In Situ Synthesis of (M:Nb,Ta)C/Ni35 Composite Coating Cladded on 40Cr Steel
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.2. Microstructural Characterization
2.3. Mechanical Tests
3. Results and Discussion
3.1. Phase Composition of Composite Coating
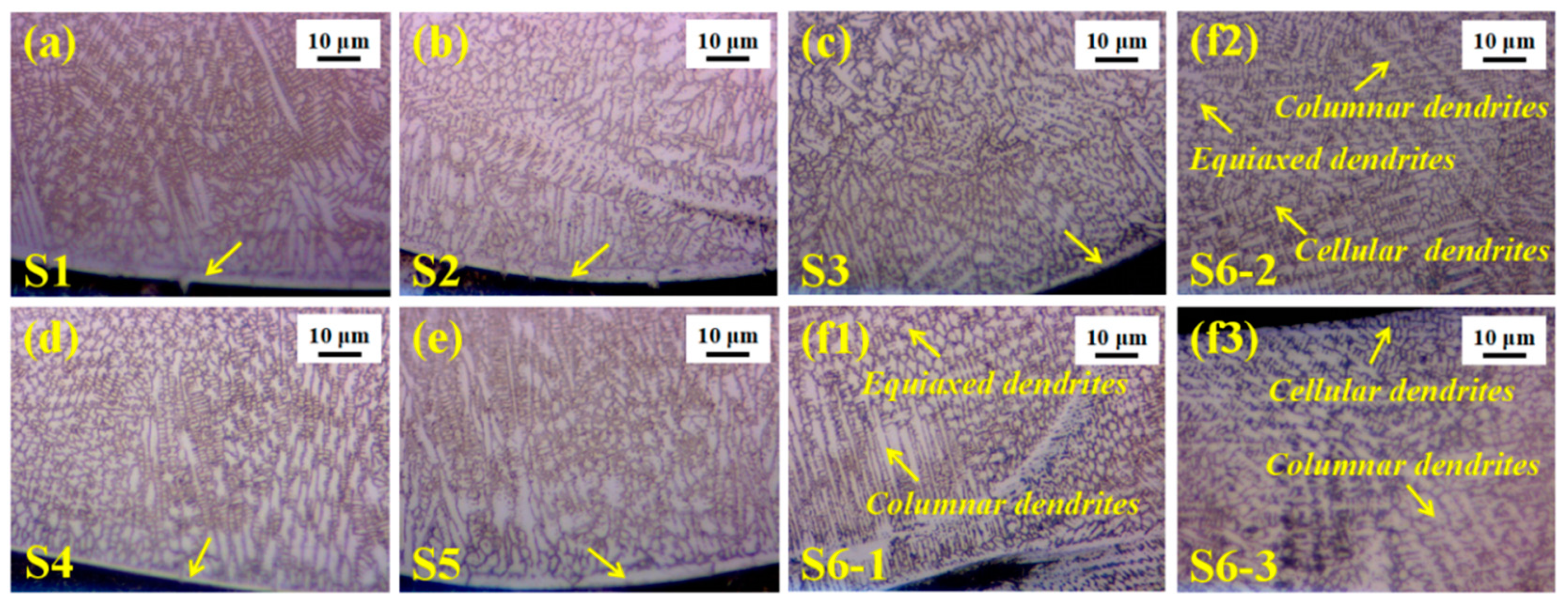
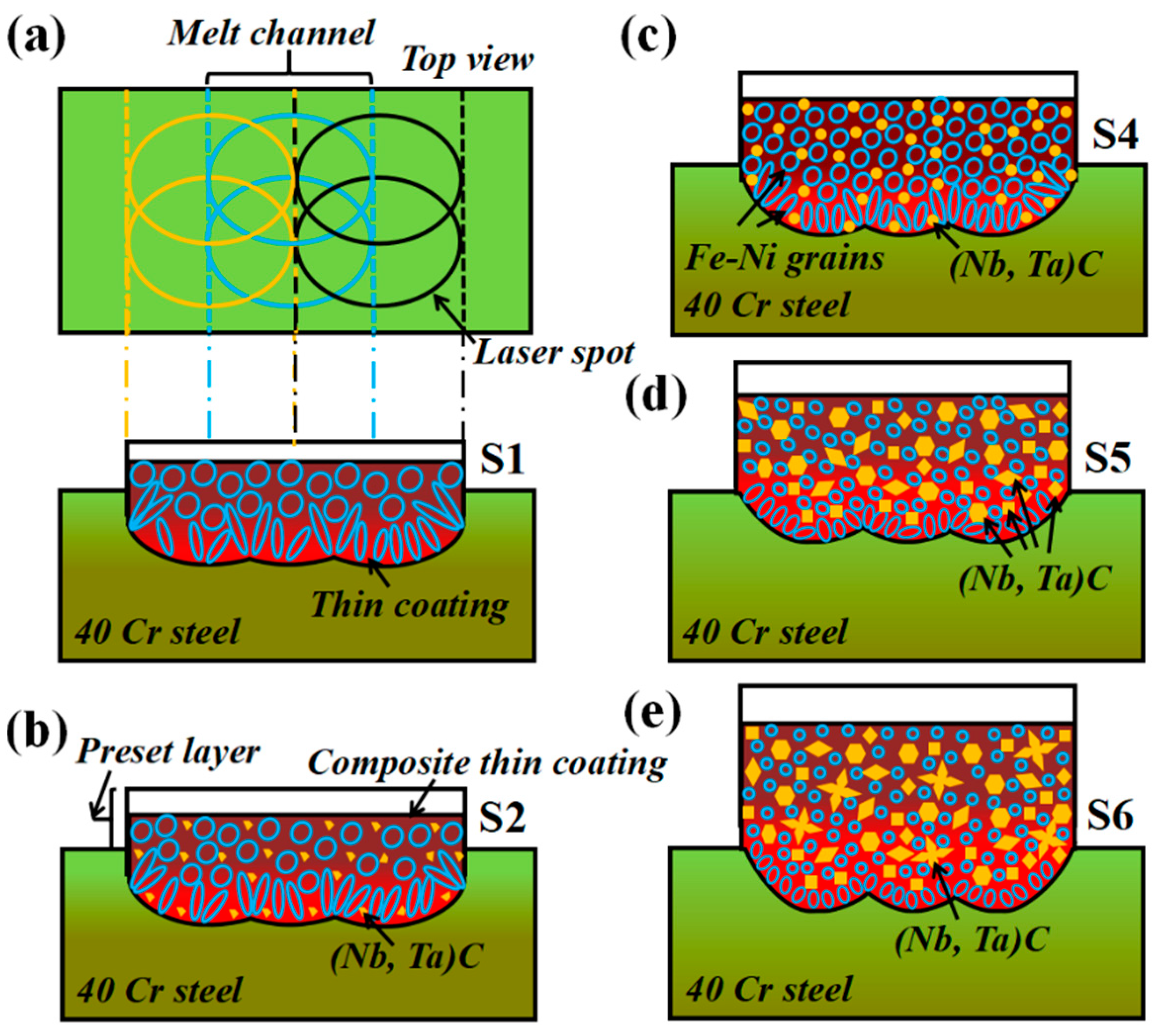
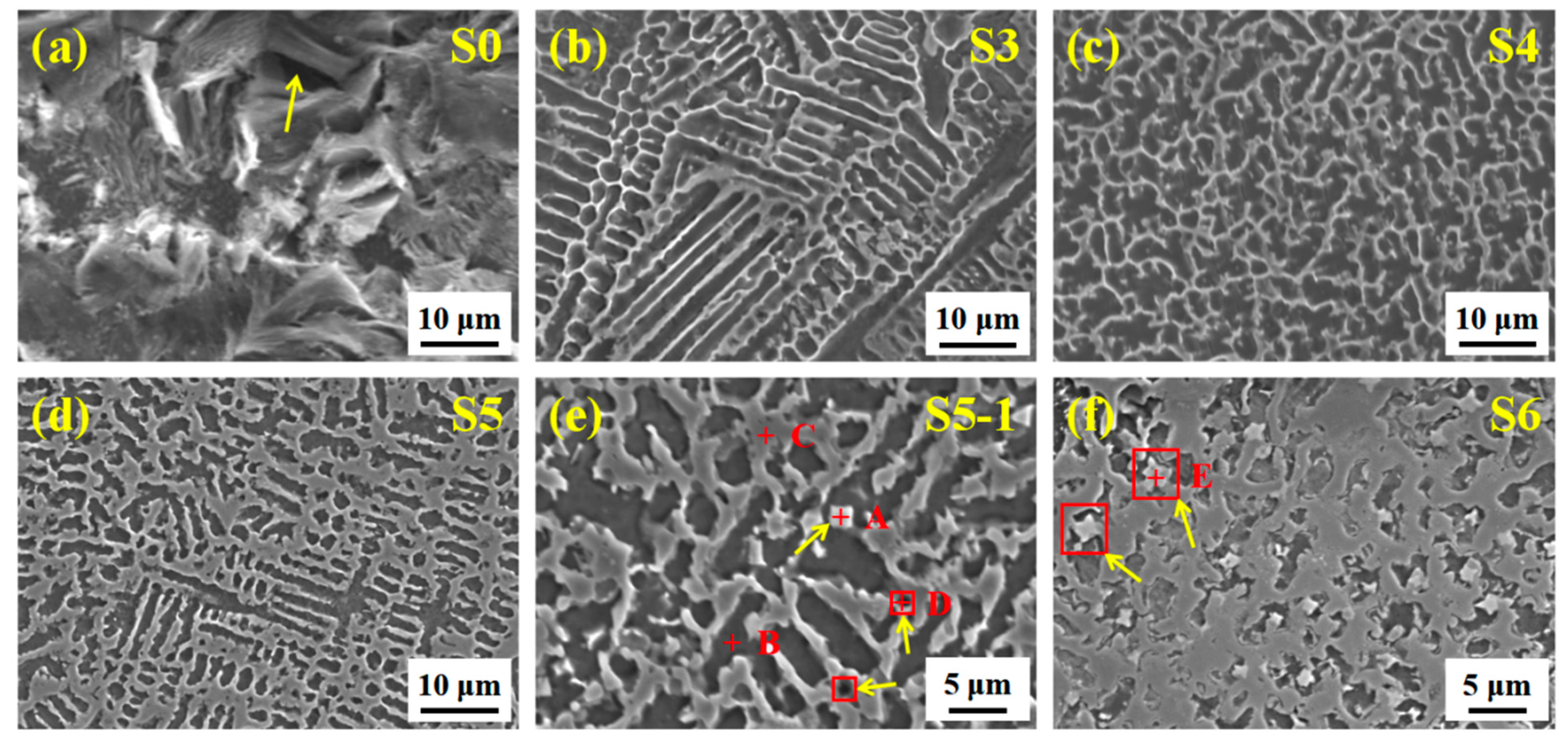
3.2. Microstructure Evolution of Composite Coating
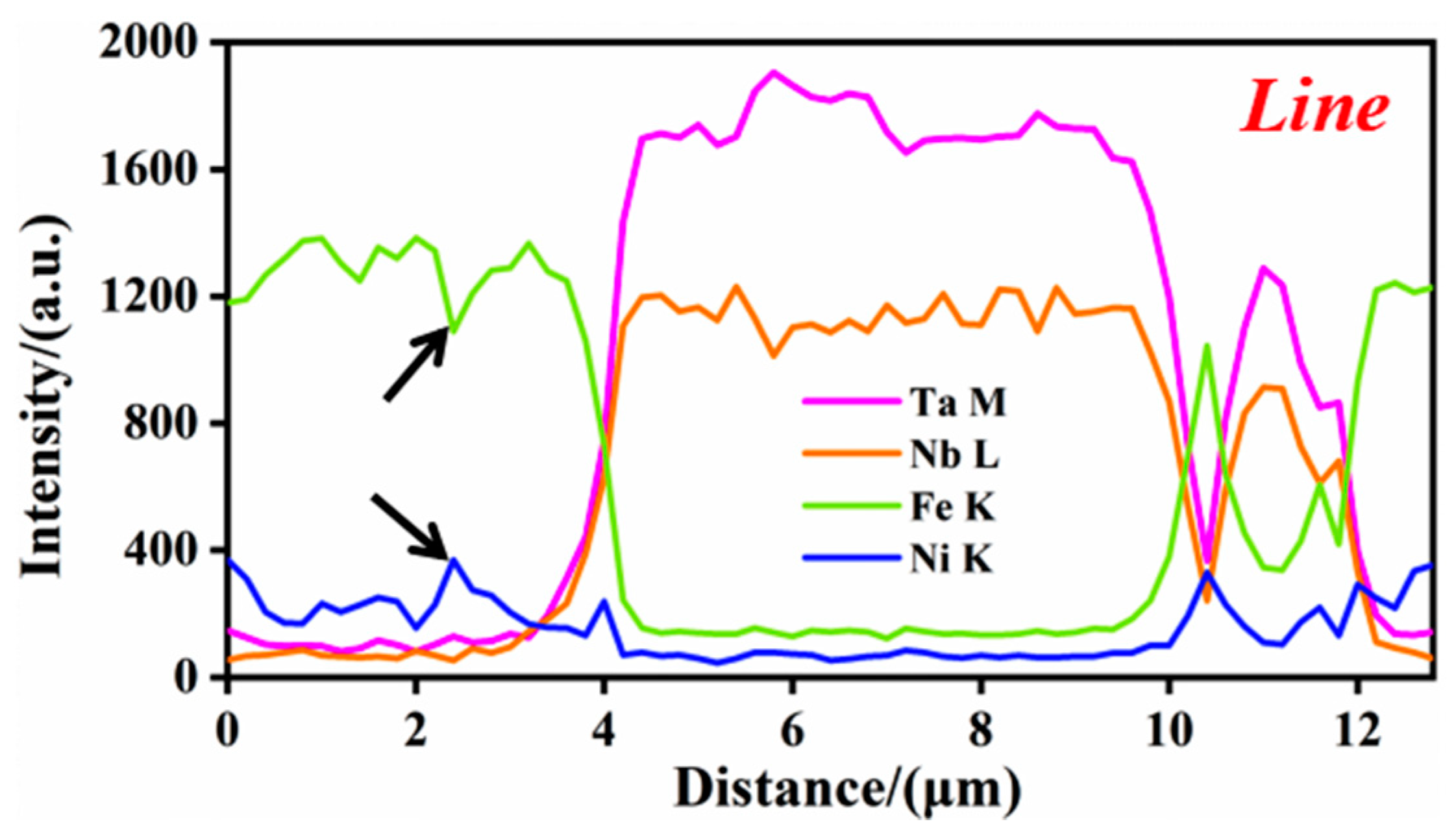
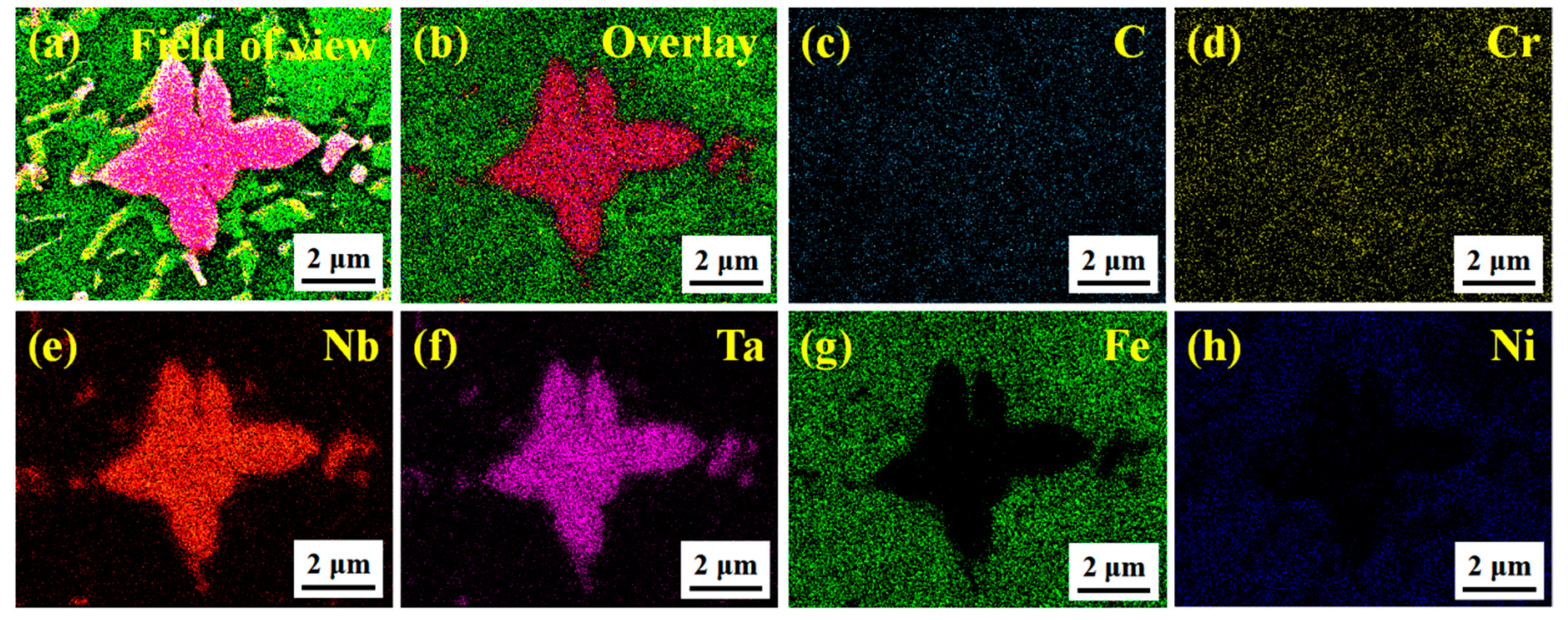
3.3. Structural Characteristics of (M:Nb,Ta)C
3.4. Formation Mechanism and Morphological Change of (M:Nb,Ta)C
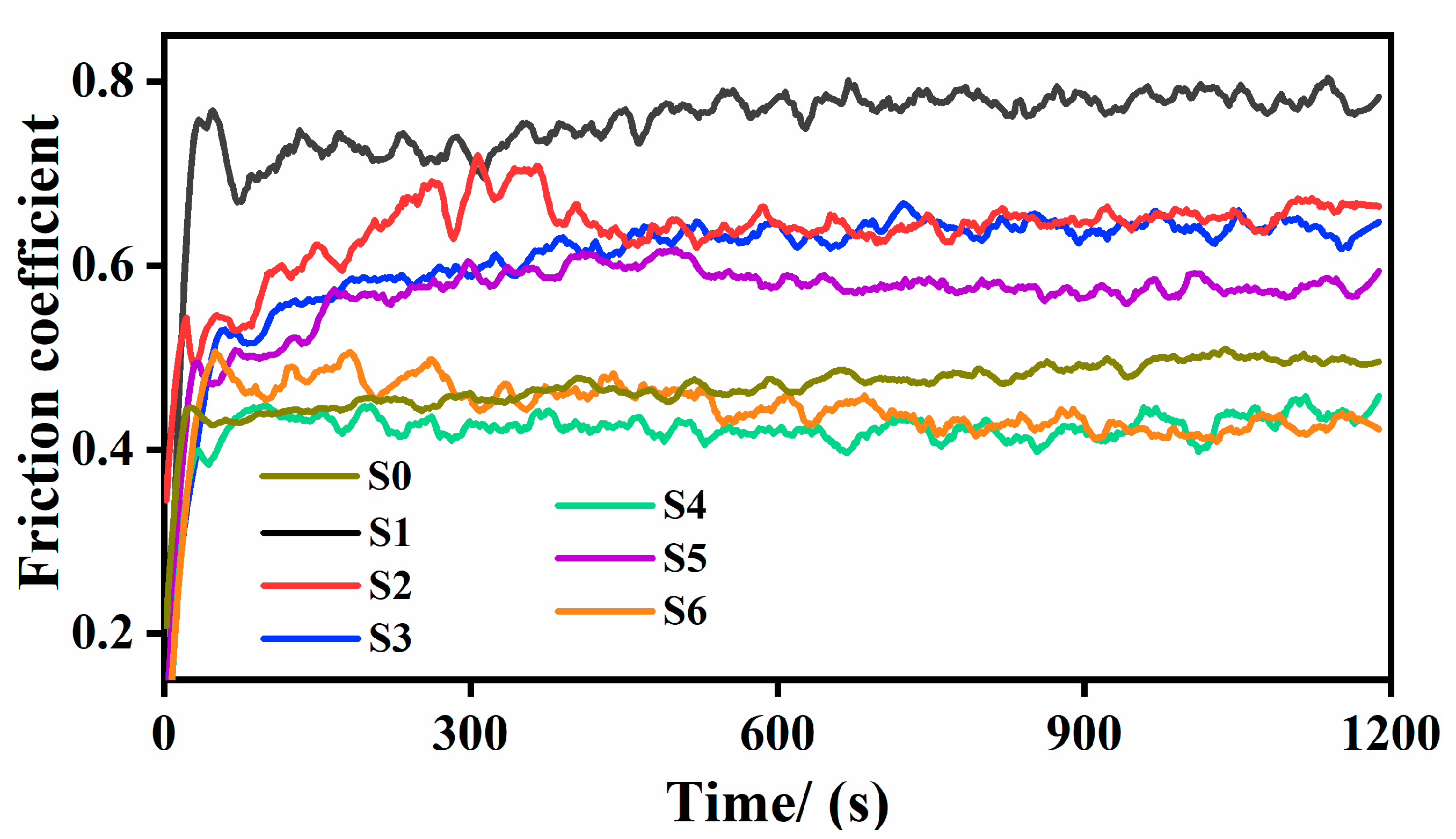
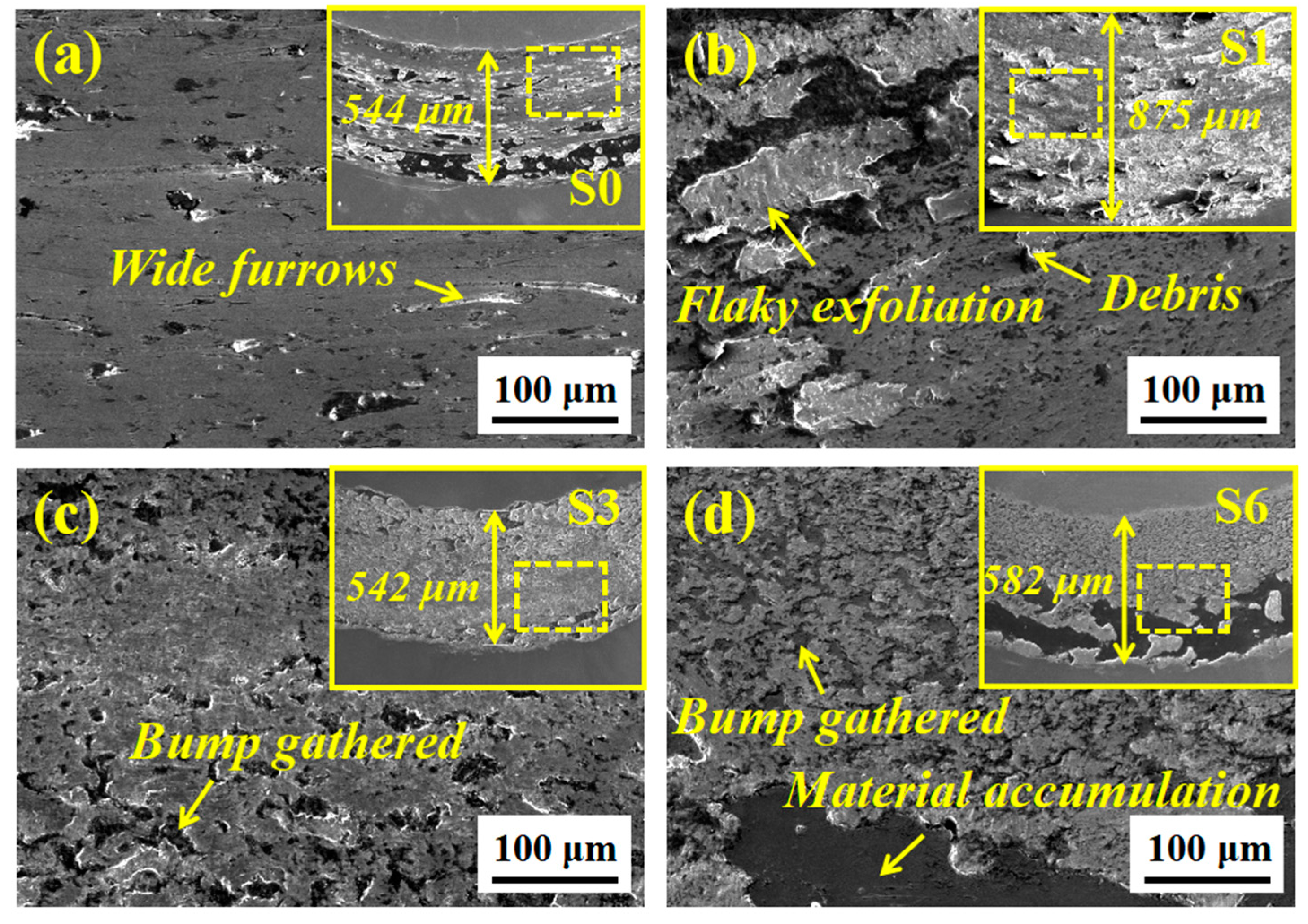
3.5. Friction and Wear Test
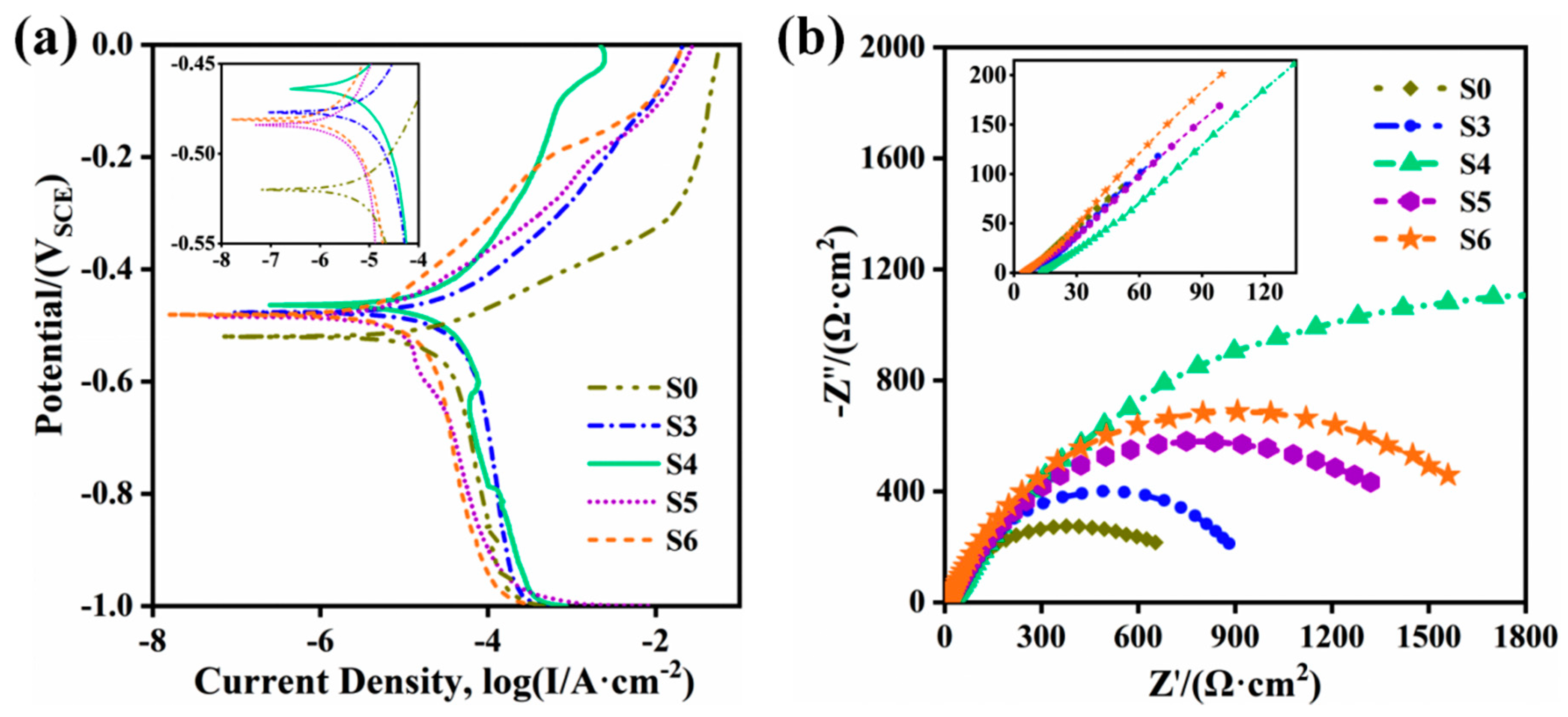
3.6. Electrochemical Corrosion Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.Y.; Guo, L.T.; Liu, X.M.; Feng, W.; Li, B.E.; Tao, X.Y.; Qiang, Y.H. Effect of laser parameters on the microstructure of bonding porcelain layer fused on titanium. Opt. Mater. 2017, 71, 136–140. [Google Scholar] [CrossRef]
- Pang, X.M.; Zhou, F.L.; Li, B.; Jiang, J.X.; Zhou, J.X. Optical thermostability and weatherability of TiN/TiC-Ni/Mo cermet-based spectral selective absorbing coating by laser cladding. Opt. Mater. 2021, 117, 111195. [Google Scholar] [CrossRef]
- Zhang, C.S.; Shen, X.H.; Wang, J.T.; Xu, C.H.; He, J.Q.; Bai, X.L. Improving surface properties of Fe-based laser cladding coating deposited on a carbon steel by heat assisted ultrasonic burnishing. J. Mater. Res. Technol. 2021, 12, 100–116. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.F.; Tian, L.H.; Liu, Z.G.; Wang, J.X.; Zhang, J.X. Properties and formation mechanism of cladding layer on high-strength low-alloy steel subjected to ultrasonic impact treatment with titanium alloy pin. Surf. Coat. Technol. 2021, 418, 127256. [Google Scholar] [CrossRef]
- Liu, J.C.; Li, L.J. Effects of powder concentration distribution on fabrication of thin-wall parts in coaxial laser cladding. Opt. Laser Technol. 2005, 37, 287–292. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hagino, H. Effects of the ambient oxygen concentration on WC-12Co cermet coatings fabricated by laser cladding. Opt. Laser Technol. 2021, 139, 106922. [Google Scholar] [CrossRef]
- Jiang, J.X.; Pang, X.M.; Zhou, J.X.; Li, B.; Zhou, F.L. Optical performance and corrosion resistance of TiN/Ni multiphase cermet by laser cladding. Opt. Laser Technol. 2021, 143, 107308. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y.; Zou, Z.D.; Shi, C.W. Effects of chromium addition on microstructure and properties of TiC-VC reinforced Fe-based laser cladding coatings. J. Alloys Compd. 2014, 614, 107–112. [Google Scholar] [CrossRef]
- Nam, S.; Kim, C.; Kim, Y.M. Microstructure evolution of in-situ (Ti,W)C-Al2O3 particle-reinforced alloy fabricated by gas tungsten arc cladding. Surf. Coat. Technol. 2018, 354, 1–9. [Google Scholar] [CrossRef]
- Wang, X.H.; Zou, Z.D.; Qu, S.Y. Microstructure of Fe-Based Alloy Hardfacing Coating Reinforced by TiC-VC Particles. J. Iron Steel Res. Int. 2006, 13, 51–55. [Google Scholar] [CrossRef]
- Gu, Y.F.; Liu, J.X.; Wang, Y.; Xue, J.X.; Wang, X.G.; Zhang, H.B.; Xu, F.F.; Zhang, G.J. Corrosion behavior of TiC-SiC composite ceramics in molten FLiNaK salt. J. Eur. Ceram. Soc. 2017, 37, 2575–2582. [Google Scholar] [CrossRef]
- Jiang, G.Q.; Cui, C.Y.; Chen, L.; Zhao, K.; Cui, X.G. Effects of powder size and preset thickness on microstructure and properties of Ni35 thin coating prepared by laser cladding. Appl. Opt. 2021, 60, 9535–9542. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G. X-ray Diffraction: A Practical Approach; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zhang, M.Y.; Li, M.; Wang, S.F.; Chi, J.; Ren, L.S.; Fang, M.; Zhou, C. Enhanced wear resistance and new insight into microstructure evolution of in-situ (Ti,Nb)C reinforced 316 L stainless steel matrix prepared via laser cladding. Opt. Lasers Eng. 2020, 128, 106043. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Wang, C.M.; Gong, Z.Q.; Wang, G.M.; Sun, H.F.; Yang, H.R. Effect of Ti on T15M composite coating fabricated by laser cladding technology. Surf. Coat. Technol. 2017, 325, 643–649. [Google Scholar] [CrossRef]
- Dong, G.; Yan, B.; Deng, Q.L.; Yu, T. Effect of niobium on the microstructure and wear resistance of nickel-based alloy coating by laser cladding. Rare Met. Mater. Eng. 2011, 40, 973–977. [Google Scholar]
- Fu, W.; Yong, Y.W.; Deng, Q.L.; Dong, G. Effect of in-situ precipitation of NbC by laser cladding on microstructure and properties of Ni25 cladding layer. In Proceedings of the 2015 Academic Annual Conference of Shanghai Laser Society, Shanghai, China, 16 December 2015. (In Chinese). [Google Scholar]
- Hu, G.X.; Cai, X.; Rong, Y.H. Fundamentals of Materials Science; Shanghai Jiaotong University Press: Shanghai, China, 2010. (In Chinese) [Google Scholar]
- Li, Q.; Zhang, D.W.; Lei, T.Q.; Chen, C.Z.; Chen, W.Z. Comparison of laser-clad and furnace-melted Ni-based alloy microstructures. Surf. Coat. Technol. 2001, 137, 122–135. [Google Scholar] [CrossRef]
- Zhang, D.W.; Lei, T.C.; Zhang, J.G.; Ouyang, J.H. The effects of heat treatment on microstructure and erosion properties of laser surface-clad Ni-base alloy. Surf. Coat. Technol. 1999, 115, 176–183. [Google Scholar] [CrossRef]
- Cao, Y.B.; Zhi, S.X.; Gao, Q.; Tian, X.T.; Geng, T.; Guan, X.; Qin, C. Formation behavior of in-situ NbC in Fe-based laser cladding coatings. Mater. Charact. 2016, 119, 159–165. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yan, H.; Zhang, P.L.; Guo, J.L.; Yu, Z.S.; Ringsberg, J.W. Improving surface resistance to wear and corrosion of nickel-aluminum bronze by laser-clad TaC/Co-based alloy composite coatings. Surf. Coat. Technol. 2021, 405, 126592. [Google Scholar] [CrossRef]





| No. | Nb:Ta:C (wt.%) | Nb–Ta–C (wt.%) | Ni35 (wt.%) |
|---|---|---|---|
| S1 | 0 | 0 | 100 |
| S2 | 1:1:2 | 3 | 97 |
| S3 | 1:1:2 | 6 | 94 |
| S4 | 1:1:2 | 9 | 91 |
| S5 | 1:1:2 | 12 | 88 |
| S6 | 1:1:2 | 15 | 85 |
| No. | Ecorr/V | Icorr/A | Rp/Ω·cm2 | Vcorr/A·cm−2 |
|---|---|---|---|---|
| S0 | −0.522 | 2.673 × 10−5 | 1010 | 0.932 × 10−3 |
| S3 | −0.474 | 3.527 × 10−5 | 1028 | 1.036 × 10−3 |
| S4 | −0.463 | 3.676 × 10−5 | 1289 | 0.722 × 10−3 |
| S5 | −0.486 | 1.039 × 10−5 | 3093 | 0.296 × 10−3 |
| S6 | −0.479 | 1.065 × 10−5 | 3559 | 0.286 × 10−3 |
| Area | Fe | Ni | Nb | Ta | C | Cr | O |
|---|---|---|---|---|---|---|---|
| A | 13.49 | 3.57 | 29.82 | 33.02 | 13.97 | 1.12 | 5.01 |
| B | 60.01 | 21.34 | 0.09 | 0.09 | 12.63 | 1.00 | 4.26 |
| C | 72.26 | 17.15 | 1.46 | 0.54 | 5.82 | 1.64 | 1.13 |
| D | 71.42 | 24.05 | 0 | 0 | 2.11 | 1.31 | 1.01 |
| E | 6.42 | 1.68 | 38.6 | 39.38 | 11.58 | 2.34 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.; Cui, C.; Chen, L.; Wu, Y.; Cui, X. In Situ Synthesis of (M:Nb,Ta)C/Ni35 Composite Coating Cladded on 40Cr Steel. Materials 2021, 14, 7437. https://doi.org/10.3390/ma14237437
Jiang G, Cui C, Chen L, Wu Y, Cui X. In Situ Synthesis of (M:Nb,Ta)C/Ni35 Composite Coating Cladded on 40Cr Steel. Materials. 2021; 14(23):7437. https://doi.org/10.3390/ma14237437
Chicago/Turabian StyleJiang, Gaoqiang, Chengyun Cui, Lu Chen, Yucheng Wu, and Xigui Cui. 2021. "In Situ Synthesis of (M:Nb,Ta)C/Ni35 Composite Coating Cladded on 40Cr Steel" Materials 14, no. 23: 7437. https://doi.org/10.3390/ma14237437





