Application of Silica Nanoparticles for Improving Growth, Yield, and Enzymatic Antioxidant for the Hybrid Rice EHR1 Growing under Water Regime Conditions
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis of Silica Nanoparticles (SiO2NPs) from Useless Materials (RH Silica)
2.2. Cultivation and Experimental Conditions
2.3. Studied Traits
2.3.1. Plant Growth Characteristics
2.3.2. Determination of Chlorophyll and Proline Content
2.3.3. Determination of the Activity of the Antioxidant Enzymes
2.4. Statistical Analysis
3. Results and Discussion
3.1. Utilized Advanced Tools in Terms of Transmission Electron Microscopy (TEM), Dynamic Light Scattering (DLS), Selected Area Diffraction (SAED), Scanning Electron Microscopy (SEM), and X-ray Diffraction (XRD) for Affirming the Preparation of Silica Oxide Nanoparticles (SiO2NPs)
3.2. Application of SiO2NPs as a Foliar for Rice Plants
3.2.1. Plant Growth Characteristics
3.2.2. Yield and Yield Attributes
3.2.3. Effects of Water Regimes, SiO2NPs Concentrations, and Their Interaction on Chlorophyll and Proline Content
3.2.4. Antioxidant Enzymes Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, S.A. Price hike of staple food, nutritional impact and consumption adjustment: Evidence from the 2005–2010 rice price increase in rural Bangladesh. Appl. Econ. 2019, 51, 743–761. [Google Scholar] [CrossRef] [Green Version]
- Seck, P.A.; Diagne, A.; Mohanty, S.; Wopereis, M.C.S. Crops that feed the world 7: Rice. Food Secur. 2012, 4, 7–24. [Google Scholar] [CrossRef]
- O’Toole, J.C. Rice and Water: The Final Frontier; Rockefeller Foundation: Bangkok, Thailand, 2004. [Google Scholar]
- Kumar, S.; Dwivedi, S.K.; Basu, S.; Kumar, G.; Mishra, J.; Koley, T.K.; Rao, K.; Choudhary, A.; Mondal, S.; Kumar, S.; et al. Anatomical, agro-morphological and physiological changes in rice under cumulative and stage specific drought conditions prevailed in eastern region of India. Field Crops Res. 2020, 245, 107658. [Google Scholar] [CrossRef]
- Ndjiondjop, M.-N.; Cisse, F.; Futakuchi, K.; Lorieux, M.; Manneh, B.; Bocco, R.; Fatondji, B. Effect of drought on rice (Oryza spp.) genotypes according to their drought tolerance level. In Proceedings of the Second Africa Rice Congress, Bamako, Mali, 22–26 March 2010; pp. 1–15. [Google Scholar]
- Bocco, R.; Lorieux, M.; Seck, P.; Futakuchi, K.; Manneh, B.; Baimey, H.; Ndjiondjop, M. Agro-morphological characterization of a population of introgression lines derived from crosses between IR 64 (Oryza sativa indica) and TOG 5681 (Oryza glaberrima) for drought tolerance. Plant Sci. 2012, 183, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datnoff, L.E.; Snyder, G.H.; Korndörfer, G.H. Silicon in Agriculture; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Ma, J.F. Silicon uptake and translocation in plants. In Proceedings of the International Plant Nutrition Colloquium XVI, Davis, CA, USA, 26–30 August 2009. [Google Scholar]
- Mehrabanjoubani, P.; Abdolzadeh, A.; Sadeghipour, H.R.; Aghdasi, M. Silicon affects transcellular and apoplastic uptake of some nutrients in plants. Pedosphere 2015, 25, 192–201. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; Rehman, M.Z.U.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. 2020, 27, 4958–4968. [Google Scholar] [CrossRef]
- Abdel-Haliem, M.E.; Hegazy, H.S.; Hassan, N.S.; Naguib, D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017, 110, 70–81. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Sun, L.; Liu, L.; Wang, L.; Hou, Y.; Wang, A.; Wang, G.; Huang, S. The effects of nanomaterial treated water on the pathogens of rice diseases and fungicides. Nanosci. Nanotechnol. Lett. 2017, 9, 957–963. [Google Scholar] [CrossRef]
- Alvarez, R.d.C.F.; de Mello Prado, R.; Felisberto, G.; Deus, A.C.F.; de Oliveira, R.L.L. Effects of soluble silicate and nanosilica application on rice nutrition in an Oxisol. Pedosphere 2018, 28, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Cáceres, M.; Vassena, C.V.; Garcerá, M.D.; Santo-Orihuela, P.L. Silica nanoparticles for insect pest control. Curr. Pharm. Des. 2019, 25, 4030–4038. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi Sarvestani, Z.; Kassaee, M.Z.; Modares Sanavi, S.A.M.; Sorooshzadeh, A. Improving growth and yield of wheat under drought stress via application of SiO2 nanoparticles. J. Agric. Sci. Technol. 2018, 20, 1479–1492. [Google Scholar]
- Moonmoon, S.; Fakir, M.; Islam, M. Effect of drought stress on grain dry weight, photosynthesis and chlorophyll in six rice genotypes. Sch. J. Agric. Vet. Sci. 2017, 4, 13–17. [Google Scholar]
- Ghorbanpour, M.; Mohammadi, H.; Kariman, K. Nanosilicon-based recovery of barley (Hordeum vulgare) plants subjected to drought stress. Environ. Sci. Nano 2020, 7, 443–461. [Google Scholar] [CrossRef]
- Sharifi Rad, J.; Karimi, J.; Mohsenzadeh, S.; Sharifi Rad, M.; Moradgholi, J. Evaluating SiO2 nanoparticles effects on developmental characteristic and photosynthetic pigment contents of Zeamays L. Bull. Environ. Pharm. Life Sci. 2014, 3, 194–201. [Google Scholar]
- Qados, A.M.S.A. Mechanism of nanosilicon-mediated alleviation of salinity stress in Faba bean (Vicia faba L.) Plants. Am. J. Exp. Agric. 2015, 7, 78–95. [Google Scholar] [CrossRef]
- Bottomley, P.J.; Angle, J.S.; Weaver, R. Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 12. [Google Scholar]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Los Baños, Philippines, 1971. [Google Scholar]
- Yoshida, S. Fundamentals of Rice Crop Science; The International Rice Research Institute: Los Baños, Philippines, 1981. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Urbanek, H.; Kuzniak-Gebarowska, E.; Herka, K. Elicitation of defence responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol. Plant. 1991, 13, 43–50. [Google Scholar]
- Van Rossum, M.W.; Alberda, M.; van der Plas, L.H. Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci. 1997, 130, 207–216. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Emam, M.M.; Khattab, H.E.; Helal, N.M.; Deraz, A.E. Effect of selenium and silicon on yield quality of rice plant grown under drought stress. Aust. J. Crop Sci. 2014, 8, 596. [Google Scholar]
- Hamoud, Y.A.; Wang, Z.; Guo, X.; Shaghaleh, H.; Sheteiwy, M.; Chen, S.; Qiu, R.; Elbashier, M.M.A. Effect of irrigation regimes and soil texture on the potassium utilization efficiency of rice. Agronomy 2019, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Howe, J.A.; Golden, B.R. Calcium silicate slag reduces drought stress in rice (Oryza sativa L.). J. Agron. Crop Sci. 2019, 205, 353–361. [Google Scholar] [CrossRef]
- Liang, Y. Effect of silicon on leaf ultrastructure, chlorophyll content and photosynthetic activity of barley under salt stress. Pedosphere 1998, 8, 289–296. [Google Scholar]
- Fauteux, F.; Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sangster, A.G.; Hodson, M.J.; Tubb, H.J. Chapter 5. Silicon deposition in higher plants. In Silicon in Agriculture; Elsevier BV: Amsterdam, The Netherlands, 2001; pp. 85–113. [Google Scholar]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant. Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 2014, 22, 2837–2845. [Google Scholar] [CrossRef]
- Tamai, K.; Ma, J.F. Reexamination of silicon effects on rice growth and production under field conditions using a low silicon mutant. Plant Soil 2008, 307, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Kheyri, N.; Norouzi, H.A.; Mobasser, H.R.; Torabi, B. Effects of silicon and zinc nanoparticles on growth, yield, and biochemical characteristics of rice. Agron. J. 2019, 111, 3084–3090. [Google Scholar] [CrossRef]
- Detmann, K.C.; Araújo, W.L.; Martins, S.C.; Fernie, A.R.; DaMatta, F.M. Metabolic alterations triggered by silicon nutrition: Is there a signaling role for silicon? Plant Signal. Behav. 2013, 8, e22523. [Google Scholar] [CrossRef] [Green Version]
- Kheir, A.M.S.; Abouelsoud, H.M.; Hafez, E.M.; Ali, O.A.M. Integrated effect of nano-Zn, nano-Si, and drainage using crop straw–filled ditches on saline sodic soil properties and rice productivity. Arab. J. Geosci. 2019, 12, 471. [Google Scholar] [CrossRef]
- Herbinger, K.; Tausz, M.; Wonisch, A.; Soja, G.; Sorger, A.; Grill, D. Complex interactive effects of drought and ozone stress on the antioxidant defence systems of two wheat cultivars. Plant Physiol. Biochem. 2002, 40, 691–696. [Google Scholar] [CrossRef]
- Saha, S.; Begum, H.H.; Nasrin, S. Effects of drought stress on growth and accumulation of proline in five rice varieties (Oryza Sativa L.). J. Asiat. Soc. Bangladesh Sci. 2019, 45, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Johari-Pireivatlou, M. Effect of soil water stress on yield and proline content of four wheat lines. Afr. J. Biotechnol. 2010, 9, 36–40. [Google Scholar]
- Lum, M.; Hanafi, M.; Rafii, Y.; Akmar, A. Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. J. Anim. Plant Sci. 2014, 24, 1487–1493. [Google Scholar]
- Rahdari, P.; Hosseini, S.M.; Tavakoli, S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Med. Plants Res. 2012, 6, 1539–1547. [Google Scholar]
- Hegazy, H.; Hassan, N.; Abdel-Haliem, M.; Naguib, D. biochemical response of rice plant to biotic and abiotic stress under silica ions and nanoparticles application. Egypt. J. Bot. 2015, 55, 79–103. [Google Scholar]
- Athar, H.-U.-R.; Khan, A.; Ashraf, M. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ. Exp. Bot. 2008, 63, 224–231. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Hasheminasab, H.; Assad, M.T.; Aliakbari, A.; Sahhafi, S.R. Influence of drought stress on oxidative damage and antioxidant defense systems in tolerant and susceptible wheat genotypes. J. Agric. Sci. 2012, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, T.; Wijsman, H.J.W.; Loon, L.C. Petunia peroxidase a: Isolation, purification and characteristics. JBIC J. Biol. Inorg. Chem. 1991, 199, 139–146. [Google Scholar] [CrossRef]
- Lagrimini, L.M. Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiol. 1991, 96, 577–583. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J. 2008, 3, 448–453. [Google Scholar]
- Almaghrabi, O.A. Impact of drought stress on germination and seedling growth parameters of some wheat cultivars. Life Sci. J. 2012, 9, 590–598. [Google Scholar]
- Lin, B.-S.; Diao, S.-Q.; Li, C.-H.; Fang, L.-J.; Qiao, S.-C.; Yu, M. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
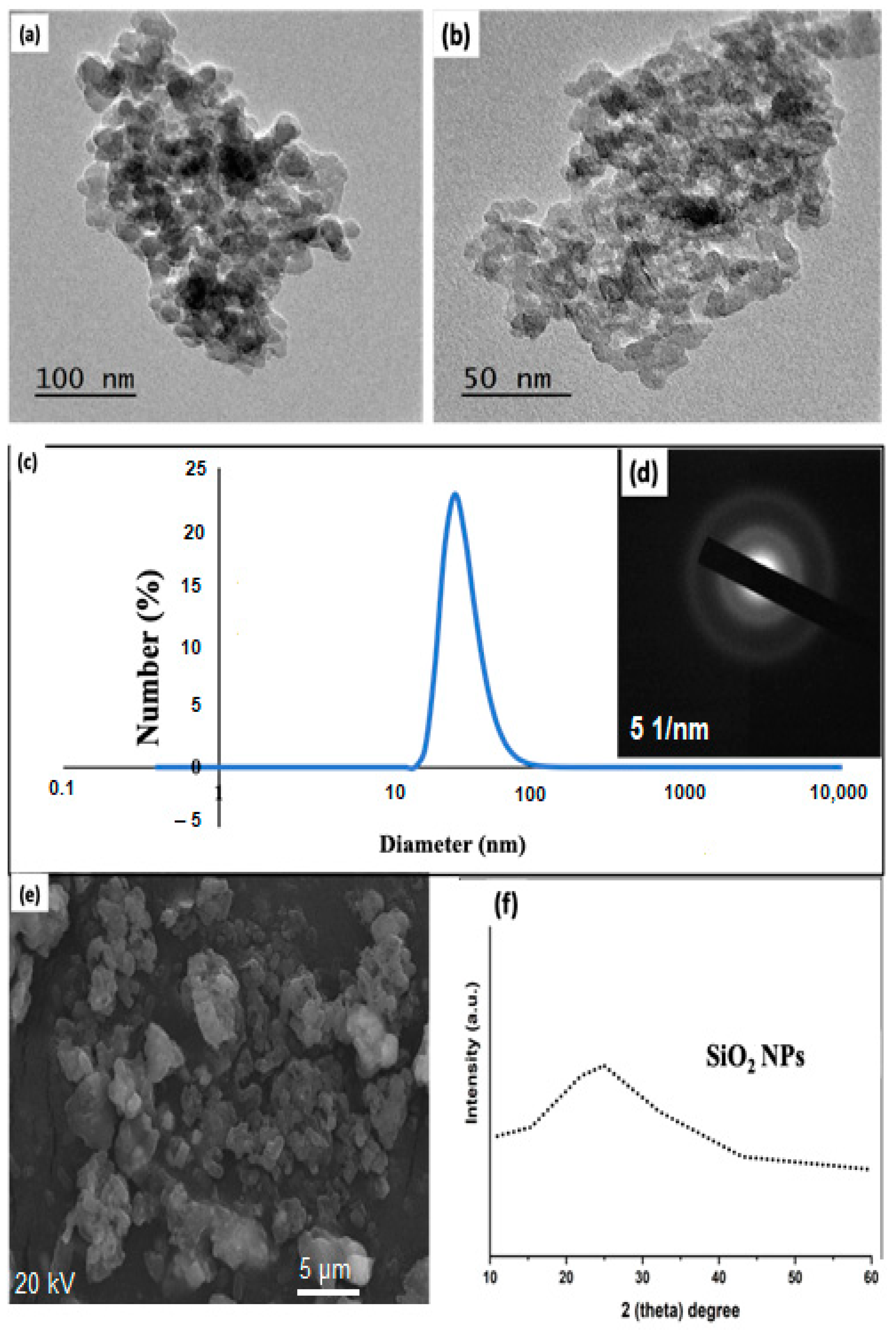
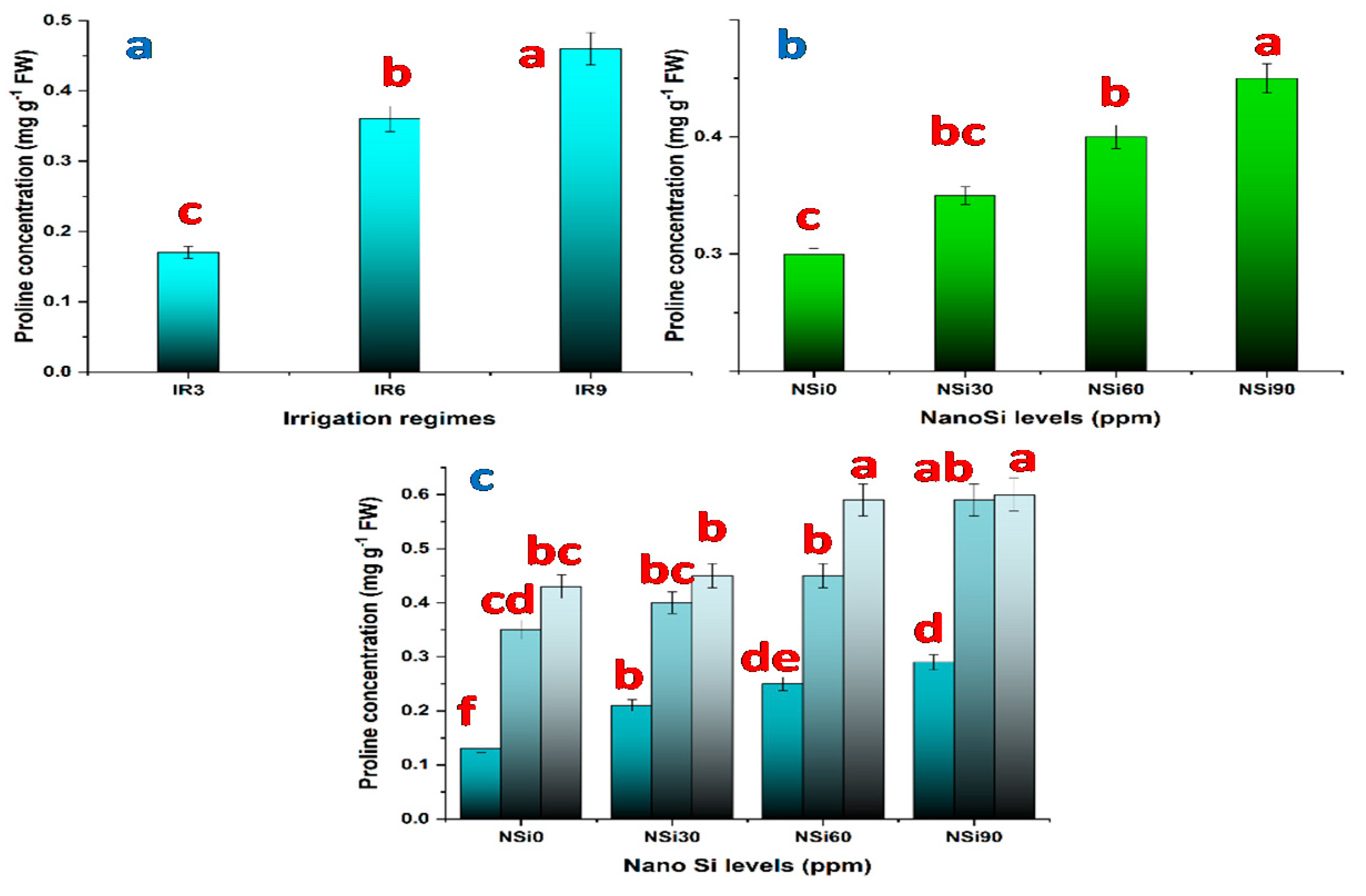



| Seasons | Texture | Sand (%) | Silt (%) | Clay (%) | pH (1:2.5 Soil Extract) | E.C. (dSm−1) | Organic Matter % | Available N (ppm) | Available P (ppm) | Available K (ppm) | Available Zn (ppm) | Available Mn (ppm) | Available Fe (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | Clayey | 12.8 | 31.5 | 55.7 | 8.33 | 3.12 | 1.45 | 18.4 | 14.7 | 322 | 0.78 | 3.44 | 3.12 |
| 2019 | Clayey | 12.0 | 32.0 | 56.0 | 8.40 | 3.48 | 1.50 | 19.1 | 15.2 | 347 | 0.95 | 3.22 | 3.70 |
| Treatments | Leaf Area Index (LAI) | Dry Matter Production (g/m2) | ||
|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | |
| Water regimes | – | – | – | – |
| IR3 | 7.07 a | 6.94 a | 1575.1 a | 1562.2 a |
| IR6 | 6.98 a | 6.87 a | 1566.0 a | 1551.7 a |
| IR9 | 5.44 b | 5.29 b | 1231.2 b | 1224.1 b |
| F test | ** | ** | ** | ** |
| SiO2NPs concentrations | – | – | – | – |
| – | – | – | – | |
| SiO2NPs-0 | 6.26 d | 6.06 d | 1415.6 d | 1399.5 d |
| SiO2NPs-30 | 6.41 c | 6.28 c | 1440.3 c | 1428.5 c |
| SiO2NPs-60 | 6.56 b | 6.49 b | 1462.2 b | 1465.7 b |
| SiO2NPs-90 | 6.77 a | 6.65 a | 1501.6 a | 1491.6 a |
| F test | ** | ** | ** | ** |
| IRs X SiO2NPs | ** | N.S | N.S | ** |
| SiO2NPs Concentrations | Water Regimes | ||
|---|---|---|---|
| 2018 | |||
| IR3 | IR6 | IR9 | |
| SiO2NPs-0 | 6.71 c | 6.75 c | 5.32 d |
| SiO2NPs-30 | 6.96 bc | 6.77 bc | 5.49 d |
| SiO2NPs-60 | 7.21 ab | 7.06 abc | 5.38 d |
| SiO2NPs-90 | 7.38 a | 7.34 a | 5.58 d |
| SiO2NPs Concentrations | Water Regimes | ||
|---|---|---|---|
| 2019 | |||
| IR3 | IR6 | IR9 | |
| SiO2NPs-0 | 1536.2 bc | 1518.0 d | 1192.3 g |
| SiO2NPs-30 | 1570.0 b | 1546.4 cd | 1235.0 f |
| SiO2NPs-60 | 1580.1 b | 1579.2 b | 1231.2 f |
| SiO2NPs-90 | 1623.2 a | 1620.4 a | 1266.6 e |
| Treatments | The Number of Panicles/m2 | The Number of Filled Grains/Panicle | The Number of Unfilled Grains/Panicles | |||
|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | |
| Water regimes IR3 IR6 IR9 F test | – 616.7 a 612.9 a 447.5 b ** | – 613.4 a 609.2 a 438.1 b ** | – 154.1 a 151.7 a 118.8 b ** | – 150.3 a 146.5 a 113.6 b ** | – 6.3 c 7.4 b 15.4 a ** | – 7.1 c 8.4 b 17.1 a ** |
| SiO2NPs concentrations SiO2NPs-0 SiO2NPs-30 SiO2NPs-60 SiO2NPs-90 F test | – 538.0 d 554.7 c 565.3 b 578.1 a ** | – 530.4 d 548.4 c 560.3 b 575.2 a ** | – 137.3 c 139.3 b 144.1 a 145.4 a ** | – 131.7 c 135.8 b 138.0 ab 140.5 a ** | – 10.9 a 10.2 b 9.1 c 8.4 d ** | – 12.7 a 11.4 b 10.3 c 9.8 c ** |
| IRs X SiO2NPs | ** | ** | N.S | N.S | N.S | N.S |
| 1000 Grain Weight (g) | Grain Yield (t/ha) | Biological Yield (t/ha) | ||||
|---|---|---|---|---|---|---|
| Treatments | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 |
| Water regimes IR3 IR6 IR9 F test | – 26.17 a 26.10 a 24.62 b ** | – 26.11 a 26.06 a 24.48 b ** | – 12.01 a 11.89 a 8.62 b ** | – 11.83 a 11.74 a 8.58 b ** | – 27.42 a 27.18 a 21.84 b ** | – 27.01 a 26.85 a 21.40 b ** |
| SiO2NPs concentrations SiO2NPs-0 SiO2NPs-30 SiO2NPs-60 SiO2NPs-90 F test | – 25.34 d 25.54 c 25.68 b 25.95 a ** | – 25.27 d 25.37 c 25.62 b 25.93 a ** | – 10.41d 10.75 c 10.92 b 11.29 a ** | – 10.23 d 10.56 c 10.93 b 11.15 a ** | – 24.30 d 25.13 c 25.86 b 26.62 a ** | – 23.90 d 24.67 c 25.48 b 26.30 a ** |
| IRs X SiO2NPs | ** | ** | ** | ** | ** | ** |
| SiO2NPs Concentrations | Water Regimes | |||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | |||||
| IR3 | IR6 | IR9 | IR3 | IR6 | IR9 | |
| SiO2NPs-0 SiO2NPs-30 SiO2NPs-60 SiO2NPs-90 | 595.1 c 618.0 b 620.6 b 634.0 a | 589.2 d 605.3 c 623.6 ab 633.7 a | 430.0 g 441.0 fg 452.3 f 466.6 e | 600.0 bc 606.3 b 614.7 ab 632.6 a | 584.0 c 605.0 b 619.3 ab 628.6 a | 407.3 f 434.0 e 447.1 de 464.3 d |
| SiO2NPs Concentrations | Water Regimes | |||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | |||||
| IR3 | IR6 | IR9 | IR3 | IR6 | IR9 | |
| SiO2NPs-0 SiO2NPs-30 SiO2NPs-60 SiO2NPs-90 | 25.76 d 26.15 bc 26.32 ab 26.46 a | 25.90 cd 25.99 cd 26.13 bc 26.37 ab | 24.37 f 24.51 f 26.69 f 25.02 e | 25.88 bc 25.91 bc 26.25 ab 26.39 a | 25.71 c 25.90 bc 26.26 ab 26.38 a | 24.22 e 24.30 e 24.37 e 25.03 d |
| SiO2NPs Concentrations | Water Regimes | |||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | |||||
| IR9 | IR6 | IR9 | IR9 | IR6 | IR9 | |
| SiO2NPs-0 SiO2NPs-30 SiO2NPs-60 SiO2NPs-90 | 11.42 d 11.94 bc 12.21 ab 12.49 a | 11.40 d 11.70 cd 12.01 bc 12.41 a | 8.35 f 8.56 ef 8.61 e 8.97 e | 11.25 e 11.73 c 12.08 ab 12.24 a | 11.29 e 11.52 d 11.98 b 12.18 a | 8.14 h 8.40 g 8.73 g 9.02 f |
| SiO2NPs Concentrations | Water Regimes | |||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | |||||
| IR3 | IR6 | IR9 | IR3 | IR6 | IR9 | |
| SiO2NPs-0 SiO2NPs-30 SiO2NPs-60 SiO2NPs-90 | 26.30 de 27.04 bcd 27.77 b 28.60 a | 25.94 e 26.70 cd 27.42 bc 28.67 a | 20.71 h 21.67 g 22.40 fg 22.58 f | 25.87 d 26.59 c 27.46 b 28.11 a | 25.65 d 26.30 c 27.15 b 28.28 a | 20.17 h 21.12 g 21.82 f 22.52 e |
| Seasons | Water Regimes | SiO2NPs Concentrations | IRs X SiO2NPs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IR3 | IR6 | IR9 | F Test | SiO2NPs-0 | SiO2NPs-30 | SiO2NPs-60 | SiO2NPs-90 | F Test | F Test | |
| 2018 | 44.67 a | 44.32 a | 36.68 b | ** | 40.98 d | 41.56 c | 42.00 b | 43.04 a | ** | ** |
| 2019 | 43.87a | 43.36 a | 36.27 b | ** | 39.86 d | 40.59 c | 41.63 b | 42.59 a | ** | ** |
| SiO2NPs Applications | Water Regimes | |||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | |||||
| IR3 | IR6 | IR9 | IR3 | IR6 | IR9 | |
| SiO2NPs-0 SiO2NPs-30 SiO2NPs-60 SiO2NPs-90 | 43.85 cd 44.24 cd 44.76 bc 45.83 a | 43.67 d 44.23 cd 44.11 cd 45.35 ab | 35.43 f 36.23 f 37.15 e 37.93 e | 42.34 d 43.33 cd 44.70 ab 45.11 a | 41.95 e 42.70 de 43.91 bc 44.90 a | 35.30 h 35.75 gh 36.30 g 37.76 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshayb, O.M.; Nada, A.M.; Ibrahim, H.M.; Amin, H.E.; Atta, A.M. Application of Silica Nanoparticles for Improving Growth, Yield, and Enzymatic Antioxidant for the Hybrid Rice EHR1 Growing under Water Regime Conditions. Materials 2021, 14, 1150. https://doi.org/10.3390/ma14051150
Elshayb OM, Nada AM, Ibrahim HM, Amin HE, Atta AM. Application of Silica Nanoparticles for Improving Growth, Yield, and Enzymatic Antioxidant for the Hybrid Rice EHR1 Growing under Water Regime Conditions. Materials. 2021; 14(5):1150. https://doi.org/10.3390/ma14051150
Chicago/Turabian StyleElshayb, Omnia M., Abdelwahed M Nada, Heba M. Ibrahim, Heba E. Amin, and Ayman M. Atta. 2021. "Application of Silica Nanoparticles for Improving Growth, Yield, and Enzymatic Antioxidant for the Hybrid Rice EHR1 Growing under Water Regime Conditions" Materials 14, no. 5: 1150. https://doi.org/10.3390/ma14051150






