Effect of (NH4)2ZrF6, Voltage and Treating Time on Corrosion Resistance of Micro-Arc Oxidation Coatings Applied on ZK61M Magnesium Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Coating Preparation Procedures
2.2. Experiment Process
2.3. Characterization
3. Results
3.1. Orthogonal Experimental Results and Analysis
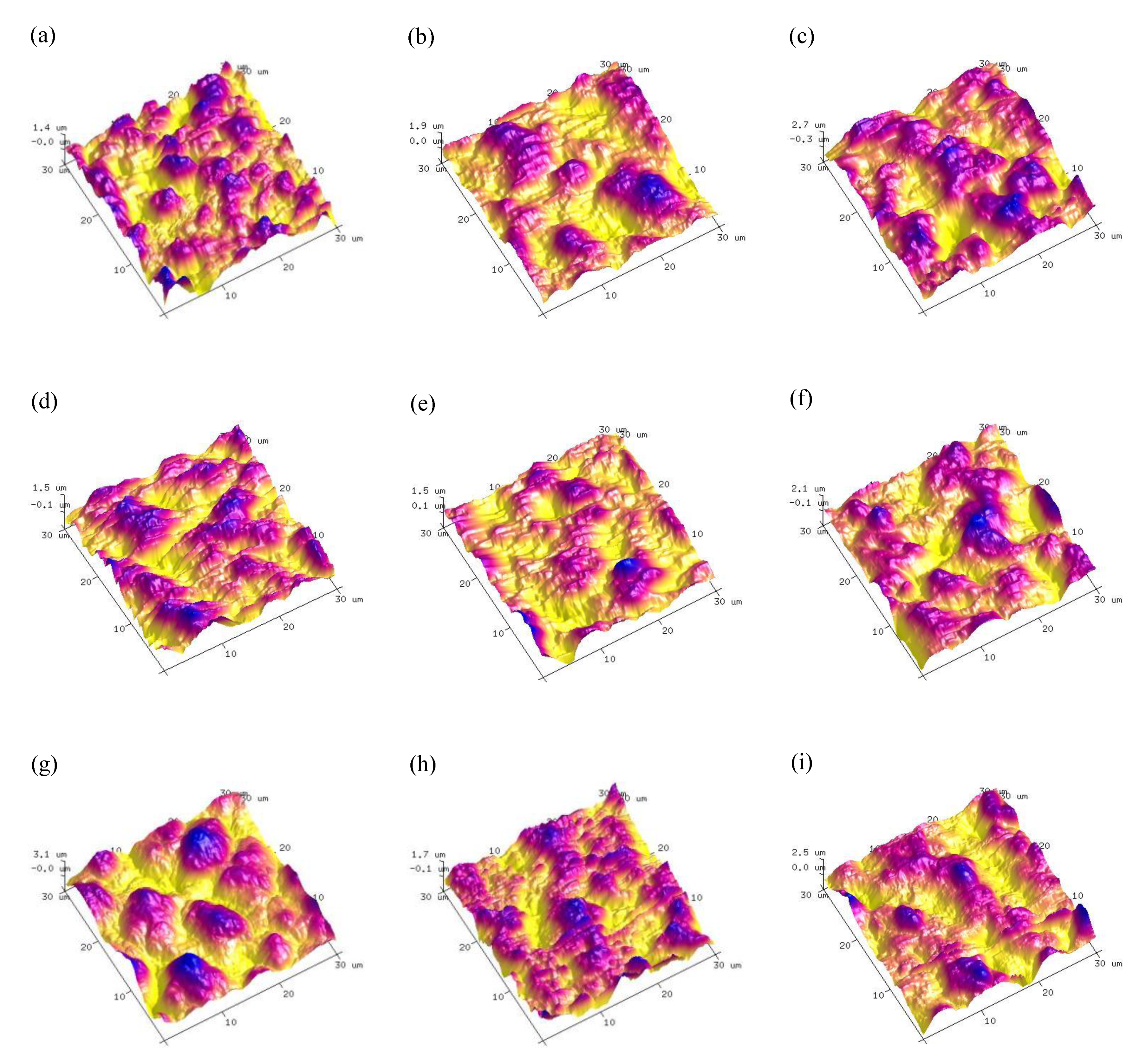
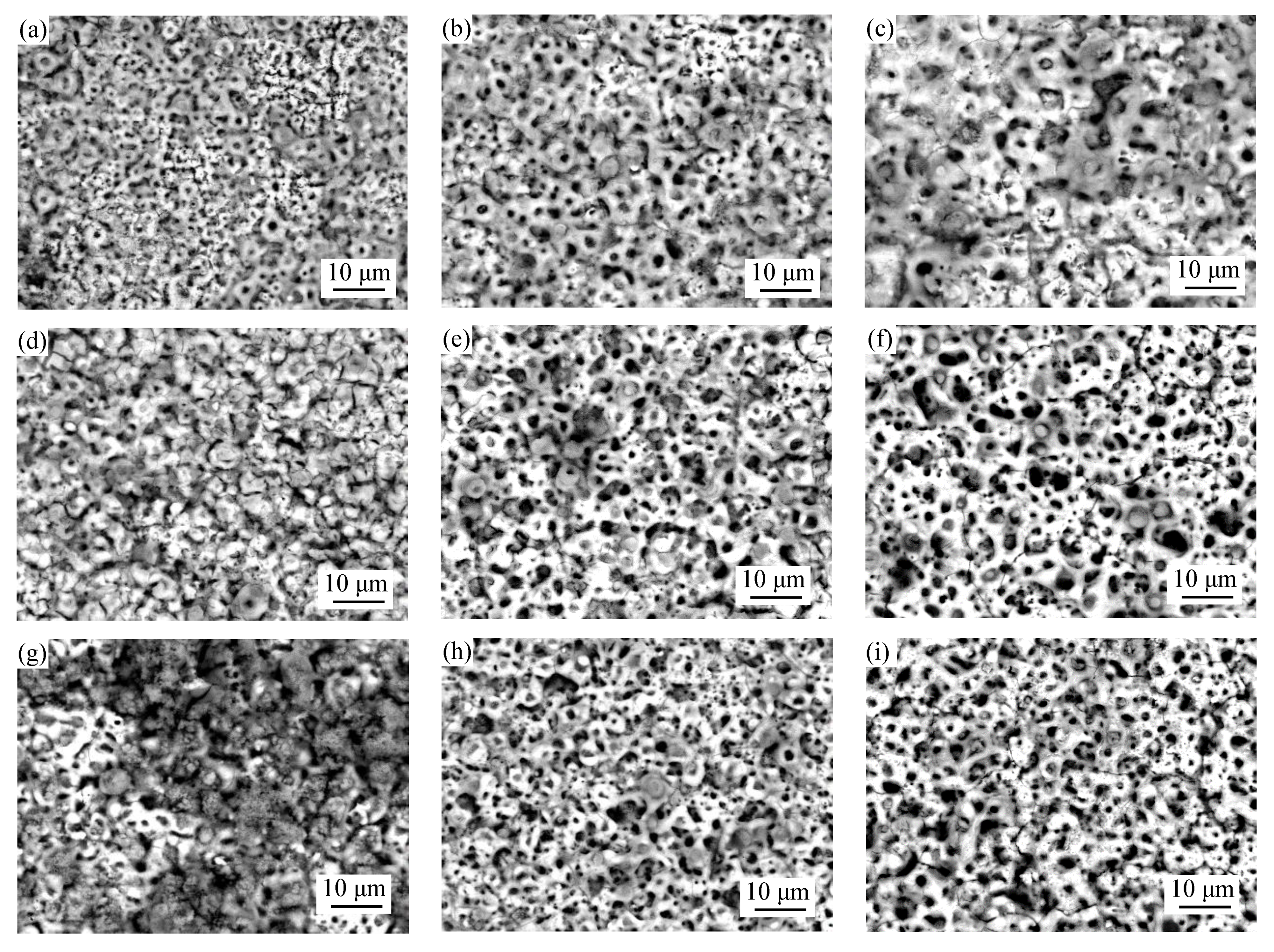
3.2. Surface Morphologies and Elemental Composition of MAO Coatings
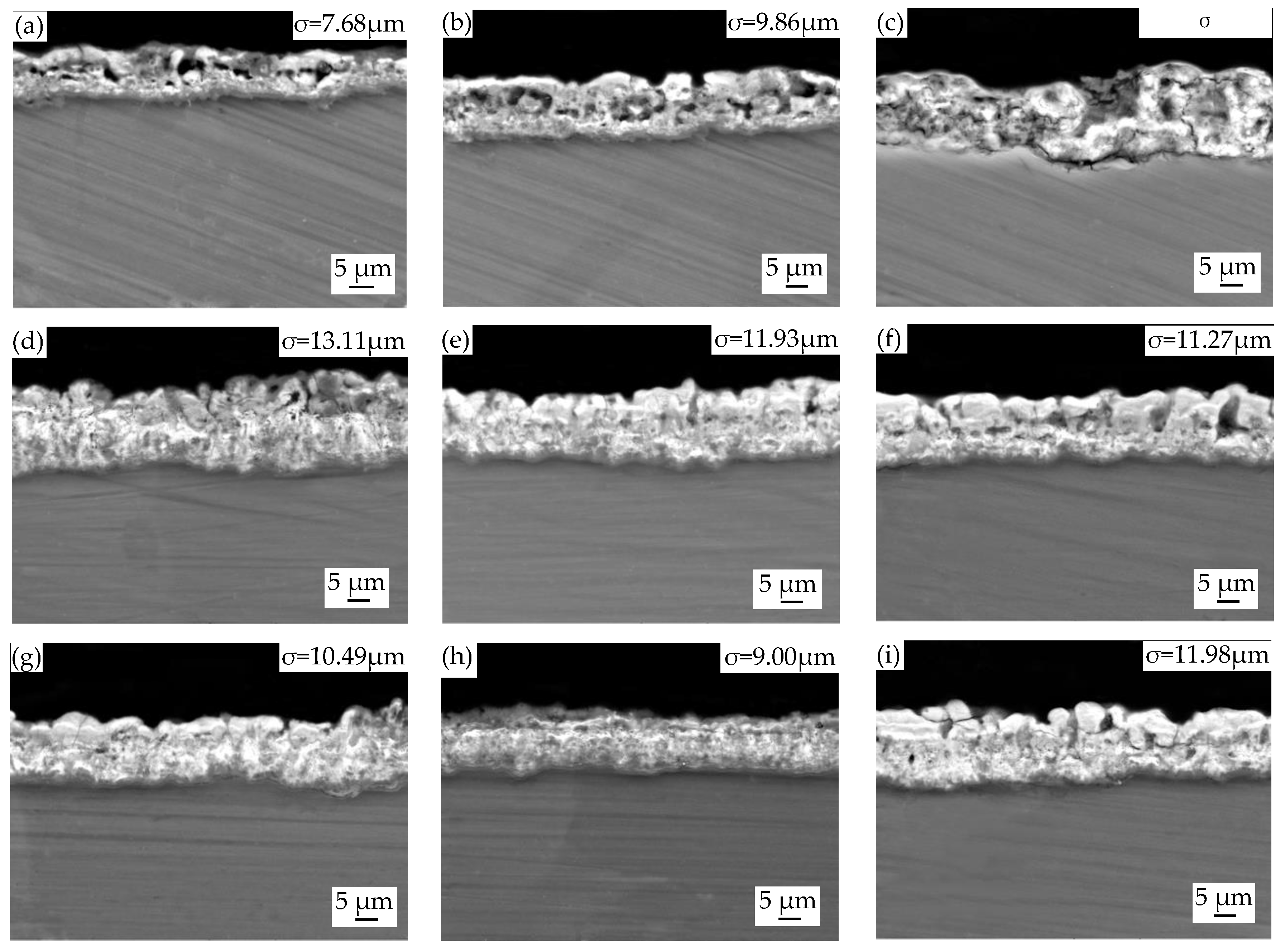
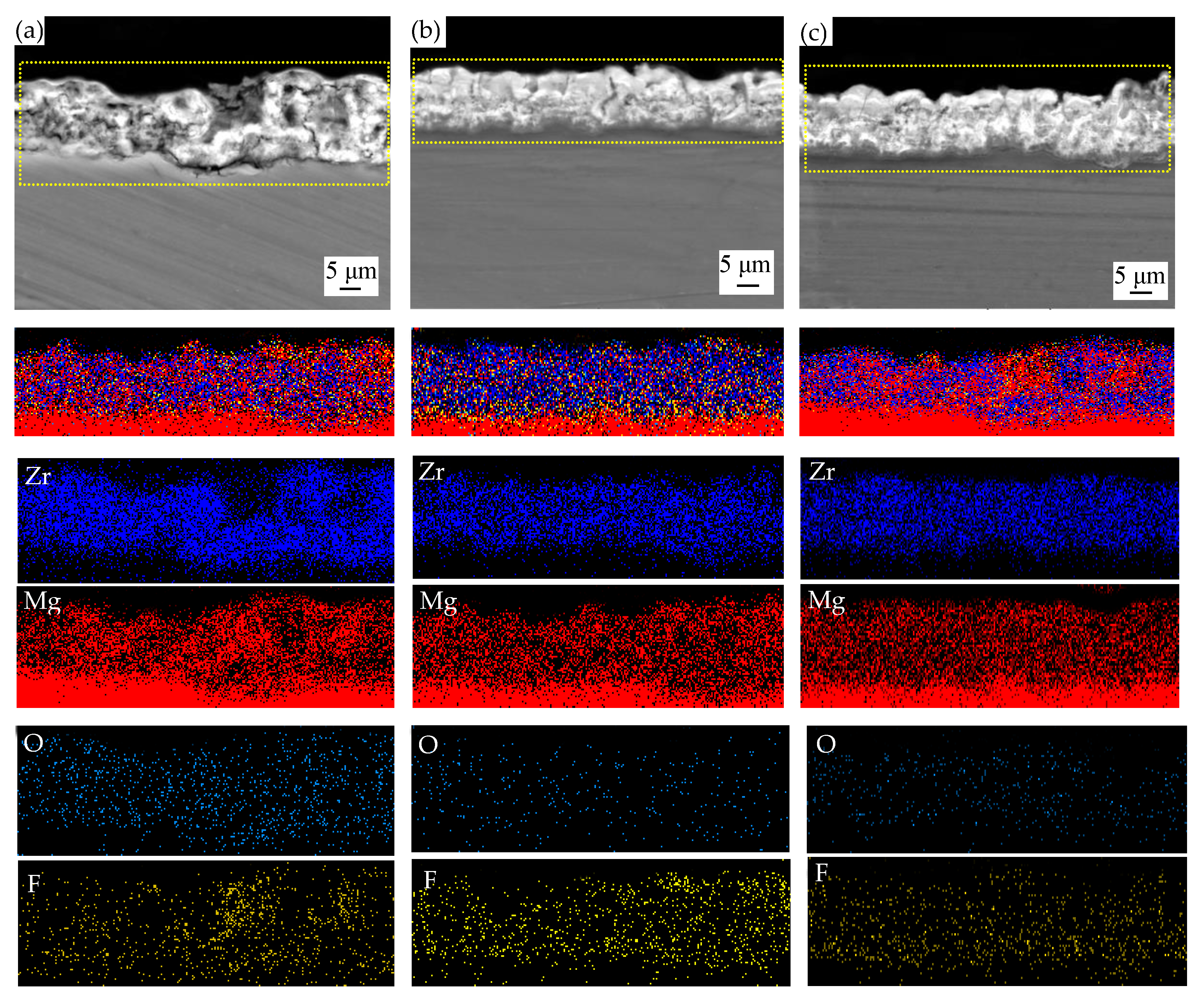
3.3. Cross-Sectional Morphologies and Elemental Composition of MAO Coatings
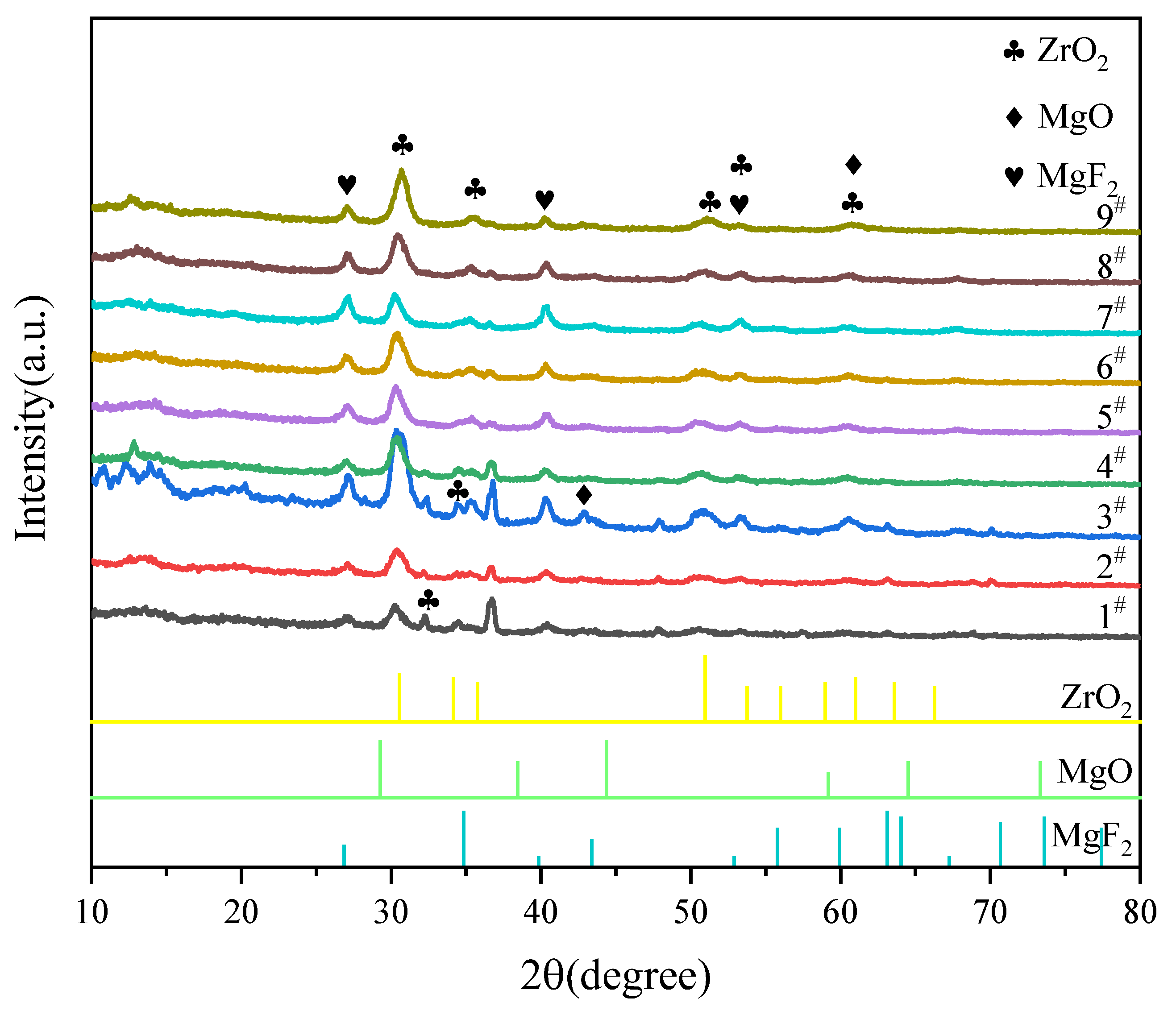
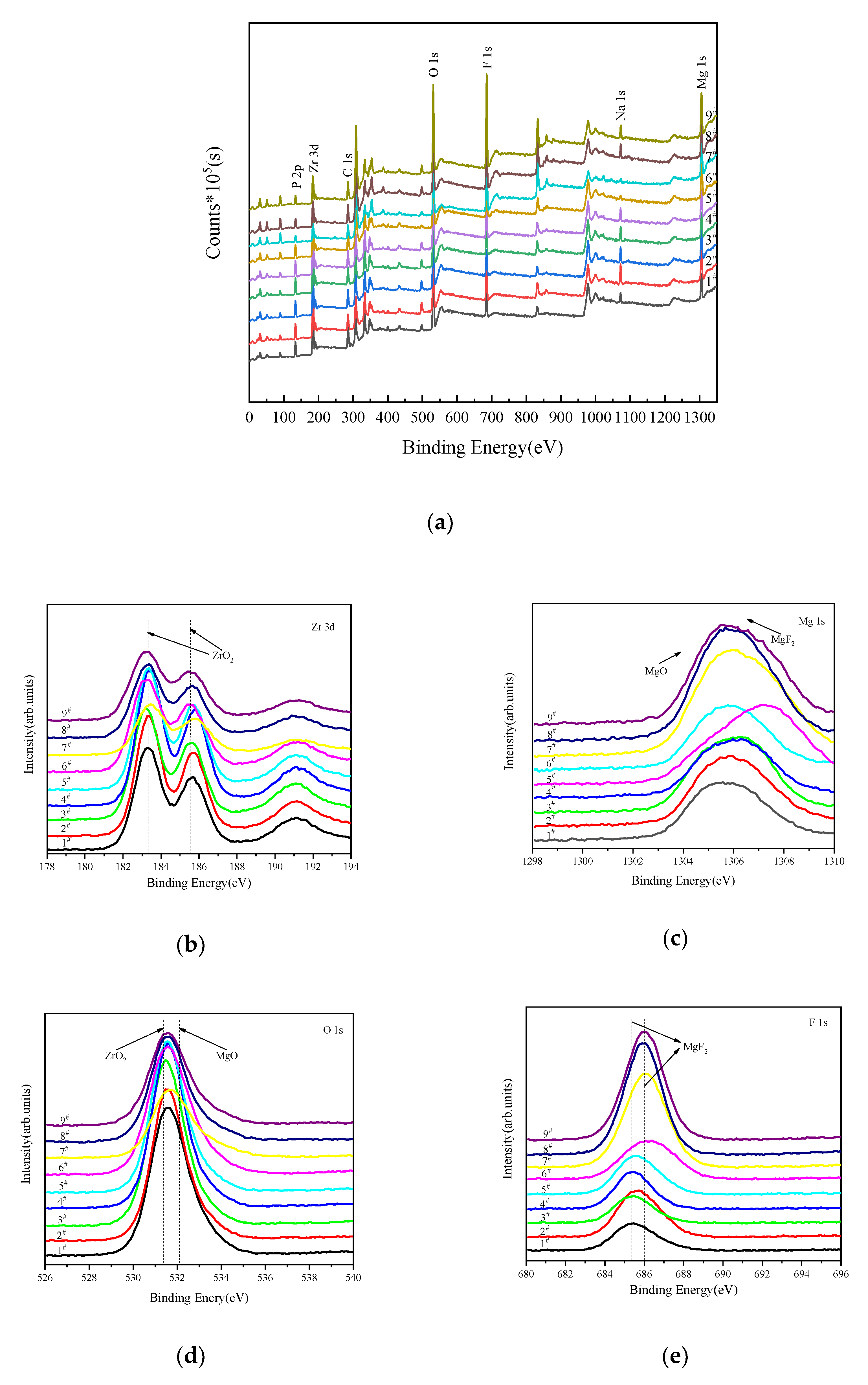
3.4. Phase and Chemical Composition of MAO Coatings
3.5. Electrochemical Corrosion Test of MAO Coatings
4. Conclusions
- The best coating was obtained from the combination of A2B2C3, which means the parameter combination is 6 g/L (NH4)2ZrF6, voltage is 450 V, treating time is 15min.
- The MAO coatings on ZK61M magnesium alloy mainly consisted of ZrO2, MgO, MgF2 and amorphous phase Mg phosphate.
- NO.5# has the best corrosion property which corrosion current density is 4 magnitudes lower compared to the substrate. This may attribute to the higher content of zirconium, compacter structure, thicker coating, and the filling effect of MgF2 in the micropores.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atapour, M.; Blawert, C.; Zheludkevich, M.L. The wear characteristics of CeO2 containing nanocomposite coating made by aluminate-based PEO on AM 50 magnesium alloy. Surf. Coat. Technol. 2019, 357, 626–637. [Google Scholar] [CrossRef]
- Tang, H.; Han, Y.; Wu, T.; Tao, W.; Jian, X.; Wu, Y.; Xu, F. Synthesis and properties of hydroxyapatite-containing coating on AZ31 magnesium alloy by micro-arc oxidation. Appl. Surf. Sci. 2017, 400, 391–404. [Google Scholar] [CrossRef]
- Han, H.; Wang, R.; Wu, Y.; Zhang, X.; Wang, D.; Yang, Z.; Su, Y.; Shen, D.; Nash, P. An investigation of plasma electrolytic oxidation coatings on crevice surface of AZ31 magnesium alloy. J. Alloys Compd. 2019, 811, 152010. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R. Preparation and corrosion resistance of a nanocomposite plasma electrolytic oxidation coating on Mg-1%Ca alloy formed in aluminate electrolyte containing titania nano-additives. J. Alloys Compd. 2016, 688, 841–857. [Google Scholar] [CrossRef]
- Ma, X.; Blawert, C.; Höche, D.; Kainer, K.U.; Zheludkevich, M.L. A model describing the growth of a PEO coating on AM50 Mg alloy under constant voltage mode. Electrochim. Acta 2017, 251, 461–474. [Google Scholar] [CrossRef]
- Lv, X.; Zou, G.; Ling, K.; Yang, W.; Mo, Q.; Li, W. Tribological properties of MAO/MoS2 self-lubricating composite coating by microarc oxidation and hydrothermal reaction. Surf. Coat. Technol. 2021, 406, 126630. [Google Scholar] [CrossRef]
- Sobrinho, P.H.; Savguira, Y.; Ni, Q.; Thorpe, S.J. Statistical analysis of the voltage-time response produced during PEO coating of AZ31B magnesium alloy. Surf. Coat. Technol. 2017, 315, 530–545. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Pardo, A.; Matykina, E. Corrosion and wear of PEO coated AZ91/SiC composites. Surf. Coat. Technol. 2017, 309, 1023–1032. [Google Scholar] [CrossRef]
- Chen, W.-w.; Wang, Z.-x.; Sun, L.; Lu, S. Research of growth mechanism of ceramic coatings fabricated by micro-arc oxidation on magnesium alloys at high current mode. J. Magnes. Alloy. 2015, 3, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.J.; Song, R.G.; Li, H.X.; Xiang, N. Effect of Various Additives on Performance of Plasma Electrolytic Oxidation Coatings Formed on AZ31 Magnesium Alloy in the Phosphate Electrolytes. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2018, 33, 703–709. [Google Scholar] [CrossRef]
- Tang, M.Q.; Shao, Y.F.; Feng, Z.Q.; Wang, W.; Li, G.; Yan, Z.W.; Zhang, R.Z. Self-sealing Microarc Oxidation Coating Mainly Containing ZrO2 and Nano Mg2Zr5O12 on AZ91D Mg Alloy. Int. J. Electrochem. Sci. 2020, 15, 12447–12461. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Q.; Wei, B.; Yu, B.; He, J.; Li, D. Study on the microstructure and corrosion resistance of ZrO2-containing ceramic coatings formed on magnesium alloy by plasma electrolytic oxidation. J. Alloys Compd. 2009, 474, 551–556. [Google Scholar] [CrossRef]
- Yao, Z.; Xu, Y.; Liu, Y.; Wang, D.; Jiang, Z.; Wang, F. Structure and corrosion resistance of ZrO2 ceramic coatings on AZ91D Mg alloys by plasma electrolytic oxidation. J. Alloys Compd. 2011, 509, 8469–8474. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Imshinetskiy, I.M.; Nadaraia, K.V.; Gnedenkov, A.S.; Sinebryukhov, S.L.; Ustinov, A.Y.; Samokhin, A.V.; Gnedenkov, S.V. Influence of ZrO2/SiO2 nanomaterial incorporation on the properties of PEO layers on Mg-Mn-Ce alloy. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Wang, S.-y.; Si, N.-c.; Xia, Y.-p.; Liu, L. Influence of nano-SiC on microstructure and property of MAO coating formed on AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1926–1934. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, J.; Liu, G.; Wei, H.; Li, D.; Yao, Z.; Jiang, Z. Effects of electric parameters on structure and thermal control property of PEO ceramic coatings on Ti alloys. Surf. Coat. Technol. 2016, 307, 1284–1290. [Google Scholar] [CrossRef]
- Xu, C.F.; Yan, X.Y.; Yang, H.W.; Yan, H. Effect of Voltage on the Microstructure and Corrosion Properties of MAO Coatings on Biodegradable ZK60 Mg Alloys. Int. J. Electrochem. Sci. 2018, 13, 3555–3565. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Zhang, J.-W.; Ye, F.; Lv, W.-G.; Lu, S.; Sun, L.; Jiang, X.-Z. Properties of Micro-Arc Oxidation Coating Fabricated on Magnesium Under Two Steps Current-Decreasing Mode. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Choi, D. Investigation of ZrO2 nanoparticles concentration and processing time effect on the localized PEO coatings formed on AZ91 alloy. J. Magnes. Alloy. 2019, 7, 555–565. [Google Scholar] [CrossRef]
- Chen, J.; Lin, W.; Liang, S.; Zou, L.; Wang, C.; Wang, B.; Yan, M.; Cui, X. Effect of alloy cations on corrosion resistance of LDH/MAO coating on magnesium alloy. Appl. Surf. Sci. 2019, 463, 535–544. [Google Scholar] [CrossRef]
- Mohedano, M.; Arrabal, R.; Mingo, B.; Pardo, A.; Matykina, E. Role of particle type and concentration on characteristics of PEO coatings on AM50 magnesium alloy. Surf. Coat. Technol. 2018, 334, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.J.; Guo, Y.Q.; Xiang, N.; Xiong, Y.; Hu, Q.; Song, R.G. A study on microstructure and corrosion resistance of ZrO2-containing PEO coatings formed on AZ31 Mg alloy in phosphate-based electrolyte. Appl. Surf. Sci. 2015, 357, 1463–1471. [Google Scholar] [CrossRef]
- Qiu, Z.Z.; Wang, R.; Zhang, Y.S.; Qu, Y.F.; Wu, X.H. Study of coating growth behavior during the plasma electrolytic oxidation of magnesium alloy ZK60. J. Mater. Eng. Perform. 2015, 24, 1483–1491. [Google Scholar] [CrossRef]
- Keyvani, A.; Zamani, M.; Bahamirian, M.; Nikoomanzari, E.; Fattah-alhosseini, A.; Sina, H. Role of incorporation of ZnO nanoparticles on corrosion behavior of ceramic coatings developed on AZ31 magnesium alloy by plasma electrolytic oxidation technique. Surf. Interfaces 2021, 22, 17. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.; Tian, J.; Liu, H.; Zhou, J.; Xu, T. Effect of potassium fluoride in electrolytic solution on the structure and properties of microarc oxidation coatings on magnesium alloy. Appl. Surf. Sci. 2005, 252, 345–351. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Piotrowski, K.; Bajorek, A.; Mlynarek, K.; Babilas, R.; Simka, W. Surface modification of biomedical MgCa4.5 and MgCa4.5Gd0.5 alloys by micro-arc oxidation. Materials 2021, 14, 1360. [Google Scholar] [CrossRef]
- Cui, X.-J.; Liu, C.-H.; Yang, R.-S.; Li, M.-T.; Lin, X.-Z. Self-sealing micro-arc oxidation coating on AZ91D Mg alloy and its formation mechanism. Surf. Coat. Technol. 2015, 269, 228–237. [Google Scholar] [CrossRef]
- Simka, W.; Sowa, M.; Socha, R.P.; Maciej, A.; Michalska, J. Anodic oxidation of zirconium in silicate solutions. Electrochim. Acta 2013, 104, 518–525. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Negrea, D.; Andrei, V.; Coaca, E.; Mihailescu, C.N.; Lungu, C.P. Characterization of Al2O3/ZrO2 composite coatings deposited on Zr-2.5Nb alloy by plasma electrolytic oxidation. Appl. Surf. Sci. 2018, 451, 169–179. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Q.; He, J.; Wei, B. Preparation and properties of composite ceramic coating containing Al2O3–ZrO2–Y2O3 on AZ91D magnesium alloy by plasma electrolytic oxidation. Curr. Appl. Phys. 2009, 9, 1341–1346. [Google Scholar] [CrossRef]
- Cao, H.; Huo, W.; Ma, S.; Zhang, Y.; Zhou, L. Microstructure and corrosion behavior of composite coating on pure Mg acquired by sliding friction treatment and micro-arc oxidation. Materials 2018, 11, 1232. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Shin, K.R.; Namgung, S.; Yoo, B.; Shin, D.H. Electrochemical response of ZrO2-incorporated oxide layer on AZ91 Mg alloy processed by plasma electrolytic oxidation. Surf. Coat. Technol. 2011, 205, 3779–3784. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Qi, Y.; Peng, Z.; Ye, Y.; Liang, J. Corrosion and tribocorrosion resistance of MAO-based composite coating on AZ31 magnesium alloy. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, W.; Liu, D.; Gao, W.; Chen, J. Preparation and corrosion behavior in marine environment of MAO coatings on magnesium alloy. Materials 2020, 13, 345. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Z.; Xu, A.; Liu, X. Understanding pitting corrosion behavior of AZ91 alloy and its MAO coating in 3.5% NaCl solution by cyclic potentiodynamic polarization. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]



| Factors | A | B | C |
|---|---|---|---|
| Levels | (NH4)2ZrF6 Concentration (g/L) | Voltage (V) | Treating Time (min) |
| 1 | 3 | 400 | 5 |
| 2 | 6 | 450 | 10 |
| 3 | 9 | 500 | 15 |
| Experimental | A | B | C | Icorr (mA/cm2) | Ra (μm) | Porosity (%) |
|---|---|---|---|---|---|---|
| 1# | 3 | 400 | 5 | 0.2604 | 0.351 | 10.957 |
| 2# | 3 | 450 | 10 | 1.1740 | 0.420 | 10.685 |
| 3# | 3 | 500 | 15 | 0.6487 | 0.635 | 7.076 |
| 4# | 6 | 400 | 10 | 1.025 | 0.370 | 8.471 |
| 5# | 6 | 450 | 15 | 0.03247 | 0.218 | 15.196 |
| 6# | 6 | 500 | 5 | 0.3394 | 0.481 | 17.602 |
| 7# | 9 | 400 | 15 | 0.4722 | 0.790 | 13.833 |
| 8# | 9 | 450 | 5 | 0.7529 | 0.522 | 16.323 |
| 9# | 9 | 500 | 10 | 0.05712 | 0.549 | 10.09 |
| k1 | 0.694 (0.469) (9.573) | 0.586 (0.504) (11.087) | 0.451 (0.451) (14.961) | |||
| k2 | 0.466 (0.356) (13.756) | 0.653 (0.387) (14.068) | 0.752 (0.446) (12.749) | |||
| k3 | 0.427 (0.620) (16.415) | 0.348 (0.555) (14.589) | 0.384 (0.548) (12.035) | |||
| R | 0.267 (0.264) (6.842) | 0.305 (0.168) (3.502) | 0.368 (0.102) (2.926) |
| Coating | Zr (at%) | Mg (at%) | O (at%) | F (at%) | Na (at%) | P (at%) | Zn (at%) |
|---|---|---|---|---|---|---|---|
| 1# | 13.27 | 30.43 | 23.91 | 15.81 | 0.87 | 14.90 | 0.81 |
| 2# | 11.27 | 32.07 | 21.88 | 18.61 | 1.00 | 13.96 | 1.22 |
| 3# | 11.54 | 32.54 | 19.48 | 23.41 | 0.98 | 11.02 | 1.03 |
| 4# | 15.72 | 26.41 | 18.50 | 28.44 | 1.35 | 8.53 | 1.05 |
| 5# | 16.26 | 27.36 | 20.16 | 24.07 | 1.15 | 10.10 | 0.90 |
| 6# | 15.87 | 29.18 | 19.86 | 25.12 | 1.18 | 8.23 | 0.57 |
| 7# | 13.48 | 30.61 | 14.11 | 33.58 | 0.88 | 6.43 | 0.91 |
| 8# | 14.07 | 29.22 | 16.49 | 32.57 | 0.91 | 5.88 | 0.87 |
| 9# | 19.86 | 26.72 | 18.67 | 28.02 | 1.08 | 4.93 | 0.73 |
| Sample | Zr 3d | Mg 1s | O 1s | F 1s |
|---|---|---|---|---|
| Binding Energy (eV) | 185.5 | 1306.5 | 531.3 | 685.3 |
| Compounds | ZrO2 | MgF2 | ZrO2 | MgF2 |
| Binding Energy (eV) | 183.3 | 1303.9 | 532.1 | 686.1 |
| Compounds | ZrO2 | MgO | MgO | MgF2 |
| Coating | Ecorr (V) | Icorr (A/cm2) | ba (mV/dec) | −bc (mV/dec) | Rp (kΩ·cm2) |
|---|---|---|---|---|---|
| ZK61M | −1.594 | 1.526 × 10−4 | 87.29 | 176.68 | 1.662 × 102 |
| 1# | −1.443 | 2.604 × 10−7 | 52.84 | 150.08 | 6.517 × 104 |
| 2# | −1.498 | 1.174 × 10−6 | 123.58 | 238.52 | 3.010 × 104 |
| 3# | −1.491 | 6.487 × 10−7 | 179.34 | 308.22 | 7.588 × 104 |
| 4# | −1.433 | 1.025 × 10−6 | 37.92 | 211.78 | 1.362 × 104 |
| 5# | −1.458 | 3.247 × 10−8 | 84.21 | 291.75 | 8.739 × 105 |
| 6# | −1.481 | 3.394 × 10−7 | 94.97 | 263.97 | 8.935 × 104 |
| 7# | −1.475 | 4.722 × 10−7 | 98.87 | 239.63 | 6.429 × 104 |
| 8# | −1.465 | 7.529 × 10−7 | 44.00 | 329.77 | 2.239 × 104 |
| 9# | −1.476 | 5.712 × 10−8 | 93.55 | 247.76 | 5.162 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, J.; Li, H.; Li, Z.; Chen, Y.; Wang, Y.; Geng, J. Effect of (NH4)2ZrF6, Voltage and Treating Time on Corrosion Resistance of Micro-Arc Oxidation Coatings Applied on ZK61M Magnesium Alloys. Materials 2021, 14, 7410. https://doi.org/10.3390/ma14237410
Yong J, Li H, Li Z, Chen Y, Wang Y, Geng J. Effect of (NH4)2ZrF6, Voltage and Treating Time on Corrosion Resistance of Micro-Arc Oxidation Coatings Applied on ZK61M Magnesium Alloys. Materials. 2021; 14(23):7410. https://doi.org/10.3390/ma14237410
Chicago/Turabian StyleYong, Jiahui, Hongzhan Li, Zhengxian Li, Yongnan Chen, Yifei Wang, and Juanjuan Geng. 2021. "Effect of (NH4)2ZrF6, Voltage and Treating Time on Corrosion Resistance of Micro-Arc Oxidation Coatings Applied on ZK61M Magnesium Alloys" Materials 14, no. 23: 7410. https://doi.org/10.3390/ma14237410





