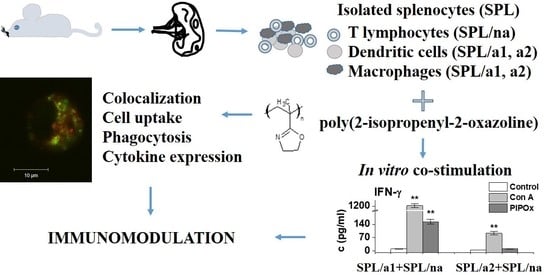
Cell-Mediated Immunoreactivity of Poly(2-isopropenyl-2-oxazoline) as Promising Formulation for Immunomodulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
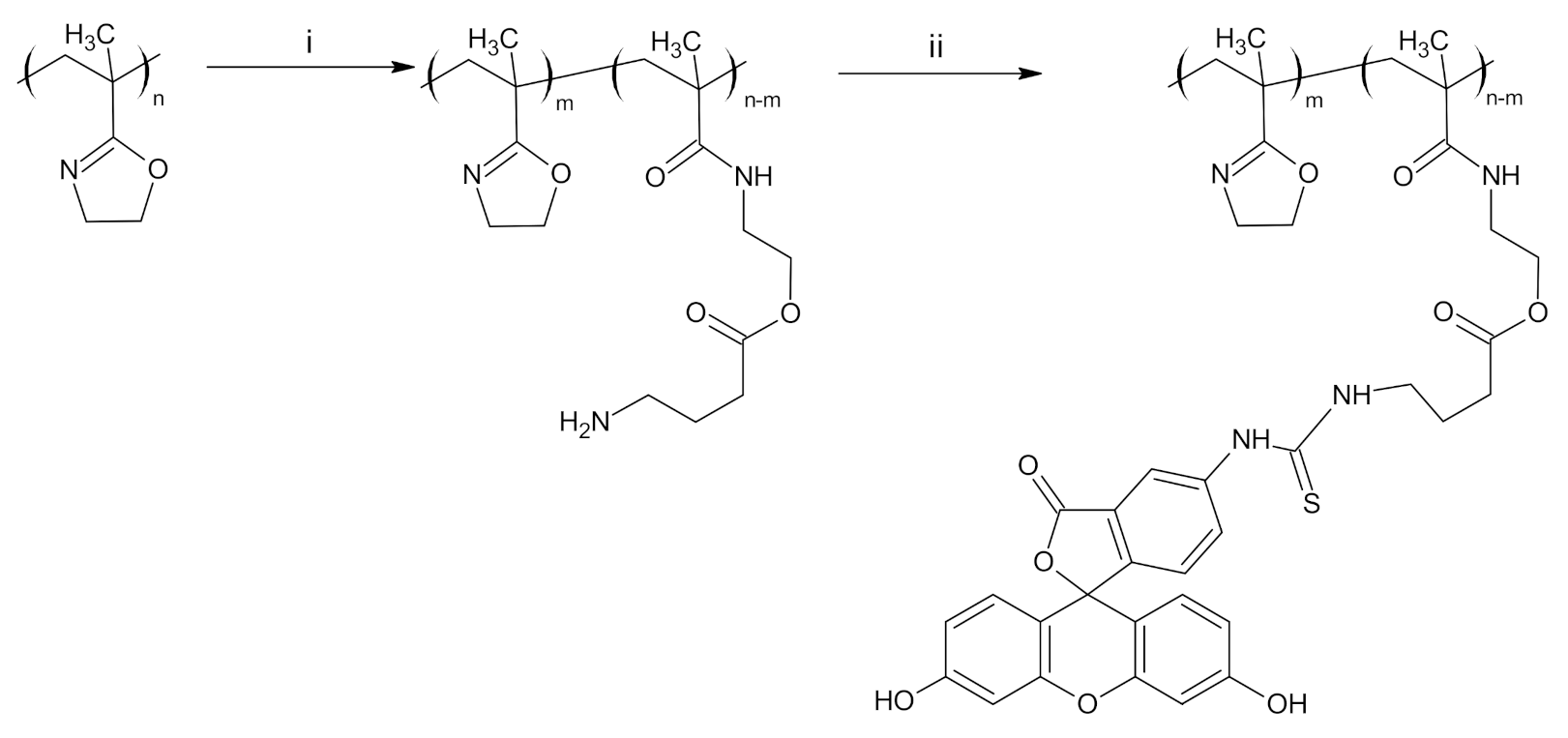
2.2. Synthesis of Polymers
2.3. Analytical Methods
2.4. Preparation of Splenocytes
2.5. Stimulation
2.6. Cytokine Secretion Assay, Immunocytometry, and ELISA
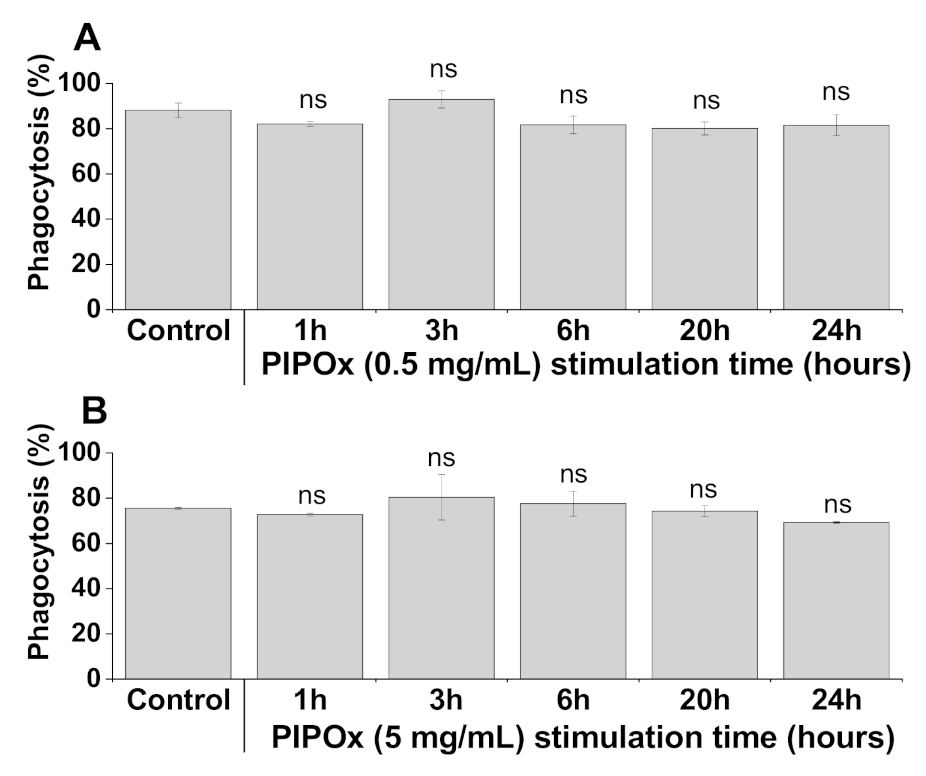
2.7. Phagocytosis
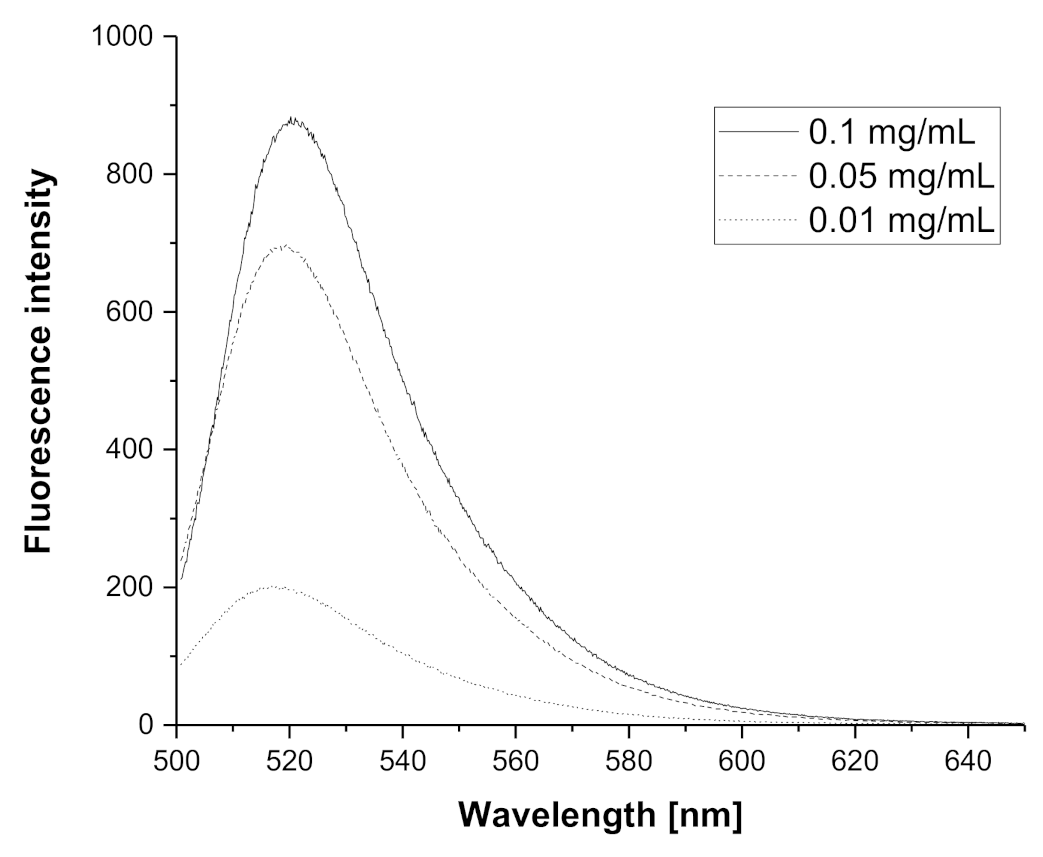
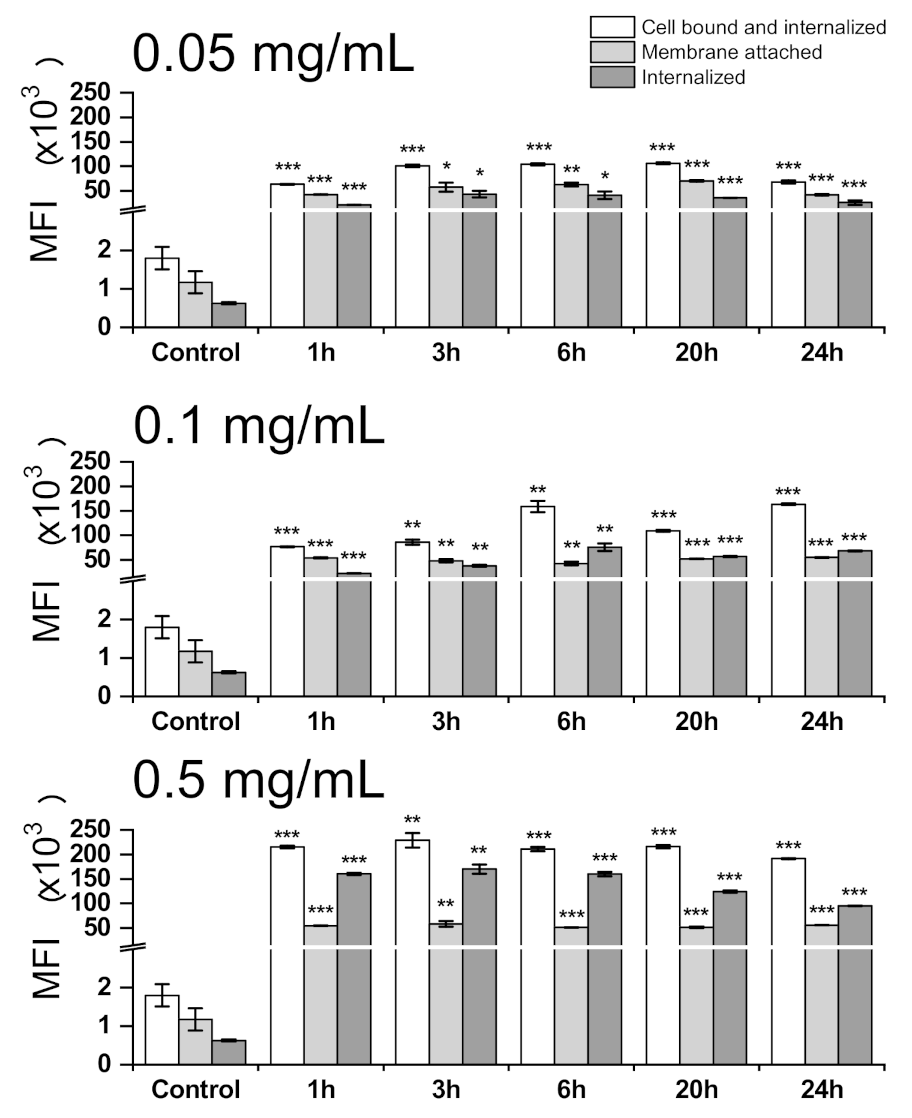
2.8. Cell Uptake and Cell Tracking of PIPOx-FITC by Fluorescence Quenching Cytofluorometric Assay
2.9. Colocalization of PIPOx-FITC in Macrophages
2.10. Statistical Analysis
3. Results
3.1. Synthesis and Labeling of PIPOx
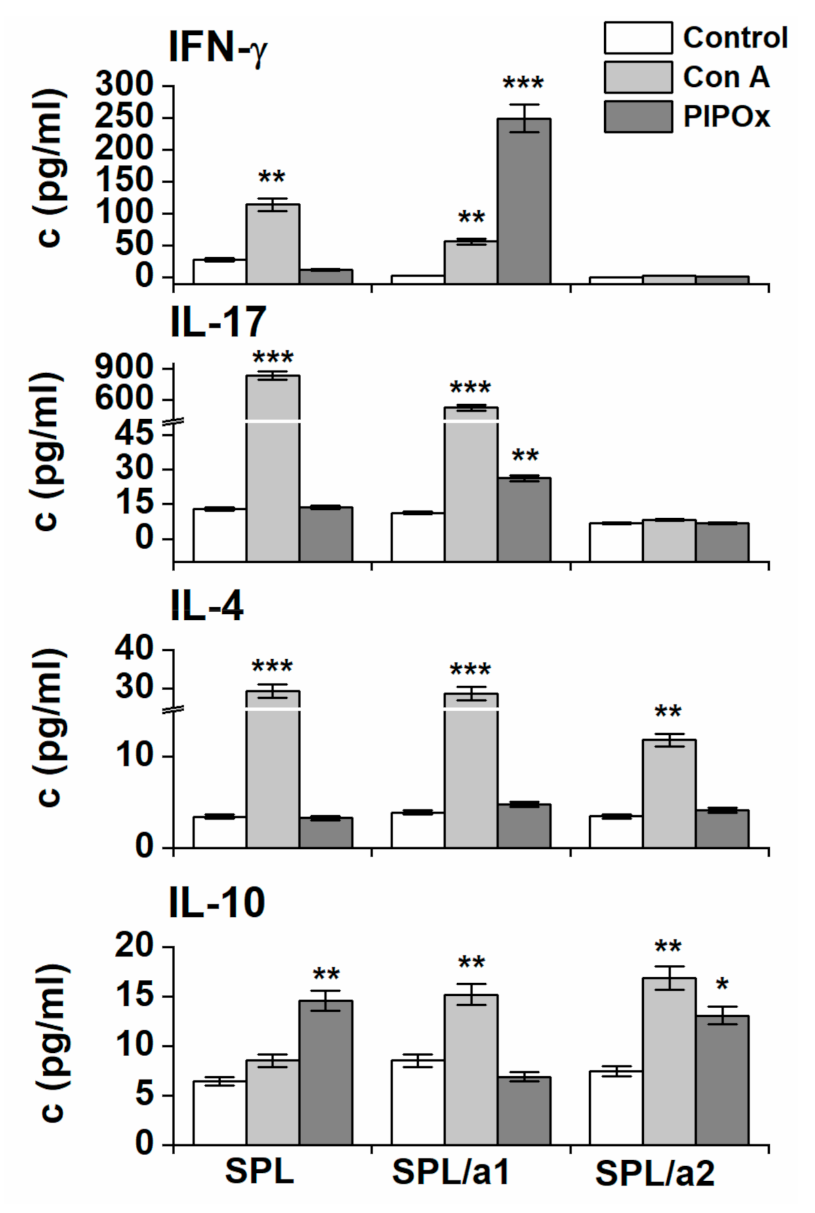
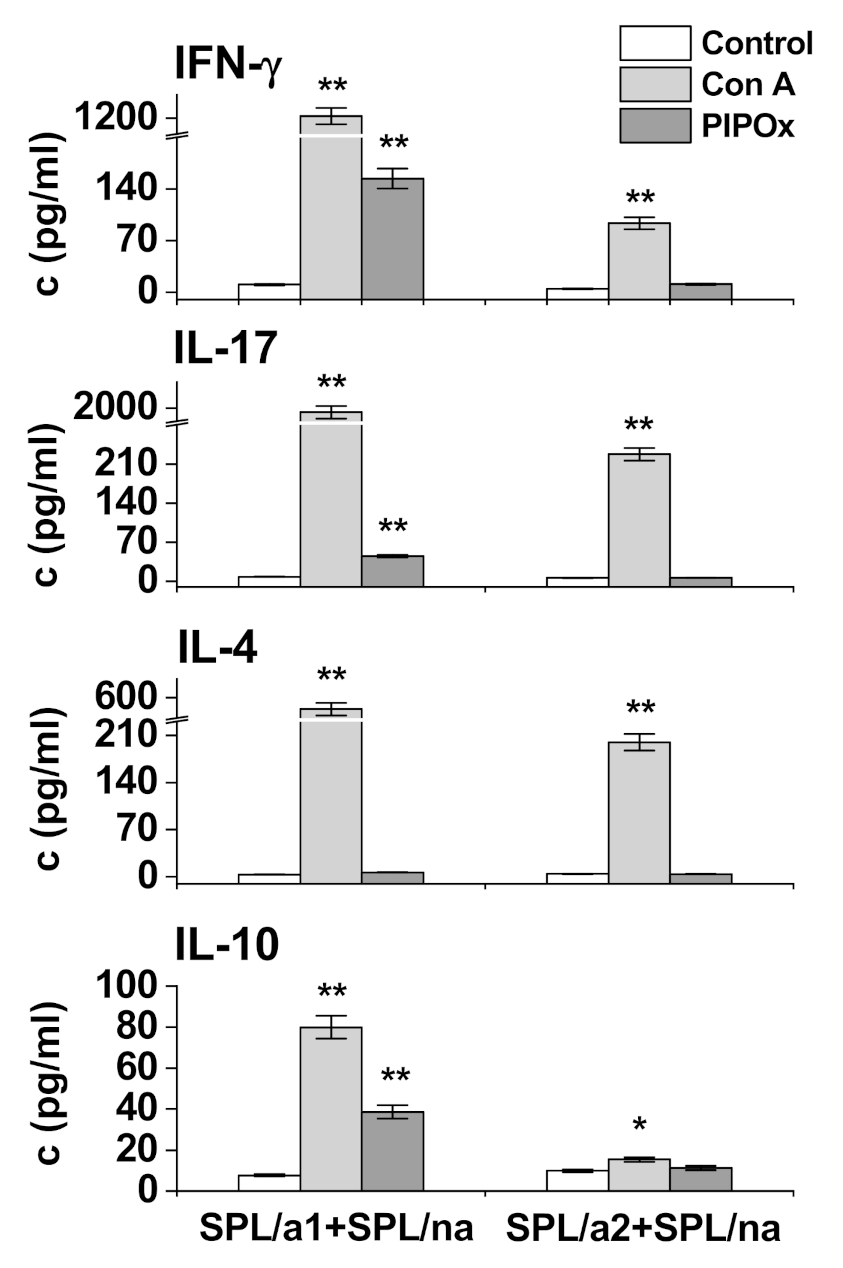
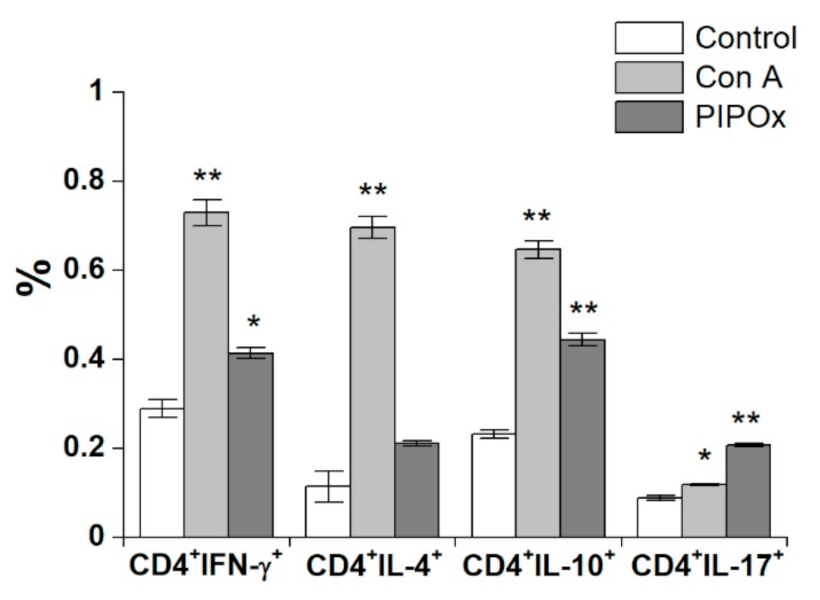
3.2. Immunomodulation Properties
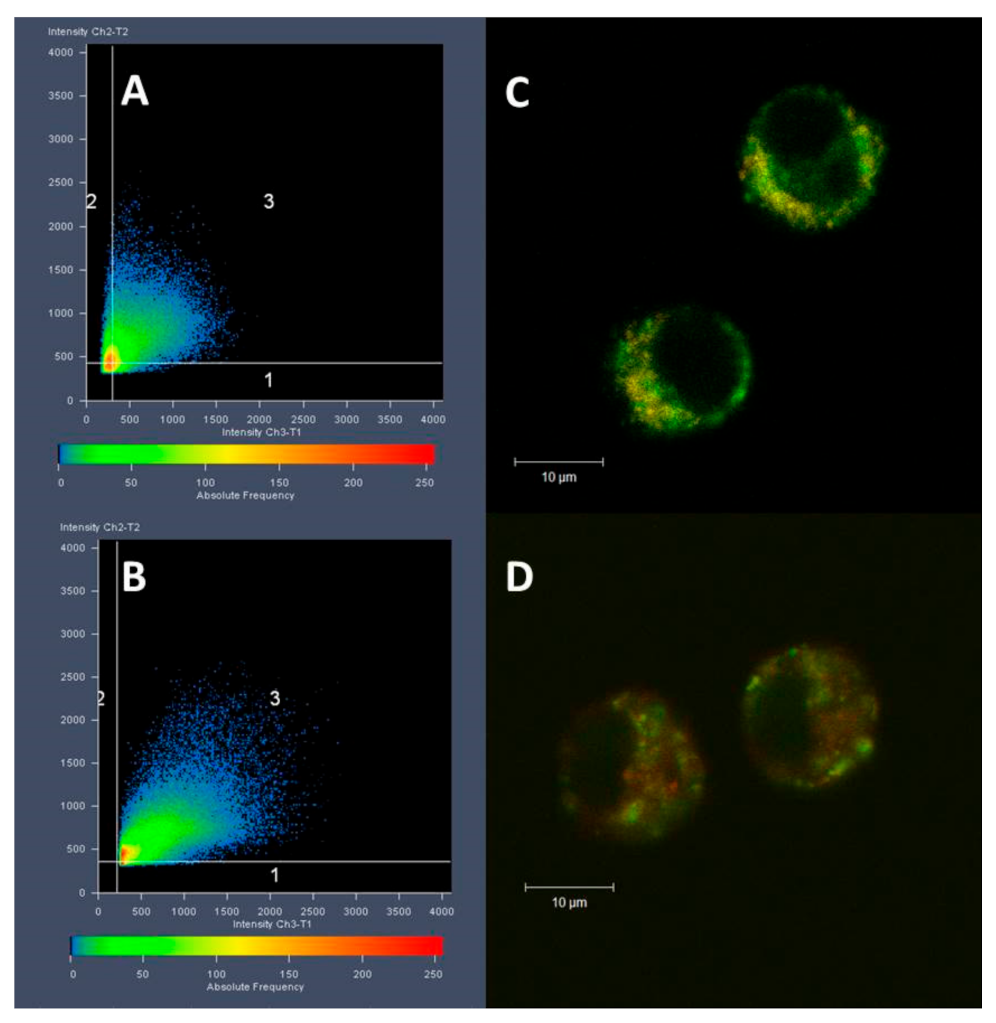
3.3. Cell Internalization and Organelles Tracking Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kronek, J.; Kroneková, Z.; Lustoň, J.; Paulovičová, E.; Paulovičová, L.; Mendrek, B. In vitro bio-immunological and cytotoxicity studies of poly(2-oxazolines). J. Mater. Sci. Mater. Med. 2011, 22, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Lorson, T.; Lübtow, M.M.; Wegener, E.; Haider, M.S.; Borova, S.; Nahm, D.; Jordan, R.; Sokolski-Papkov, M.; Kabanov, A.V.; Luxenhofer, R. Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update. Biomaterials 2018, 178, 204–280. [Google Scholar] [CrossRef]
- Sedláček, O.; Monnery, B.D.; Filippov, S.K.; Hoogenboom, R.; Hrubý, M. Poly(2-oxazoline)s—Are they more advantageous for biomedical applications than other polymers? Macromol. Rapid Commun. 2012, 33, 1648–1662. [Google Scholar] [CrossRef]
- Adams, N.; Schubert, U.S. Poly(2-oxazolines) in biological and biomedical application contexts. Adv. Drug Deliv. Rev. 2007, 59, 1504–1520. [Google Scholar] [CrossRef]
- Verbraeken, B.; Monnery, B.D.; Lava, K.; Hoogenboom, R. The chemistry of poly(2-oxazoline)s. Eur. Polym. J. 2017, 88, 451–469. [Google Scholar] [CrossRef]
- Aoi, K.; Okada, M. Polymerization of oxazolines. Prog. Polym. Sci. 1996, 21, 151–208. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly (ethylene oxide) and poly (2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Jutková, A.; Šrámková, P.; Lenkavská, L.; Huntošová, V.; Chorvát, D.; Miškovský, P.; Jancura, D.; Kronek, J. Unravelling the Excellent Chemical Stability and Bioavailability of Solvent Responsive Curcumin-Loaded 2-Ethyl-2-oxazoline-grad-2-(4-dodecyloxyphenyl)-2-oxazoline Copolymer Nanoparticles for Drug Delivery. Biomacromolecules 2018, 19, 2459–2471. [Google Scholar] [CrossRef] [PubMed]
- Lava, K.; Verbraeken, B.; Hoogenboom, R. Poly(2-oxazoline)s and click chemistry: A versatile toolbox toward multi-functional polymers. Eur. Polym. J. 2015, 65, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, M.; Sano, Y. Reactivity of N-alkylated cyclic iminoether salts having vinyl groups, 2) Polymerization and copolymerization of 2-isopropenyl-3-methyl-2-oxazolinium salts. Makromol. Chem. 1986, 187, 1807–18017. [Google Scholar] [CrossRef]
- Miyamoto, M.; Sano, Y.; Kimura, Y.; Saegusa, T. “Spontaneous” vinyl polymerization of 2-vinyl-2-oxazolines. Macromolecules 1985, 18, 1641–1648. [Google Scholar] [CrossRef]
- Kagiya, T.; Matsuda, T.; Zushi, K. Radical Copolymerization of 2-lsopropenyl-2-oxazoline with Styrene in the Presence of Lewis Acids. J. Macromol. Sci. Chem. 1972, 6, 1349–1372. [Google Scholar] [CrossRef]
- Palem, R.R.; Ganesh, S.D.; Saha, N.; Kronek, J.; Saha, P. ‘Green’ synthesis of silver polymer Nanocomposites of poly(2-isopropenyl-2-oxazoline-co-N vinylpyrrolidone) and its catalytic activity. J. Polym. Res. 2018, 25, 152. [Google Scholar] [CrossRef]
- Kagiya, T.; Matsuda, T. Selective Polymerization of 2-Isopropenyl-2-oxazoline and Cross-linking Reaction of the Polymers. Polym. J. 1972, 3, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, G.; Uzunova, V.; Hartweg, M.; Beyer, V.; Napier, R.; Becer, C.R. The effect of linker length on ConA and DC-SIGN binding of S-glucosyl functionalized poly(2-oxazoline)s. Polym. Chem. 2018, 9, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Kagiya, T.; Matsuda, T.; Nakato, M.; Hirata, R. Polymerization of 2-Oxazolines. IV. The Structure and the Reactivity of 2-Substituted-2-oxazolines and Oxazolinium Perchlorates. J. Macromol. Sci. Chem. 1972, 6, 1631–1652. [Google Scholar] [CrossRef]
- Kroneková, Z.; Mikulec, M.; Petrenčíková, N.; Paulovičová, E.; Paulovičová, L.; Jančinová, V.; Nosál’, R.; Reddy, P.S.; Shimoga, G.D.; Chorvát, D.; et al. Ex Vivo and In Vitro Studies on the Cytotoxicity and Immunomodulative Properties of Poly(2-isopropenyl-2-oxazoline) as a New Type of Biomedical Polymer. Macromol. Biosci. 2016, 16, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Y.; Chen, E.Y.X. Synthesis of Pyridine- and 2-Oxazoline-Functionalized Vinyl Polymers by Alane-Based Frustrated Lewis Pairs. Synlett 2014, 25, 1534–1538. [Google Scholar]
- Feng, H.; Changez, M.; Hong, K.; Mays, J.W.; Kang, N.G. 2-Isopropenyl-2-oxazoline: Well-Defined Homopolymers and Block Copolymers via Living Anionic Polymerization. Macromolecules 2017, 50, 54–62. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Thill, B.P.; Fazio, M.J. Ionic Oligomerization and Polymerization of 2-Alkenyl-2-oxazolines. Polym. J. 1980, 12, 661–675. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Salzinger, S.; Soller, B.S.; Rieger, B. Rare Earth Metal-Mediated Group-Transfer Polymerization: From Defined Polymer Microstructures to High-Precision Nano-Scaled Objects. J. Am. Chem. Soc. 2013, 135, 8810–8813. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Neuwirth, T.; Kempe, K.; Ozkahraman, B.; Tamahkar, E.; Mert, H.; Becer, C.R.; Schubert, U.S. 2-Isopropenyl-2-oxazoline: A versatile monomer for functionalization of polymers obtained via RAFT. Macromolecules 2012, 45, 20–27. [Google Scholar] [CrossRef]
- Raus, V.; Hološ, A.; Kronek, J.; Mosnáček, J. Well-Defined Linear and Grafted Poly(2-isopropenyl-2-oxazoline)s Prepared via Copper-Mediated Reversible-Deactivation Radical Polymerization Methods. Macromolecules 2020, 53, 2077–2087. [Google Scholar] [CrossRef]
- Nishikubo, T.; Kameyama, A.; Tokai, H. Synthesis of Polymers in Aqueous Solutions. Selective Addition Reaction of Poly(2isopropenyl-2-oxazoline) with Thiols and Carboxylic Acids in Aqueous Solutions. Polym. J. 1996, 28, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Jerca, F.A.; Jerca, V.V.; Anghelache, A.M.; Vuluga, D.M.; Hoogenboom, R. Poly(2-isopropenyl-2-oxazoline) as a versatile platform towards thermoresponsive copolymers. Polym. Chem. 2018, 9, 3473–3478. [Google Scholar] [CrossRef]
- Kronek, J.; Šrámková, P.; Kroneková, Z.; Zahoranová, A.; Petrenčíková, N.; Kleinová, A.; Mrlík, M.; Mosnáček, J. Functional polymers based on unsaturated 2-oxazolines: From thermosensitive polymers to hydrogels. Abstr. Pap. Am. Chem. Soc. 2016, 252, 2. [Google Scholar]
- Xu, X.; Jerca, F.A.; Jerca, V.V.; Hoogenboom, R. Covalent Poly(2-Isopropenyl-2-Oxazoline) Hydrogels with Ultrahigh Mechanical Strength and Toughness through Secondary Terpyridine Metal-Coordination Crosslinks. Adv. Funct. Mater. 2019, 29, 1904886. [Google Scholar] [CrossRef] [Green Version]
- Jerca, F.A.; Anghelache, A.M.; Ghibu, E.; Cecoltan, S.; Stancu, I.C.; Trusca, R.; Vasile, E.; Teodorescu, M.; Vuluga, D.M.; Hoogenboom, R.; et al. Poly(2-isopropenyl-2-oxazoline) Hydrogels for Biomedical Applications. Chem. Mater. 2018, 30, 7938–7949. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Jerca, F.A.; Van Hecke, K.; Jerca, V.A.; Hoogenboom, R. High compression strength single network hydrogels with pillar[5]arene junction points. Mater. Horiz. 2020, 7, 566–573. [Google Scholar] [CrossRef] [Green Version]
- Leiske, M.N.; Mahmoud, A.M.; Warne, N.M.; Goos, J.A.C.M.; Pascual, S.; Montembault, V.; Fontaine, L.; Davis, T.P.; Whittaker, M.R.; Kempe, K. Poly(2-isopropenyl-2-oxazoline)—A structural analogue to poly(vinyl azlactone) with Orthogonal Reactivity. Polym. Chem. 2020, 11, 5681–5692. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Bhise, N.S.; Evangelista, M.B.; Rouwkema, J.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Vrana, N.E.; Khademhosseini, A. Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response. Trends Biotechnol. 2016, 34, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Mallapragada, S.K.; Narasimhan, B. Immunomodulatory biomaterials. Int. J. Pharm. 2008, 364, 265–271. [Google Scholar] [CrossRef]
- Katholnig, K.; Linke, M.; Pham, H.; Hengstschläger, M.; Weichhart, T. Immune responses of macrophages and dendritic cells regulated by mTOR signaling. Biochem. Soc. Trans. 2013, 41, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. The macrophage: Past, present and future. Eur. J. Immunol. 2007, 37, S9–S17. [Google Scholar] [CrossRef]
- Price, J.V.; Vance, R.E. The macrophage paradox. Immunity 2014, 41, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Rostama, H.M.; Singh, S.; Salazar, F.; Magennis, P.; Hook, A.; Singhb, T.; Vrana, N.E.; Alexander, M.R.; Ghaemmaghami, A.M. The impact of surface chemistry modification on macrophage polarization. Immunobiology 2016, 221, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, Y.; Jiang, Y.; Shao, J.; Sun, X.; Chen, J.; Dong, L.; Zhang, J. Anti-tumor immune responses of tumor-associated macrophages via toll-like receptor 4 triggered by cationic polymers. Biomaterials 2013, 34, 746–755. [Google Scholar] [CrossRef]
- CXP Software, Flow Cytometry Software; Beckman Coulter Inc.: Fullerton, CA, USA.
- ZEN software, Digital Microscope Software; Zeiss: Jena, Germany, 2014.
- Reddy, S.T.; Swartz, M.A.; Hubbell, J.A. Targeting dendritic cells with biomaterials: Developing the next generation of vaccines. Trends Immunol. 2006, 27, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Savitsky, K.; Yu, X. Combined strategies for tumor immunotherapy with nanoparticles. Clin. Transl. Oncol. 2019, 21, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Li, X.; Wen, M.; Geng, P.; Ren, X.; Wang, Z.; Chen, Z. Doxorubicin-Loaded Bi-PEG Nanoparticles as Novel Chemo-Photothermal Nanoagents for Efficiently Killing Cancer Cells. J. Nanosci. Nanotechnol. 2020, 20, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J. Dendritic cell, toll-like receptor, and the immune system. J. Cancer Mol. 2006, 2, 213–215. [Google Scholar]
- Gündel, D.; Allmeroth, M.; Reime, S.; Zentel, R.; Thews, O. Endocytotic uptake of HPMA-based polymers by different cancer cells: Impact of extracellular acidosis and hypoxia. Int. J. Nanomed. 2017, 12, 5571–5584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolonko, E.M.; Kiessling, L.L. A Polymeric Domain That Promotes Cellular Internalization. J. Am. Chem. Soc. 2008, 130, 5626–5627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alconcel, S.N.S.; Baas, A.S.; Maynard, H.D. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym.Chem. 2011, 2, 1442–1448. [Google Scholar] [CrossRef]
- Mero, A.; Fang, Z.; Pasut, G.; Veronese, F.M.; Viegas, T.X. Selective conjugation of poly(2-ethyl 2-oxazoline) to granulocyte colony stimulating factor. J. Control. Release 2012, 159, 353–361. [Google Scholar] [CrossRef]
- Kronek, J.; Lustoň, J.; Kroneková, Z.; Paulovičová, E.; Farkaš, P.; Petrenčíková, N.; Paulovičová, L.; Janigová, I. Synthesis and bioimmunological efficiency of poly(2-oxazolines) containing a free amino group. J. Mater. Sci.: Mater. Med. 2010, 21, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Kronek, J.; Paulovičová, E.; Kroneková, Z.; Paulovičová, L.; Lustoň, J. Immunomodulatory efficiency of poly(2-oxazolines). J. Mater. Sci. Mater. Med. 2012, 23, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
| Cell Population | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD11c+ (%) | CD14+ (%) |
|---|---|---|---|---|---|
| Splenocytes | 30.8 ± 2.2 | 22.3 ± 1.8 | 11.8 ± 2.6 | 8.1 ± 1.7 | 5.3 ± 1.3 |
| 1st adherence period | 16.9 ± 3.1 | 15.7 ± 2.2 | 7.0 ± 1.3 | 23.3 ± 2.3 | 10.0 ± 3.1 |
| 2nd adherence period | 14.1 ± 2.9 | 14.7 ± 3.1 | 6.5 ± 1.2 | 35.4 ± 3.4 | 9.1 ± 2.5 |
| non-adherent cells | 33.2 ± 2.1 | 28.3 ± 3.1 | 12.4 ± 2.3 | 5.3 ± 1.2 | 3.3 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulovičová, E.; Kroneková, Z.; Paulovičová, L.; Majerčíková, M.; Kronek, J. Cell-Mediated Immunoreactivity of Poly(2-isopropenyl-2-oxazoline) as Promising Formulation for Immunomodulation. Materials 2021, 14, 1371. https://doi.org/10.3390/ma14061371
Paulovičová E, Kroneková Z, Paulovičová L, Majerčíková M, Kronek J. Cell-Mediated Immunoreactivity of Poly(2-isopropenyl-2-oxazoline) as Promising Formulation for Immunomodulation. Materials. 2021; 14(6):1371. https://doi.org/10.3390/ma14061371
Chicago/Turabian StylePaulovičová, Ema, Zuzana Kroneková, Lucia Paulovičová, Monika Majerčíková, and Juraj Kronek. 2021. "Cell-Mediated Immunoreactivity of Poly(2-isopropenyl-2-oxazoline) as Promising Formulation for Immunomodulation" Materials 14, no. 6: 1371. https://doi.org/10.3390/ma14061371