||
| Aluminum | 0.22 | 0.03 | 63,600 tons: 56% China | |
| Anode | Copper | 0.46 | 0.08 | 20,400 tons: 29% Chile, 12% Peru |
| Graphite | 1.1 | 20.43 | 1,120,000 tons: 62% China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Han, X.; Li, Z. Enabling Intelligent Recovery of Critical Materials from Li-Ion Battery through Direct Recycling Process with Internet-of-Things. Materials 2021, 14, 7153. https://doi.org/10.3390/ma14237153
Lu Y, Han X, Li Z. Enabling Intelligent Recovery of Critical Materials from Li-Ion Battery through Direct Recycling Process with Internet-of-Things. Materials. 2021; 14(23):7153. https://doi.org/10.3390/ma14237153
Chicago/Turabian StyleLu, Yingqi, Xu Han, and Zheng Li. 2021. "Enabling Intelligent Recovery of Critical Materials from Li-Ion Battery through Direct Recycling Process with Internet-of-Things" Materials 14, no. 23: 7153. https://doi.org/10.3390/ma14237153






