Probing the Use of Silane-Grafted Fumed Silica Nanoparticles to Produce Stable Transformer Oil-Based Nanofluids
Abstract
:1. Introduction
2. Experimental Methods
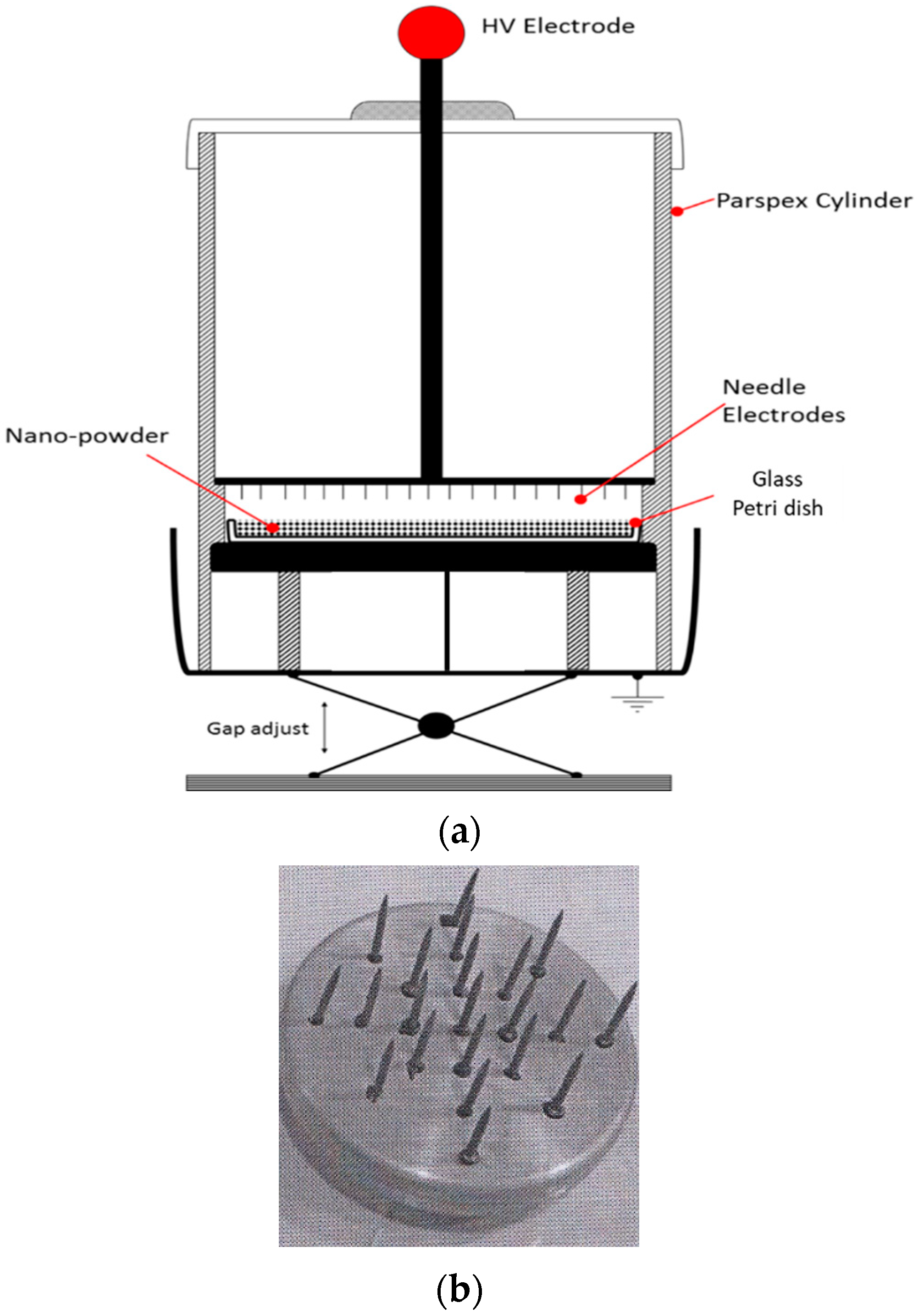
2.1. Plasma Reactor
2.2. Characterization of Nanoparticles
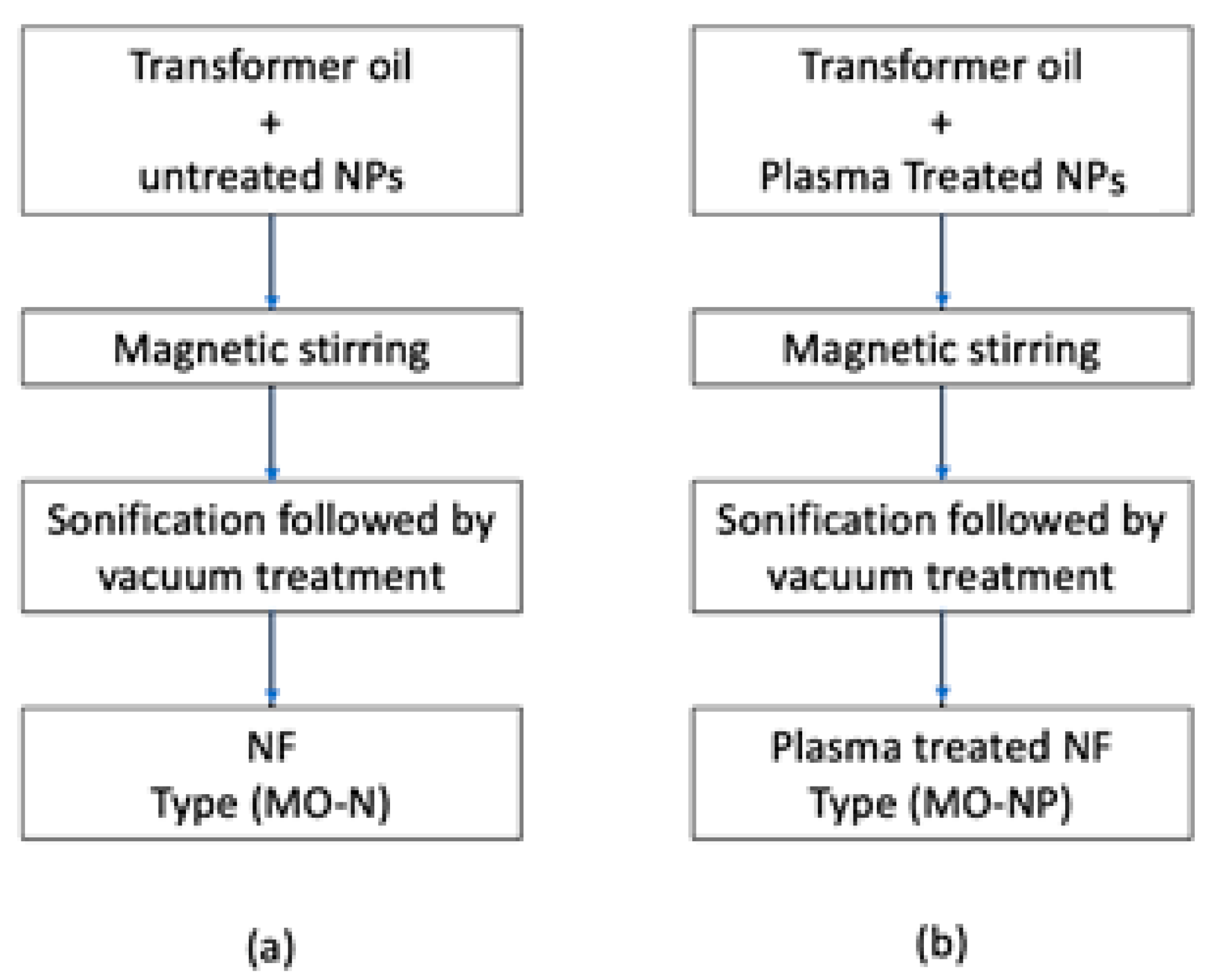
2.3. Nanofluid Preparation
2.4. Measurement of Breakdown Voltage
2.5. Measurement of Dielectric Constant () and Dissipation Factor (tan δ)
3. Results and Discussion
3.1. Surface Analysis of Nanoparticles
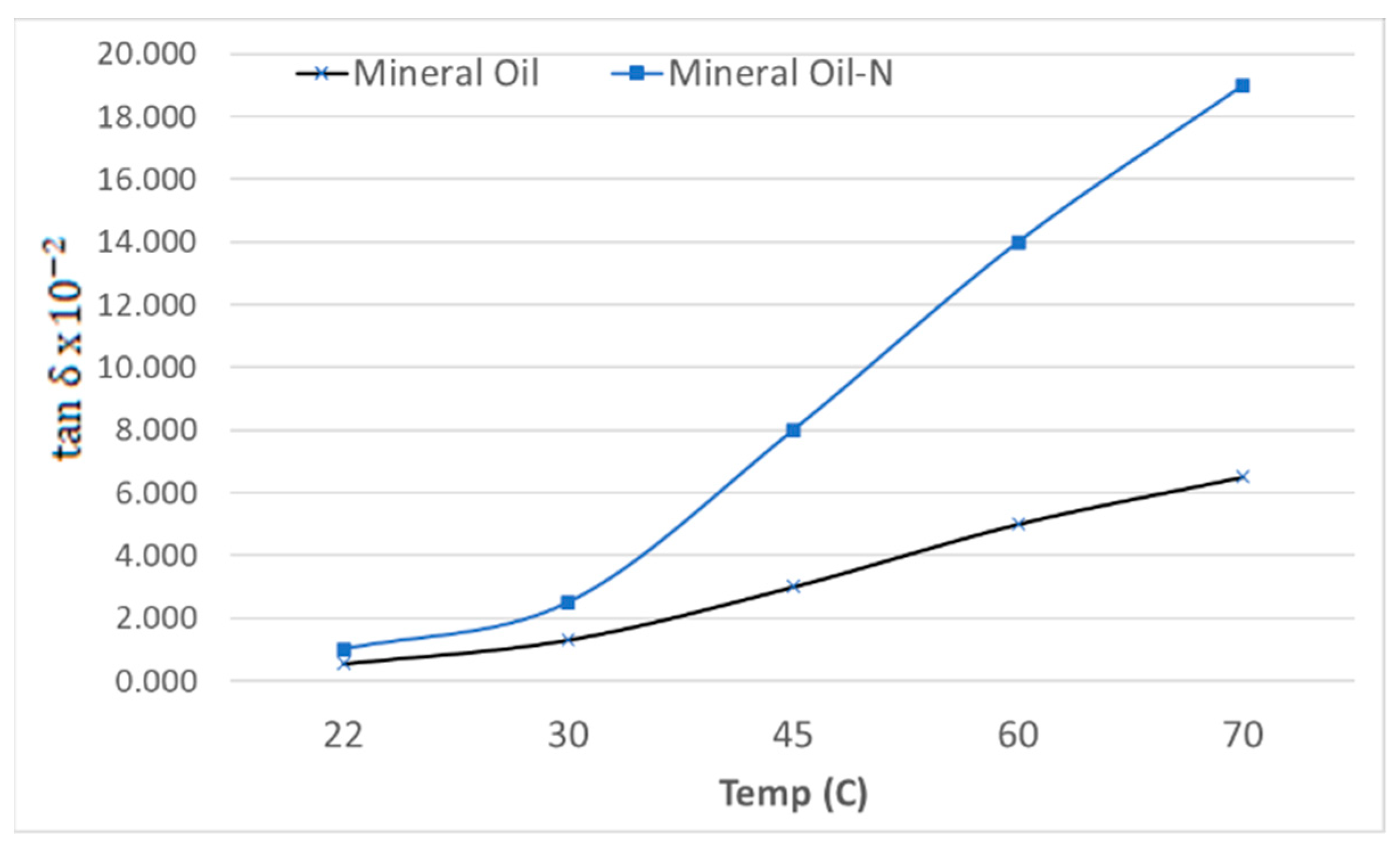
3.2. Dielectric Properties: Tan δ and Measurement
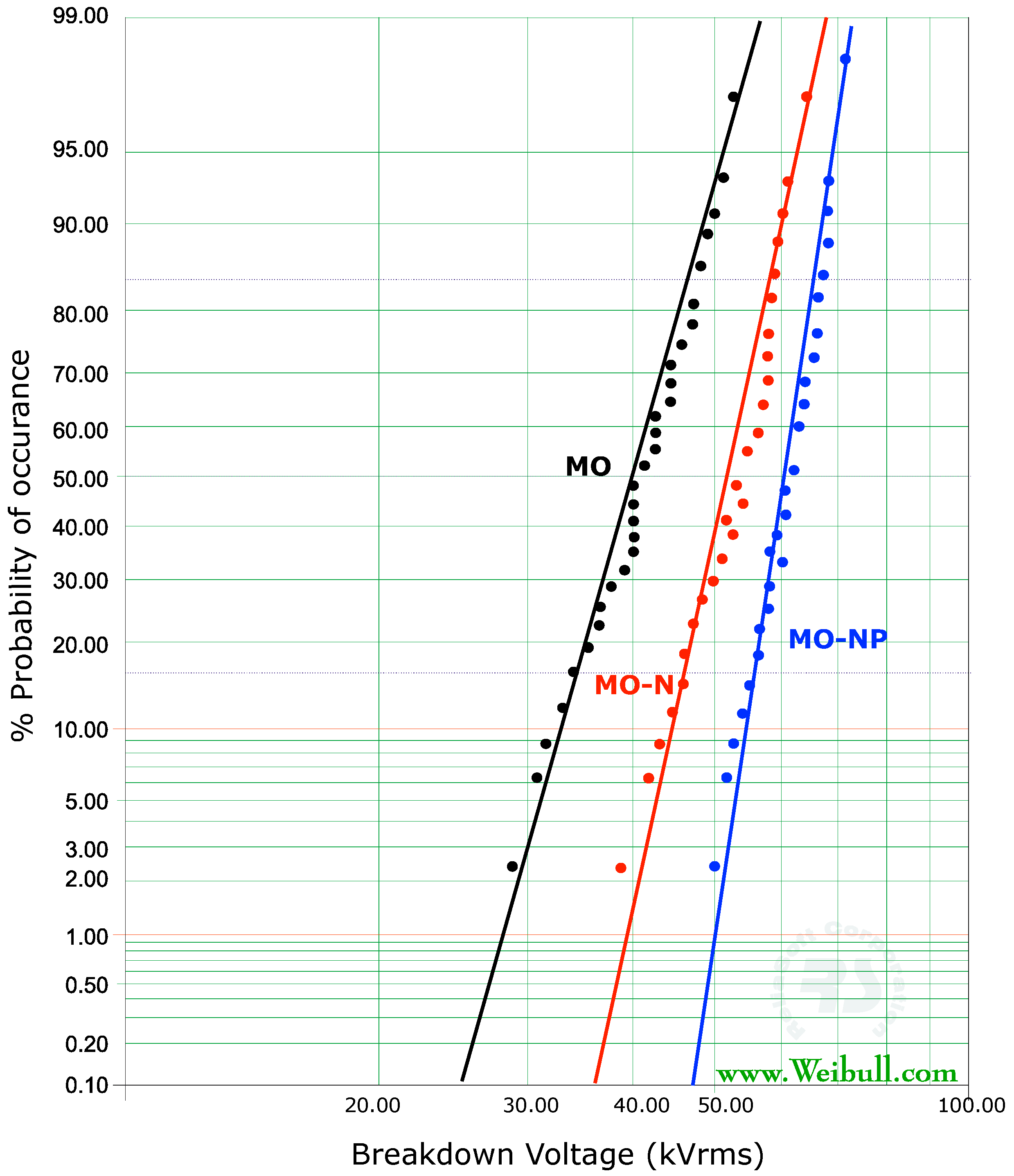
3.3. AC Breakdown Voltage
- = Scale parameter (=63.2% of data)
- = Shape parameter
- = Breakdown voltage
- n = total number of data points
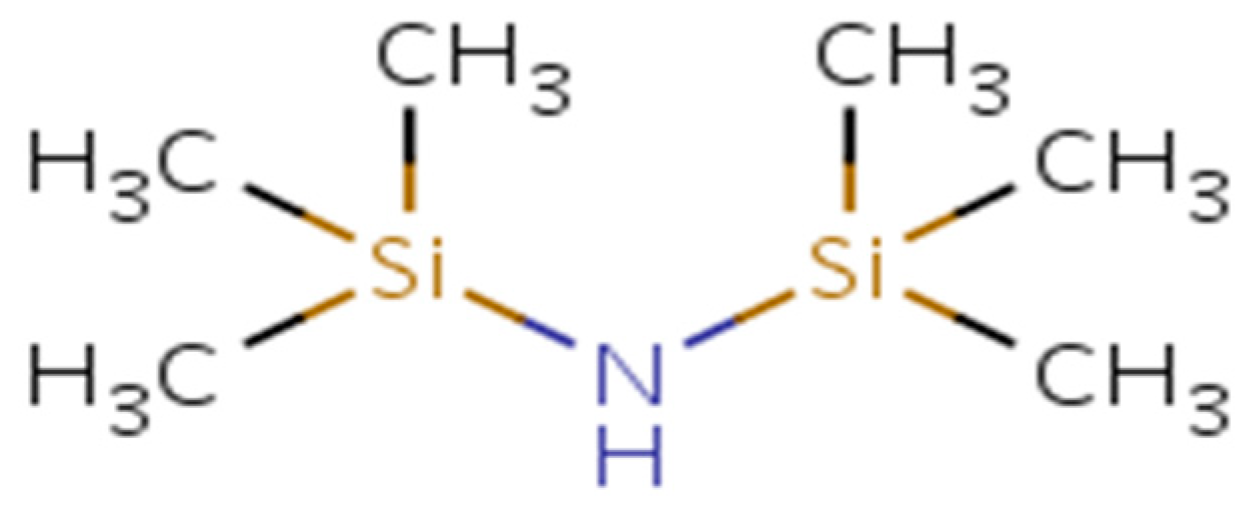
3.4. Role of Amino-Based Silane Coated Fumed Silica NPS
- (a)
- Silica modified with unpolar silanes was easier to disperse in composite, while the ones modified with polar silanes agglomerated in the polymer matrix.
- (b)
- The nanocomposites filled with unpolar silanes increased the space-charge trap density, while the polar silanes caused an increase in charge trap depth and showed less injected charge, thus causing suppression of space-charge accumulation by introducing N2-rich polar functional groups. Thus, according to these results, amino-modified fumed silica is a promising candidate for polymeric nanocomposite for HVDC cable and capacitors applications.
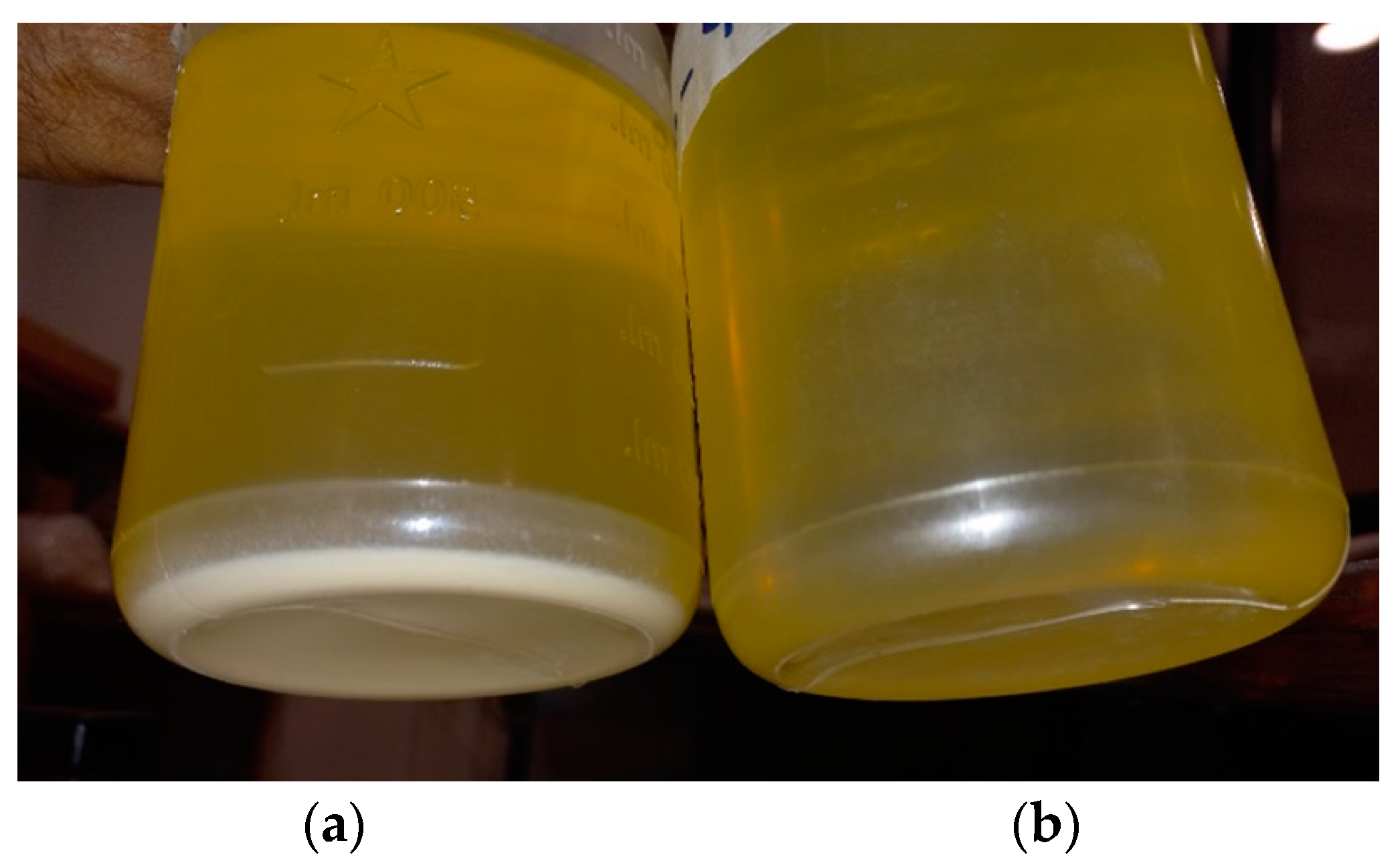
3.5. Shelf Life of MO-NP Oil Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal conductivity of fluids with nanoparticles. Int. Mech. Eng. Congr. Expo. 1995, 99–105. [Google Scholar]
- Kumar, L.H.; Kazi, S.; Masjuki, H.; Zubir, M. A review of recent advances in green nanofluids and their application in thermal systems. Chem. Eng. J. 2021, 429, 132321. [Google Scholar] [CrossRef]
- Godson, L.; Raja, B.; Lal, D.M.; Wongwises, S. Enhancement of heat transfer using nanofluids—An overview. Renew. Sustain. Energy Rev. 2010, 14, 629–641. [Google Scholar] [CrossRef]
- Kadim, E.J.; Noorden, Z.A.; Adzis, Z.; Azis, N. Nanoparticles Application in High Voltage Insulation Systems. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1380–1399. [Google Scholar] [CrossRef]
- Dhar, P.; Katiyar, A.; Maganti, L.S.; Pattamatta, A.; Das, S.K. Superior dielectric breakdown strength of graphene and carbon nanotube infused nano-oils. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 943–956. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Rytoluoto, I.; Anyszka, R.; Mahtabani, A.; Saarimaki, E.; Lahti, K.; Paajanen, M.; Dierkes, W.; Blume, A. Silica Surface-Modification for Tailoring the Charge Trapping Properties of PP/POE Based Dielectric Nanocomposites for HVDC Cable Application. IEEE Access 2020, 8, 87719–87734. [Google Scholar] [CrossRef]
- Praeger, M.; Hosier, I.L.; Vaughan, A.S.; Swingler, S.G. The effects of surface hydroxyl groups in polyethylene-silica nanocomposites. In Proceedings of the 2015 IEEE Electrical Insulation Conference (EIC), Seattle, WA, USA, 7–10 June 2015; pp. 201–204. [Google Scholar] [CrossRef]
- Lazauskas, A.; Baltrusaitis, J.; Grigaliūnas, V.; Jucius, D.; Guobienė, A.; Prosyčevas, I.; Narmontas, P. Characterization of Plasma Polymerized Hexamethyldisiloxane Films Prepared by Arc Discharge. Plasma Chem. Plasma Process. 2013, 34, 271–285. [Google Scholar] [CrossRef]
- De Almeida, M.; Caiado, K.; Sartoratto, P.; e Silva, D.C.; Pereira, A.; Morais, P. Preparation and size-modulation of silica-coated maghemite nanoparticles. J. Alloys Compd. 2010, 500, 149–152. [Google Scholar] [CrossRef]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.P.C.; Elsinawi, A. Ionic liquid-based nanofluids (ionanofluids) for thermal applications: An experimental thermophysical characterization. Pure Appl. Chem. 2019, 91, 1309–1340. [Google Scholar] [CrossRef]
- Shafahi, M.; Bianco, V.; Vafai, K.; Manca, O. An investigation of the thermal performance of cylindrical heat pipes using nanofluids. Int. J. Heat Mass Transf. 2010, 53, 376–383. [Google Scholar] [CrossRef]
- Wickramasinghe, K.; Sasahara, H.; Rahim, E.A.; Perera, G. Green Metalworking Fluids for sustainable machining applications: A review. J. Clean. Prod. 2020, 257, 120552. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced nanomaterials in oil and gas industry: Design, application and challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Xian, H.W.; Sidik, N.A.C.; Najafi, G. Recent state of nanofluid in automobile cooling systems. J. Therm. Anal. Calorim. 2018, 135, 981–1008. [Google Scholar] [CrossRef]
- Moravej, M.; Bozorg, M.V.; Guan, Y.; Li, L.K.; Doranehgard, M.H.; Hong, K.; Xiong, Q. Enhancing the efficiency of a symmetric flat-plate solar collector via the use of rutile TiO2-water nanofluids. Sustain. Energy Technol. Assess. 2020, 40, 100783. [Google Scholar] [CrossRef]
- Khan, N.S.; Gul, T.; Khan, M.A.; Bonyah, E.; Islam, S. Mixed convection in gravity-driven thin film non-Newtonian nanofluids flow with gyrotactic microorganisms. Results Phys. 2017, 7, 4033–4049. [Google Scholar] [CrossRef]
- Segal, V.; Hjortsberg, A.; Rabinovich, A.; Nattrass, D.; Raj, K. AC (60 Hz) and impulse breakdown strength of a colloidal fluid based on transformer oil and magnetite nanoparticles. In Proceedings of the Conference Record of the 1998 IEEE International Symposium on Electrical Insulation (Cat. No.98CH36239), Arlington, VA, USA, 7–10 June 1998; Volume 2, pp. 619–622. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Yao, W.; Wang, F.; Wan, F.; Tan, Y.; Mehmood, M.A. Electrical and thermal properties of insulating oil-based nanofluids: A comprehensive overview. IET Nanodielectrics 2019, 2, 27–40. [Google Scholar] [CrossRef]
- Beroual, A.; Khaled, U. Effect of Nanoparticles’ Mixtures on AC Breakdown Voltage of Mineral Oil. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1216–1222. [Google Scholar] [CrossRef]
- Thabet, A.; Allam, M.; Shaaban, S.A. Investigation on enhancing breakdown voltages of transformer oil nanofluids using multi-nanoparticles technique. IET Gener. Transm. Distrib. 2018, 12, 1171–1176. [Google Scholar] [CrossRef]
- Thirugnanam, A.R.P.R.; Siluvairaj, W.I.M.; Karthik, R. Performance studies on dielectric and physical properties of eco-friendly based natural ester oils using semi-conductive nanocomposites for power transformer application. IET Sci. Meas. Technol. 2018, 12, 323–327. [Google Scholar] [CrossRef]
- Suhaimi, S.N.; Rahman, A.R.A.; Din, M.F.M.; Hassan, M.Z.; Ishak, M.T.; Bin Jusoh, M.T. A Review on Oil-Based Nanofluid as Next-Generation Insulation for Transformer Application. J. Nanomater. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Zakaria, I.H.; Ahmad, M.H.; Abdul-Malek, Z.; Sidik, M.A.B.; Nawawi, Z.; Jambak, M.I. AC breakdown strength performance of plasma treated mineral oil-based nanofluids. In Proceedings of the 2017 International Conference on Electrical Engineering and Computer Science (ICECOS), Palembang, Indonesia, 22–23 August 2017; pp. 333–337. [Google Scholar] [CrossRef]
- He, X.; Rytöluoto, I.; Anyszka, R.; Mahtabani, A.; Saarimäki, E.; Lahti, K.; Paajanen, M.; Dierkes, W.; Blume, A. Surface Modification of Fumed Silica by Plasma Polymerization of Acetylene for PP/POE Blends Dielectric Nanocomposites. Polymers 2019, 11, 1957. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Mahtabani, A.; Rytoluoto, I.; Saarimaki, E.; Lahti, K.; Paajanen, M.; Anyszka, R.; Dierkes, W.; Blume, A. Surface Modification of Fumed Silica by Dry Silanization for PP-based Dielectric Nanocomposites. In Proceedings of the 2019 2nd International Conference on Electrical Materials and Power Equipment (ICEMPE), Guangzhou, China, 7–10 April 2019; pp. 254–259. [Google Scholar] [CrossRef] [Green Version]
- Mahtabani, A.; Rytöluoto, I.; Anyszka, R.; He, X.; Saarimäki, E.; Lahti, K.; Paajanen, M.; Dierkes, W.; Blume, A. On the Silica Surface Modification and Its Effect on Charge Trapping and Transport in PP-Based Dielectric Nanocomposites. ACS Appl. Polym. Mater. 2020, 2, 3148–3160. [Google Scholar] [CrossRef]
- Jin, H.; Andritsch, T.; Tsekmes, I.A.; Kochetov, R.; Morshuis, P.; Smit, J.J. Properties of Mineral Oil based Silica Nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1100–1108. [Google Scholar] [CrossRef]
- Evonik Operations GmbH, AEROSIL.R-812. Available online: www.coating-additives.com (accessed on 15 August 2021).
- Rong, M.; Liu, D.; Wang, N.; Su, B.; Wang, X.; Wu, Y. A New Structure Optimization Method for the Interneedle Distance of a Multineedle-to-Plane Barrier Discharge Reactor. IEEE Trans. Plasma Sci. 2010, 38, 966–972. [Google Scholar] [CrossRef]
- Qureshi, M.I.; Qureshi, B. Effect of Plasma treatment of TiO2 nanoparticles formulated nanofluid on the dielectric strength of Rice Barn Oil. J. Engg. Res. 2022. accepted to appear. [Google Scholar]
- Al-Ammar, E.; Qureshi, M.I. Probing the Use of Green Insulating Oils in Transformers Based on Their Statistical Breakdown Data. In Proceedings of the EEPAC Conference, Sharjah, United Arab Emirates, 10–12 November 2009. [Google Scholar]
- Al-arainy, A.; Qureshi, M.I.; Malik, N.H. Fundamentals of High Voltage Engineering; King Saud University Publishing and Press: Riyadh, Saudi Arabia, 2012. [Google Scholar]
- Berg, M.V. Frontal Nanotechnology Research; Nova Sci. Publishers: Hauppauge, NY, USA, 2007; Chapter 8; pp. 189–219. [Google Scholar]
- Dong, M.; Shen, L.P.; Wang, H.B.; Miao, J. Investigation on the Electrical Conductivity of Transformer Oil-Based AlN Nanofluid. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Minea, A.A. A Review on Electrical Conductivity of Nanoparticle-Enhanced Fluids. Nanomaterials 2019, 9, 1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, J.; Dong, M.; Ren, M.; Wu, X.; Shen, L.; Wang, H. Effect of nanoparticle polarization on relative permittivity of transformer oil-based nanofluids. J. Appl. Phys. 2013, 113, 204103. [Google Scholar] [CrossRef]
- Voorthuyzen, J.A.; Keskin, K.; Bergveld, P. Investigations of the surface conductivity of silicon dioxide and methods to reduce it. Surf. Sci. 1987, 187, 201–211. [Google Scholar] [CrossRef] [Green Version]
- 62539 First Edition 2007-07 IEEE 930-IEC/IEEE Guide for the Statistical Analysis of Electrical Insulation Breakdown Data (Adoption of IEEE Std 930-2004). 2007. Available online: https://ieeexplore.ieee.org/document/4288250 (accessed on 11 September 2021).
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhou, J.; Li, X.; Zhou, Y.; Tu, Y. Effect of electron shallow trap on breakdown performance of transformer oil-based nanofluids. J. Appl. Phys. 2011, 110, 104104. [Google Scholar] [CrossRef]
- Lv, Y.; Du, Y.; Li, C.; Qi, B.; Zhong, Y.; Chen, M. TiO2 nanoparticle induced space charge decay in thermal aged transformer oil. Appl. Phys. Lett. 2013, 102, 132902. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Z. Charge trapping and detrapping in polymeric materials. J. Appl. Phys. 2009, 106, 123707. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, M.; Lv, Y.; Li, C.; Sun, Q. Effect of Al2O3 nanorods on the performance of oil-impregnated pressboard insulation. Electr. Eng. 2019, 102, 715–724. [Google Scholar] [CrossRef]
- Zhang, W.; Dehghani-Sanij, A.A.; Blackburn, R. IR study on hydrogen bonding in epoxy resin–silica nanocomposites. Prog. Nat. Sci. 2008, 18, 801–805. [Google Scholar] [CrossRef]
- Dissado, L.A.; Fothergill, J.C. Electrical Degradation and Breakdown in Polymers; IET: London, UK, 1992; Volume 9, ISBN 9780863411960. [Google Scholar]
- Liang, N.; Liao, R.; Xiang, M.; Mo, Y.; Yuan, Y. Influence of Amine Compounds on the Thermal Stability of Paper-Oil Insulation. Polymers 2018, 10, 891. [Google Scholar] [CrossRef] [Green Version]
- Bagwe, R.P.; Hilliard, L.R.; Tan, W. Surface Modification of Silica Nanoparticles to Reduce Aggregation and Nonspecific Binding. Langmuir 2006, 22, 4357–4362. [Google Scholar] [CrossRef] [Green Version]
- Crutcher, R.; Warner, K. Polar Compounds: System Integrity and Diagnostic Applications. In Proceedings of the Tech Conference, Sacramento, CA, USA, 20–21 April 2015; pp. 1–6. [Google Scholar]




| Characteristic | Value |
|---|---|
| pH | 7–7.5 |
| Moisture Content | <0.5% |
| Color | White |
| Surface Area | 260 m2/g |
| Avg. Particle size | 7 nm |
| Composition | wt % |
| SiO2 | 99.8 |
| Al2O3 | ≥0.05 |
| Fe2O3 | ≥0.01 |
| TiO2 | ≥0.03 |
| Atomic Content (%) | |||
|---|---|---|---|
| Sample | Si | O | (O/Si) Ratio |
| Untreated | 24 | 75 | 3.12 |
| Plasma Treated | 32 | 68 | 2.12 |
| %P | MO | MO-N | % Increase | MO-NP | % Increase |
|---|---|---|---|---|---|
| 5 | 32 | 43 | +34 | 51 | +60 |
| 50 | 42 | 53 | +24 | 62 | +47 |
| 63.23 | 52 | 56 | +8 | 64 | +23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, M.I.; Qureshi, B. Probing the Use of Silane-Grafted Fumed Silica Nanoparticles to Produce Stable Transformer Oil-Based Nanofluids. Materials 2021, 14, 7649. https://doi.org/10.3390/ma14247649
Qureshi MI, Qureshi B. Probing the Use of Silane-Grafted Fumed Silica Nanoparticles to Produce Stable Transformer Oil-Based Nanofluids. Materials. 2021; 14(24):7649. https://doi.org/10.3390/ma14247649
Chicago/Turabian StyleQureshi, Muhammad I., and Basit Qureshi. 2021. "Probing the Use of Silane-Grafted Fumed Silica Nanoparticles to Produce Stable Transformer Oil-Based Nanofluids" Materials 14, no. 24: 7649. https://doi.org/10.3390/ma14247649







