Electrorheological Fluids of GO/Graphene-Based Nanoplates
Abstract
:1. Introduction
2. GO-Based ER Fluids
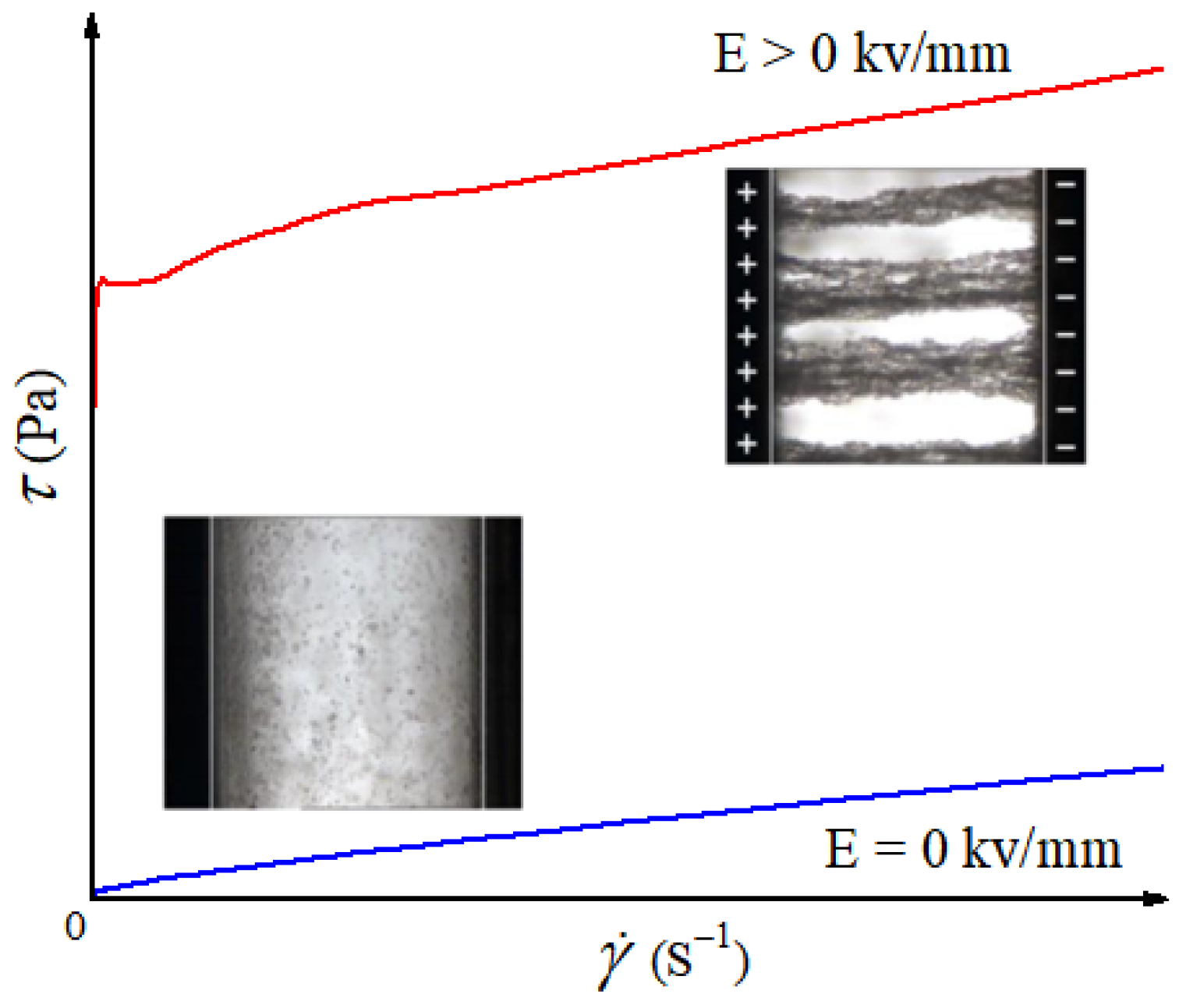
2.1. Pure GO
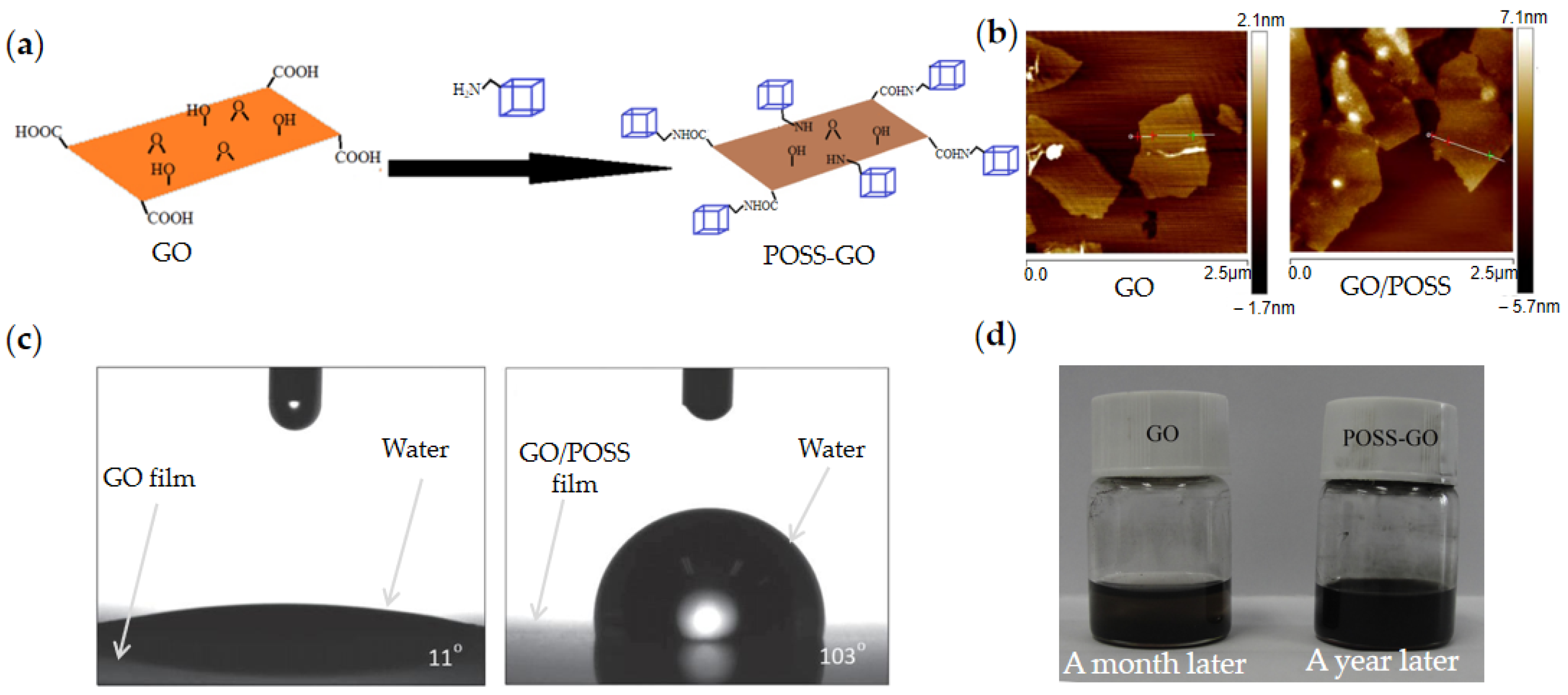
2.2. Molecular-Modified GO
2.3. Nanoparticle-Modified GO
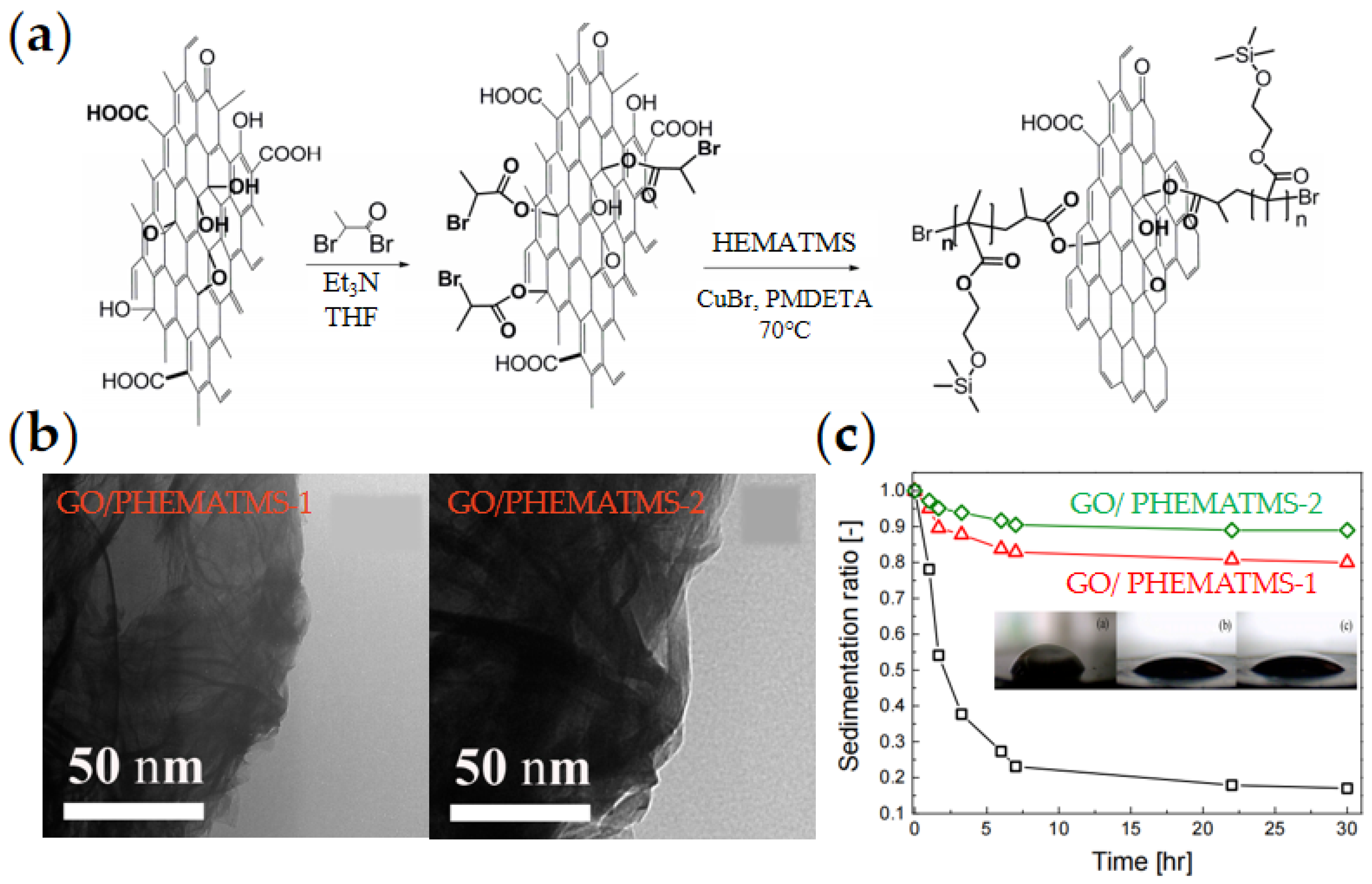
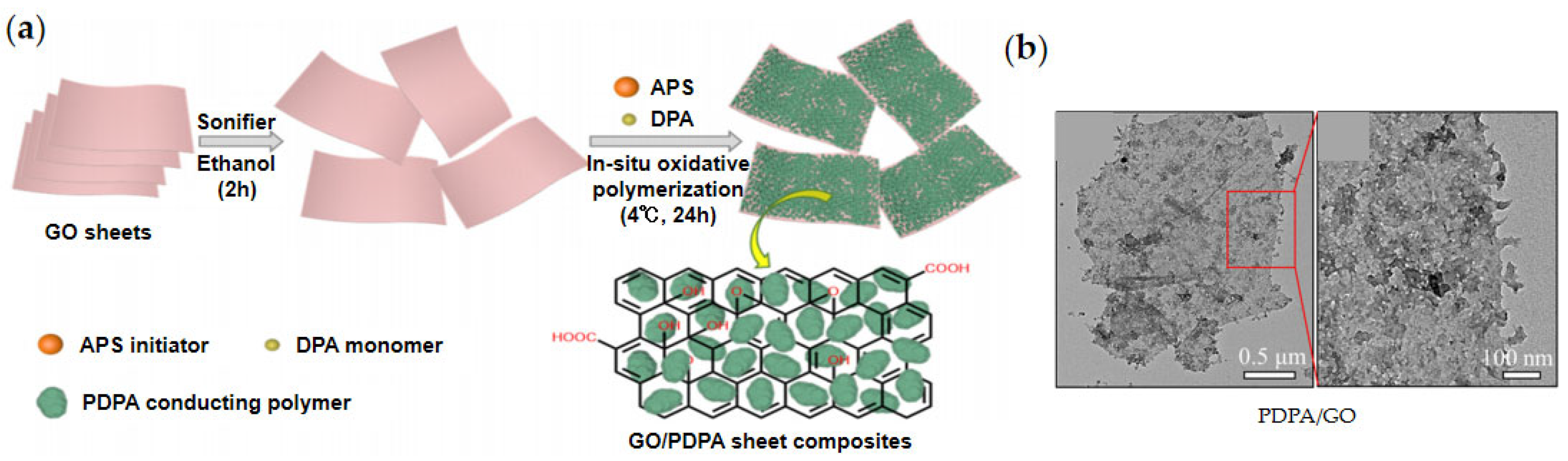
2.4. Organic-Modified GO
3. Graphene Composite ER Fluids
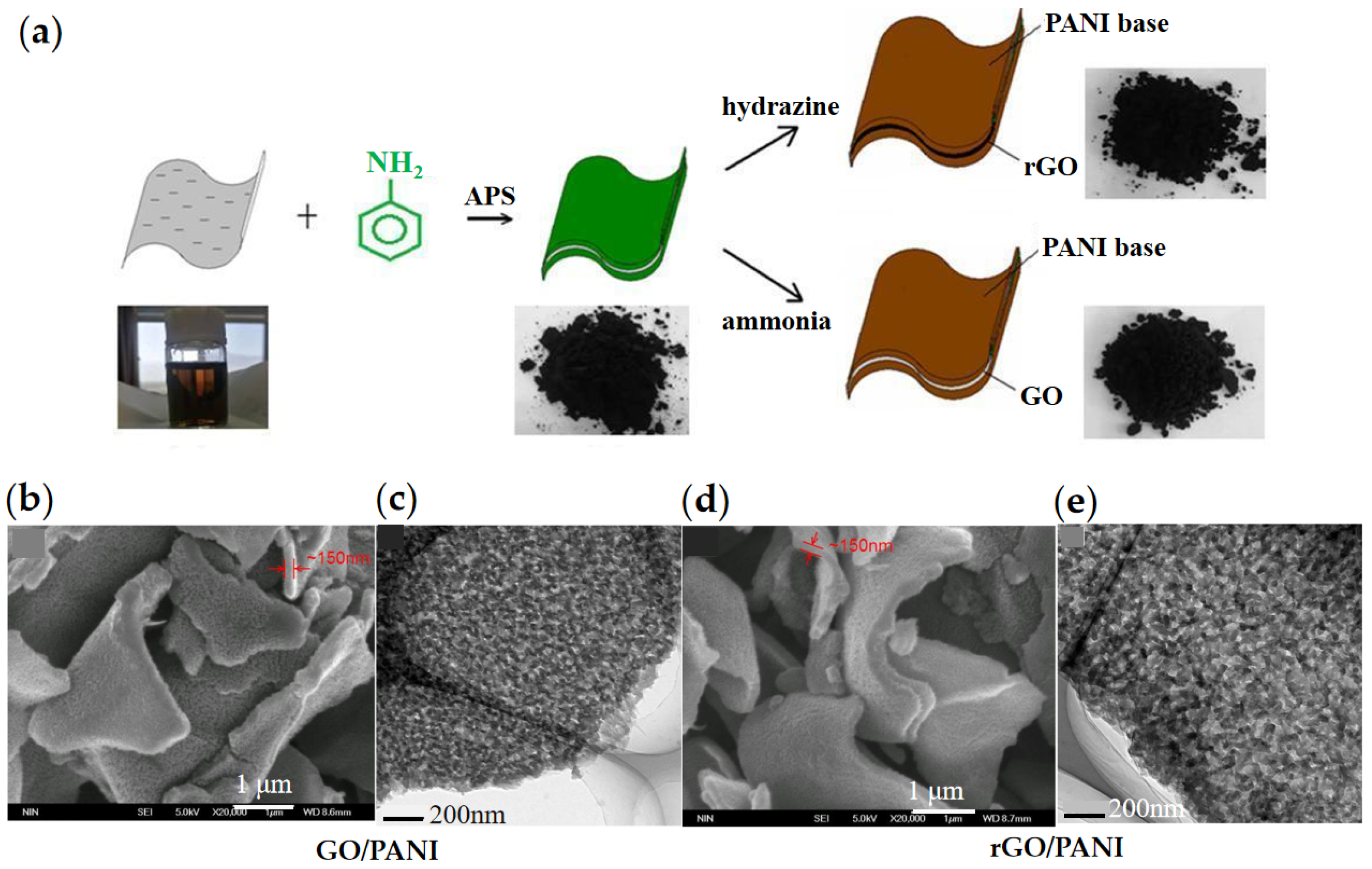
3.1. Graphene/Organic Composites
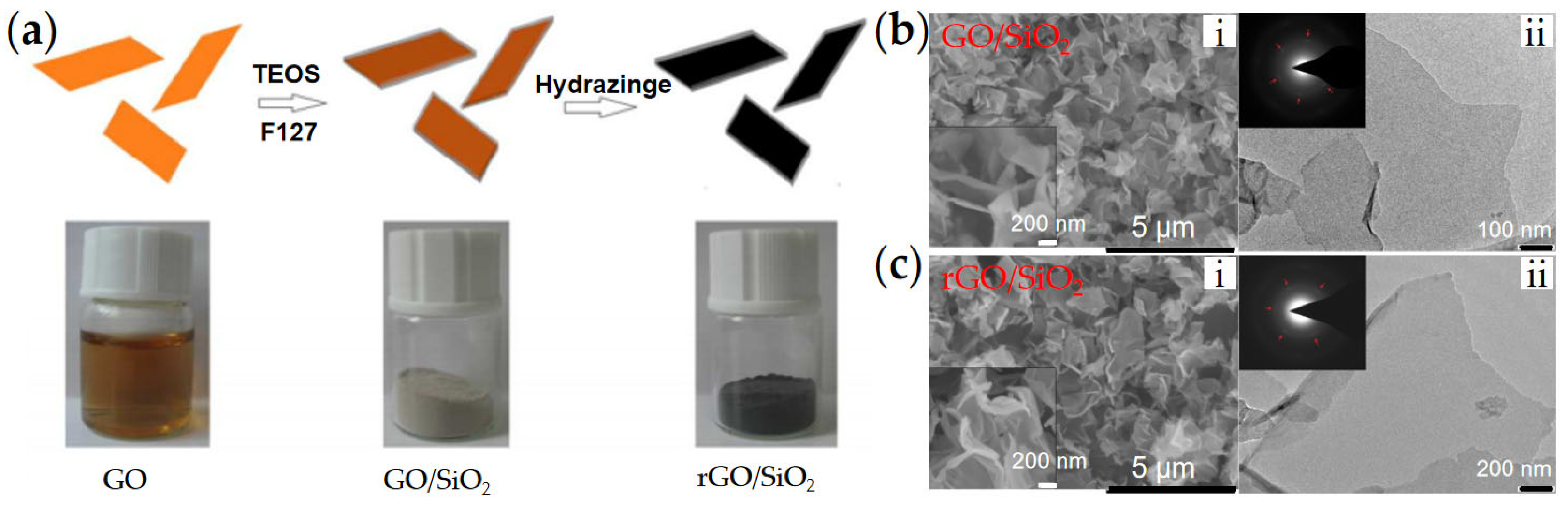
3.2. Graphene/SiO2
3.3. Graphene/C
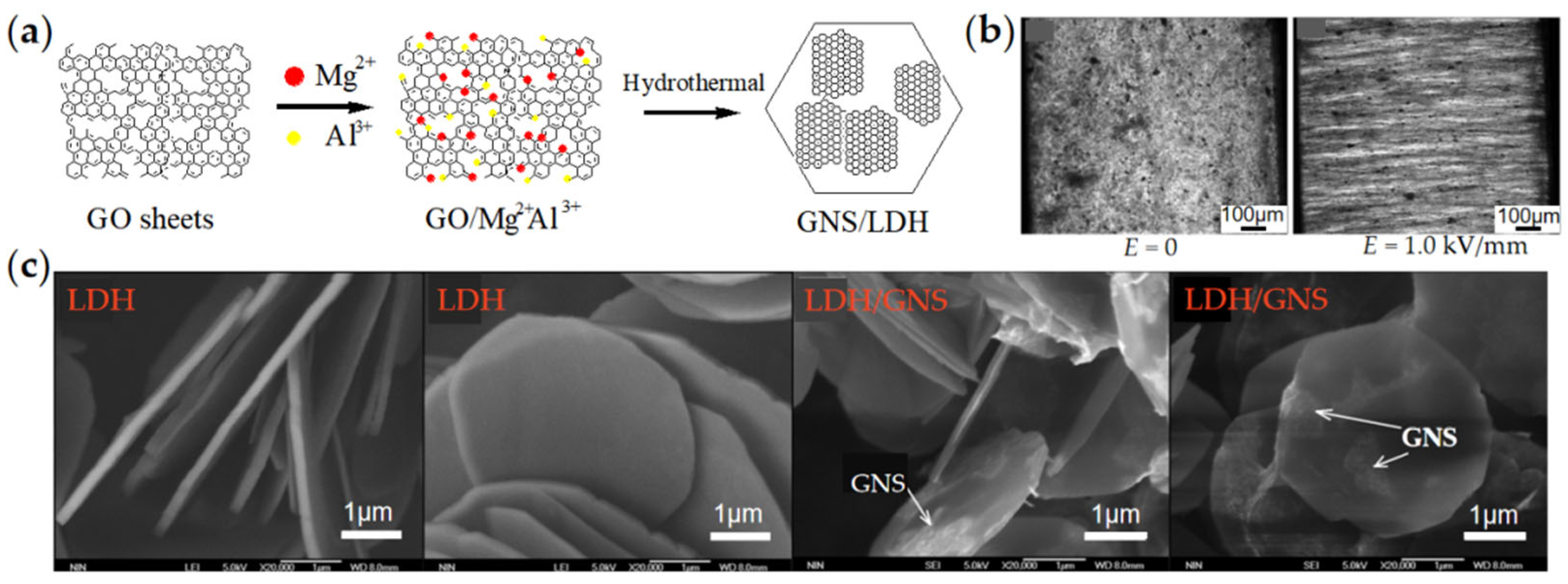
3.4. LDH/Graphene
4. ER Property of Graphene and GO-Based Composites
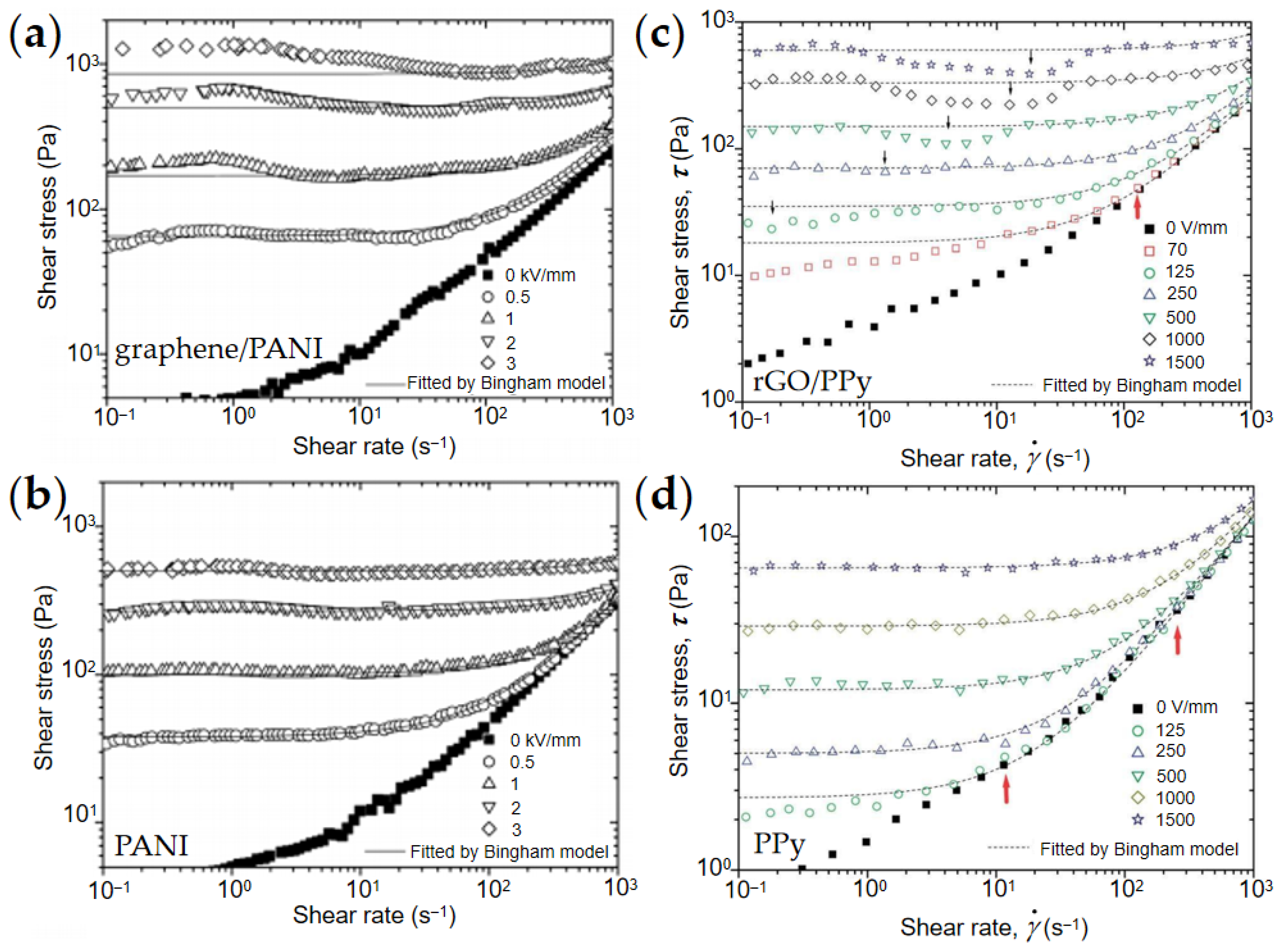
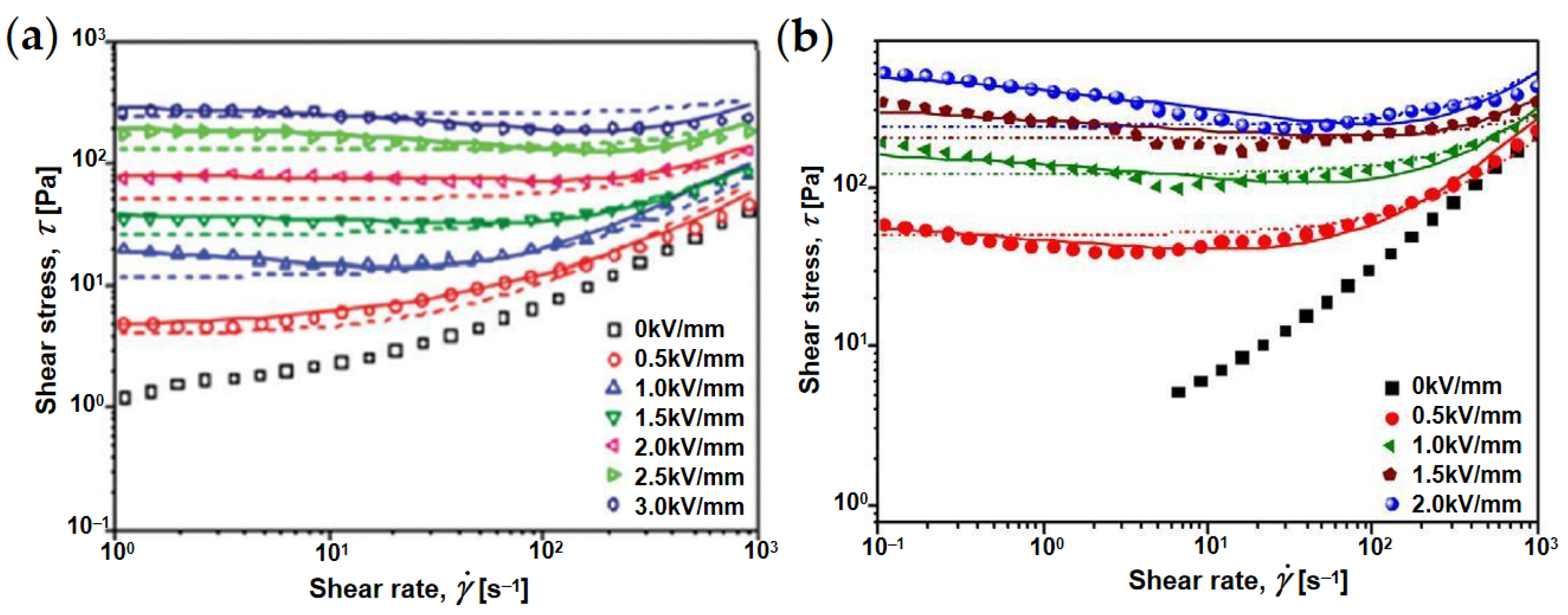
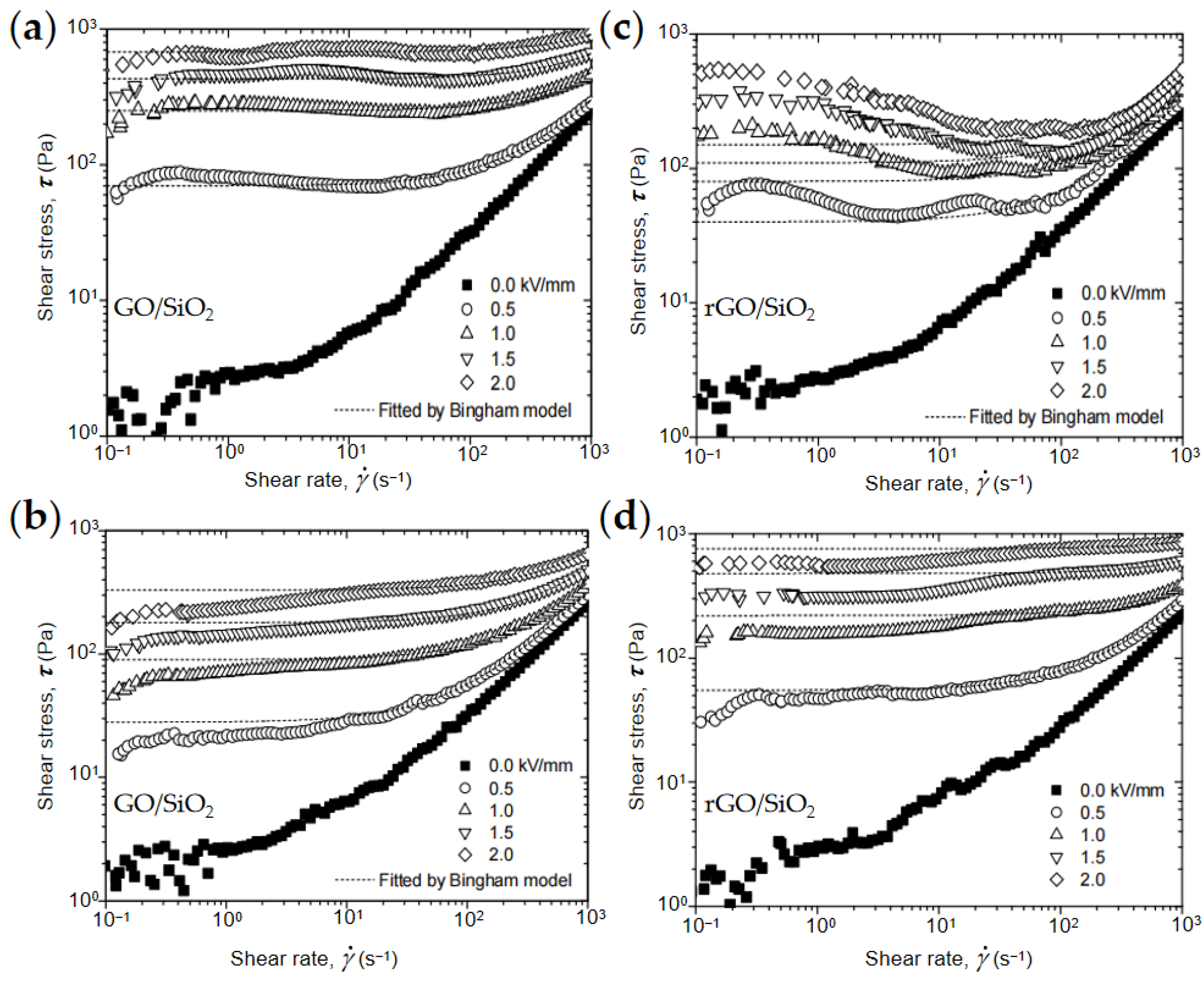
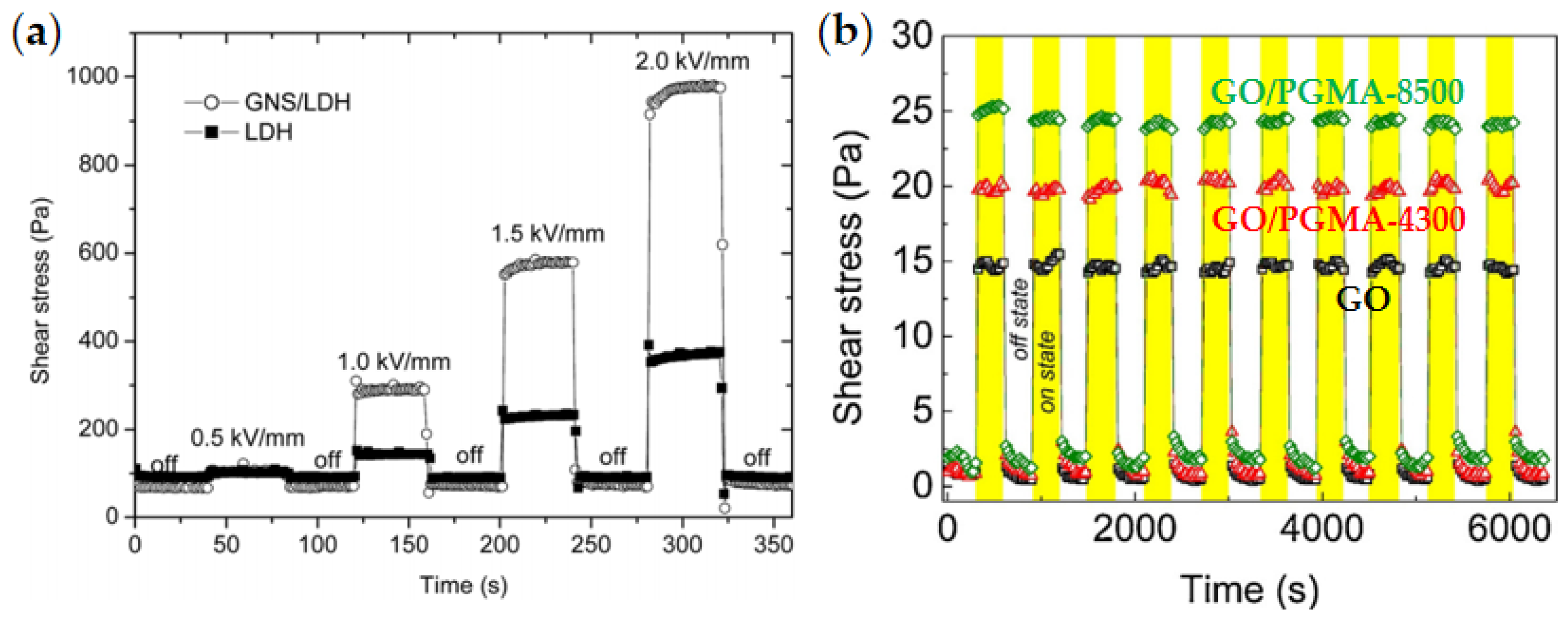
4.1. Rheological Property
4.2. Yield Stress
4.3. Dynamic Viscoelasticity
4.4. Reversibility of ER Effect
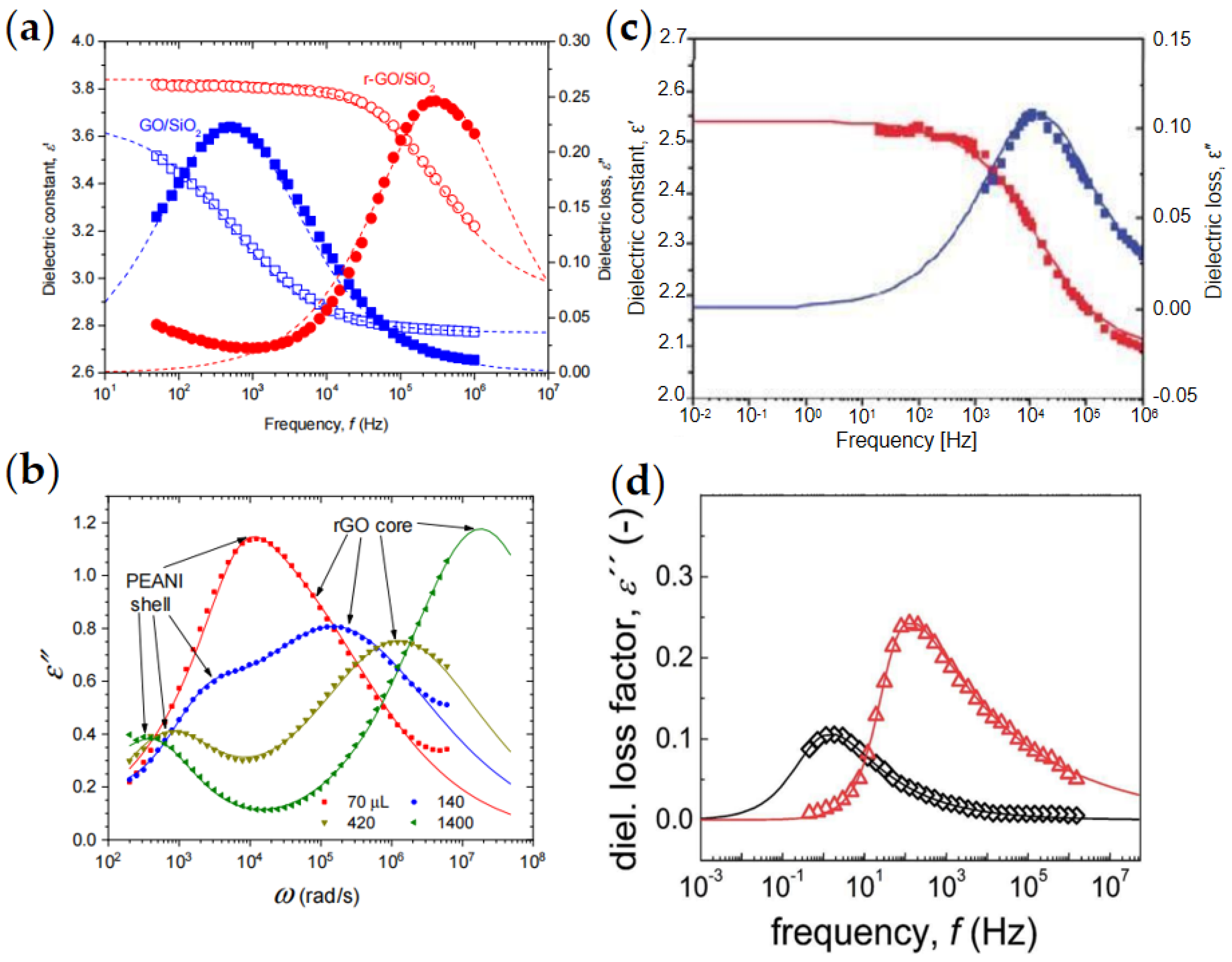
5. Dielectric Properties
6. Conclusions
- (1).
- In the material preparation, due to the problems of dispersion and aggregation of GO/graphene, the repeatability of the synthesis of GO/graphene-based nanoplates is sometimes relatively low. This restrains the mass preparation and wide application of graphene-based ER materials.
- (2).
- The material performance of GO/graphene-based nanoplate ER materials is relatively singular, and there is little development of multifunctional smart GO/graphene-based ER materials. Multifunctional GO/graphene-based ER materials may be more suitable for the requirements of future technologies for smart materials, for example, to develop GO/graphene-based nanoplate ER materials with dual responses to electric and magnetic fields.
- (3).
- In applications, present research on GO/graphene-based ER materials is usually limited to field-controlled rheological properties. In addition to electrical properties, graphene also has excellent thermal, optical, and mechanical properties, which are conducive to expanding the application fields of GO/graphene-based ER materials. For example, by combining ER effect and the high thermal conductivity of graphene, it is possible to realize the electric field control of heat conduction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanway, R. Smart fluids: Current and future developments. Mater. Sci. Technol. 2004, 20, 931–939. [Google Scholar] [CrossRef]
- Yoon, C.M.; Jang, Y.; Noh, J.; Kim, J.; Jang, J. Smart Fluid System Dually Responsive to Light and Electric Fields: An Electrophotorheological Fluid. ACS Nano 2017, 11, 9789–9801. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Noh, J.; Hong, S.; Kim, Y.K.; Jang, J. Dual Stimuli-Responsive Smart Fluid of Graphene Oxide-Coated Iron Oxide/Silica Core/Shell Nanoparticles. Chem. Mater. 2016, 28, 2624–2633. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat. Rev. Mater. 2017, 2, 17046. [Google Scholar] [CrossRef]
- Sakhaee-Pour, A.; Ahmadian, M.T.; Vafai, A. Potential application of single-layered graphene sheet as strain sensor. Solid State Commun. 2008, 147, 336–340. [Google Scholar] [CrossRef]
- Choi, S.M.; Jhi, S.H.; Son, Y.W. Controlling energy gap of bilayer graphene by strain. Nano Lett. 2010, 10, 3486–3489. [Google Scholar] [CrossRef] [Green Version]
- Cocco, G.; Cadelano, E.; Colombo, L. Gap opening in graphene by shear strain. Phys. Rev. B 2010, 81, 241412. [Google Scholar] [CrossRef] [Green Version]
- Acik, M.; Lee, G.; Mattevi, C.; Chhowalla, M.; Cho, K.; Chabal, Y.J. Unusual infrared-absorption mechanism in thermally reduced graphene oxide. Nat. Mater. 2010, 9, 840–845. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, T.; Zhao, L.; Sun, W.; Liu, X.; Tong, Z. Fast self-healing of graphene oxide-hectorite clay-poly(N,N-dimethylacrylamide) hybrid hydrogels realized by near-infrared irradiation. ACS Appl. Mater. Interfaces 2014, 6, 22855–22861. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.; Kim, J.; Kim, T.; Kim, W.J. Photothermally triggered cytosolic drug delivery via endosome disruption using a functionalized reduced graphene oxide. ACS Nano 2013, 7, 6735–6746. [Google Scholar] [CrossRef]
- Zhu, J.; Andres, C.; Xu, J.D.; Ramamoorthy, A.; Tsotsis, T.; Kotov, N. Pseudonegative thermal expansion and the state of water in graphene oxide layered assemblies. ACS Nano 2012, 6, 8357–8365. [Google Scholar] [CrossRef]
- Ren, L.; Qiu, J.; Wang, S. Thermo-adaptive functionality of graphene/polydimethylsiloxane nanocomposites. Smart Mater. Struct. 2012, 21, 105032. [Google Scholar] [CrossRef]
- Ojha, R.P.; Lemieux, P.A.; Dixon, P.K.; Liu, A.J.; Durian, D.J. Statistical mechanics of a gas-fluidized particle. Nature 2004, 427, 521–523. [Google Scholar] [CrossRef]
- Zhang, W.L.; Choi, H.J. Graphene oxide based smart fluids. Soft Matter 2014, 10, 6601–6608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Choi, H.J. Graphene/graphene oxide: A new material for electrorheological and magnetorheological applications. J. Intell. Mater. Syst. Struct. 2015, 26, 1826–1835. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, J.; Choi, H.J. Graphene and Graphene Oxide Composites and Their Electrorheological Applications. J. Nanomater. 2015, 2015, 574637. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.Z.; Kim, J.N.; Choi, H.J. Graphene Oxide and Its Inorganic Composites: Fabrication and Electrorheological Response. Materials 2019, 12, 2185. [Google Scholar] [CrossRef] [Green Version]
- Halsey, T.C. Electrorheological Fluids. Science 1992, 258, 761–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.D.; Choi, H.J. Electrorheological fluids: Smart soft matter and characteristics. Soft Matter 2012, 8, 11961. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Liu, Y.; Zhao, X.; Yin, J. Interfacial Polarization and Electroresponsive Electrorheological Effect of Anionic and Cationic Poly(ionic liquids). ACS Appl. Polym. Mater. 2019, 1, 2862–2874. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Liu, Y.; Zhao, X.; Yin, J. Nonmonotonic Influence of Size of Quaternary Ammonium Countercations on Micromorphology, Polarization, and Electroresponse of Anionic Poly(ionic liquid)s. J. Phys. Chem. B 2020, 124, 2920–2929. [Google Scholar] [CrossRef]
- Sung, K.G.; Seong, M.S.; Choi, S.-B. Performance evaluation of electronic control suspension featuring vehicle ER dampers. Meccanica 2012, 48, 121–134. [Google Scholar] [CrossRef]
- Djokoto, S.S.; Dragasius, E.; Jurenas, V.; Agelin-Chaab, M. Controlling of Vibrations in Micro-Cantilever Beam Using a Layer of Active Electrorheological Fluid Support. IEEE Sens. J. 2020, 20, 4072–4079. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Fang, Y.; Cao, C. Ultra-Precision Processing of Conductive Materials via Electrorheological Fluid-Assisted Polishing. Adv. Eng. Mater. 2020, 23, 2001109. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.-S.; Yang, H.-R.; Zhang, Y. An integrated tool for five-axis electrorheological fluid-assisted polishing. Int. J. Mach. Tools Manuf. 2010, 50, 737–740. [Google Scholar] [CrossRef]
- Delgado-Canto, M.A.; Fernandez-Silva, S.D.; Roman, C.; Garcia-Morales, M. On the Electro-Active Control of Nanocellulose-Based Functional Biolubricants. ACS Appl. Mater. Interfaces 2020, 12, 46490–46500. [Google Scholar] [CrossRef]
- Agrawal, N.; Sharma, S.C. Effect of the ER lubricant behaviour on the performance of spherical recessed hydrostatic thrust bearing. Tribol. Int. 2021, 153, 106621. [Google Scholar] [CrossRef]
- Sheng, P.; Wen, W. Electrorheological Fluids: Mechanisms, Dynamics, and Microfluidics Applications. Annu. Rev. Fluid Mech. 2012, 44, 143–174. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wen, W.; Sheng, P. Smart electroresponsive droplets in microfluidics. Soft Matter 2012, 8, 11589. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kang, S.-R.; Cha, S.W.; Choi, S.-B. An electrorheological spherical joint actuator for a haptic master with application to robot-assisted cutting surgery. Sens. Actuators Phys. 2016, 249, 163–171. [Google Scholar] [CrossRef]
- Zatopa, A.; Walker, S.; Menguc, Y. Fully Soft 3D-Printed Electroactive Fluidic Valve for Soft Hydraulic Robots. Soft Robot. 2018, 5, 258–271. [Google Scholar] [CrossRef]
- Khanicheh, A.; Mintzopoulos, D.; Weinberg, B.; Tzika, A.A.; Mavroidis, C. MR_CHIROD v.2: Magnetic resonance compatible smart hand rehabilitation device for brain imaging. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 91–98. [Google Scholar] [CrossRef]
- Han, Y.-M.; Oh, J.-S.; Kim, S.; Choi, S.-B. Design of multi-degree motion haptic mechanisms using smart fluid-based devices. Mech. Based Des. Struct. Mach. 2016, 45, 135–144. [Google Scholar] [CrossRef]
- Winslow, W.M. Method and means for translating electrical impulses into mechanical force. US Patent 2417850, 25 March 1947. [Google Scholar]
- Weiss, K.D.; Carlson, J.D.; Coulter, J.P. Review: Material Aspects of Electrorheological Systems. J. Intell. Mater. Syst. Struct. 2016, 4, 13–34. [Google Scholar] [CrossRef]
- Khusid, B.; Acrivos, A. Effects of conductivity in electric-field-induced aggregation in electrorheological fluids. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1995, 52, 1669–1693. [Google Scholar] [CrossRef]
- Block, H.; Kelly, J.P. Electro-rheology. J. Phys. D Appl. Phys. 1988, 21, 1661–1677. [Google Scholar] [CrossRef]
- Gast, A.P.; Zukoski, C.F. Electrorheological fluids as colloidal suspensions. Adv. Colloid Interface Sci. 1989, 30, 153–202. [Google Scholar] [CrossRef]
- Block, H.; Kelly, J.P.; Qin, A.; Waton, T. Materials and Mechanisms in Electrorheology. Langmuir 1990, 6, 6–14. [Google Scholar] [CrossRef]
- Filisko, F.E.; Radzilowski, L.H. An intrinsic mechanism for the activity of alumino-silicate based electrorheological materials. J. Rheol. 1990, 34, 539–552. [Google Scholar] [CrossRef]
- Hao, T.; Xu, Z.M.; Xu, Y.Z. Correlation of the Dielectric Properties of Dispersed Particles with the Electrorheological Effect. J. Colloid Interface Sci. 1997, 190, 334–340. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.; Guo, J.; Liu, F.; Li, Z.; Xu, G.; Cui, P. Fabrication of uniform core-shell structural calcium and titanium precipitation particles and enhanced electrorheological activities. Nanotechnology 2009, 20, 055604. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Q.; Guan, J.G.; Guo, J.S. Three Ways to Improve Electrorheological Properties of Polyaniline-Based Suspensions. Appl. Polym. 1997, 64, 1641–1647. [Google Scholar] [CrossRef]
- Wu, S.Z.; Zeng, F.; Shen, J.R. The Electrorheological Properties of Polypyrrole Suspensions. Polym. J. 1998, 30, 451–454. [Google Scholar] [CrossRef] [Green Version]
- Bloodworth, R.; Wendt, E. ER-Fluids Based on Polyurethane Dispersions: Structure and Properties. In Progress in Electrorheology; Springer: Boston, MA, USA, 1995; pp. 185–193. [Google Scholar]
- Dong, Y.; Yin, J.; Zhao, X. Microwave-synthesized poly(ionic liquid) particles: A new material with high electrorheological activity. J. Mater. Chem. A 2014, 2, 9812–9819. [Google Scholar] [CrossRef]
- Wen, W.; Huang, X.; Yang, S.; Lu, K.; Sheng, P. The giant electrorheological effect in suspensions of nanoparticles. Nat. Mater. 2003, 2, 727–730. [Google Scholar] [CrossRef]
- Gong, X.; Wu, J.; Huang, X.; Wen, W.; Sheng, P. Influence of liquid phase on nanoparticle-based giant electrorheological fluid. Nanotechnology 2008, 19, 165602. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; He, J.; Persello, J. Giant electrorheological effects of aluminum-doped TiO2 nanoparticles. Particuology 2010, 8, 442–446. [Google Scholar] [CrossRef]
- Asano, K.; Suto, H.; Yatsuzuka, K. Influence of the Particle Configuration on Electrorheological Effect. J. Electrost. 1997, 40–41, 573–578. [Google Scholar] [CrossRef]
- Kor, Y.K.; See, H. The electrorheological response of elongated particles. Rheol. Acta 2010, 49, 741–756. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, X. Titanate nano-whisker electrorheological fluid with high suspended stability and ER activity. Nanotechnology 2006, 17, 192–196. [Google Scholar] [CrossRef]
- Lozano, K.; Hernandez, C.; Petty, T.W.; Sigman, M.B.; Korgel, B. Electrorheological analysis of nano laden suspensions. J. Colloid Interface Sci. 2006, 297, 618–624. [Google Scholar] [CrossRef]
- Feng, P.; Wan, Q.; Fu, X.Q.; Wang, T.H.; Tian, Y. Anomalous electrorheological behavior of ZnO nanowires. Appl. Phys. Lett. 2005, 87, 033114. [Google Scholar] [CrossRef]
- Liu, Y.D.; Fang, F.F.; Choi, H.J. Silica nanoparticle decorated polyaniline nanofiber and its electrorheological response. Soft Matter 2011, 7, 2782. [Google Scholar] [CrossRef]
- Liu, Y.D.; Kim, H.Y.; Kim, J.E.; Kim, I.G.; Choi, H.J.; Park, S.J. Enhanced effect of dopant on polyaniline nanofiber based electrorheological response. Mater. Chem. Phys. 2014, 147, 843–849. [Google Scholar] [CrossRef]
- Yin, J.; Xia, X.; Xiang, L.; Zhao, X. Conductivity and polarization of carbonaceous nanotubes derived from polyaniline nanotubes and their electrorheology when dispersed in silicone oil. Carbon 2010, 48, 2958–2967. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, K.; Liu, F.; Guo, J.; Liu, X.; Xu, G.; Cui, P. Facile approach to large-scale synthesis of 1D calcium and titanium precipitate (CTP) with high electrorheological activity. ACS Appl Mater. Interfaces 2010, 2, 621–625. [Google Scholar] [CrossRef]
- Wu, J.; Jin, T.; Liu, F.; Guo, J.; Cui, P.; Cheng, Y.; Xu, G. Preparation of rod-like calcium titanyl oxalate with enhanced electrorheological activity and their morphological effect. J. Mater. Chem. C 2014, 2, 5629. [Google Scholar] [CrossRef]
- Noh, J.; Yoon, C.M.; Jang, J. Enhanced electrorheological activity of polyaniline coated mesoporous silica with high aspect ratio. J. Colloid Interface Sci. 2016, 470, 237–244. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, X.; Xia, X.; Xiang, L.; Qiao, Y. Electrorheological fluids based on nano-fibrous polyaniline. Polymer 2008, 49, 4413–4419. [Google Scholar] [CrossRef]
- Xia, X.; Yin, J.; Qiang, P.; Zhao, X. Electrorheological properties of thermo-oxidative polypyrrole nanofibers. Polymer 2011, 52, 786–792. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, C.M.; Hong, J.Y.; Jang, J. Enhanced electrorheological performance of a graphene oxide-wrapped silica rod with a high aspect ratio. J. Mater. Chem. C 2014, 2, 6010. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Lee, E.; Jang, J. Electro-responsive and dielectric characteristics of graphene sheets decorated with TiO2 nanorods. J. Mater. Chem. A 2013, 1, 117–121. [Google Scholar] [CrossRef]
- Li, L.; Yin, J.; Liu, Y.; Zhao, X. Graphene oxide vs. reduced graphene oxide as core substrate for core/shell-structured dielectric nanoplates with different electro-responsive characteristics. J. Mater. Chem. C 2015, 3, 5098–5108. [Google Scholar] [CrossRef]
- Yin, J.; Chang, R.; Kai, Y.; Zhao, X. Highly stable and AC electric field-activated electrorheological fluid based on mesoporous silica-coated graphene nanosheets. Soft Matter 2013, 9, 3910. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J.; Kim, S.G. Electrorheology of Graphene Oxide. ACS Appl. Mater. Interfaces 2012, 4, 2267–2272. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Hong, J.Y.; Jang, J. Highly stable, concentrated dispersions of graphene oxide sheets and their electro-responsive characteristics. Soft Matter 2012, 8, 7348. [Google Scholar] [CrossRef]
- Dhar, P.; Katiyar, A.; Pattamatta, A.; Das, S.K. Large electrorheological phenomena in graphene nano-gels. Nanotechnology 2017, 28, 035702. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guan, Y.; Liu, Y.; Yin, J.; Zhao, X. Highly stable nanofluid based on polyhedral oligomeric silsesquioxane-decorated graphene oxide nanosheets and its enhanced electro-responsive behavior. Nanotechnology 2016, 27, 195702. [Google Scholar] [CrossRef]
- Ilčíková, M.; Mrlík, M.; Babayan, V.; Kasák, P. Graphene oxide modified by betaine moieties for improvement of electrorheological performance. RSC Adv. 2015, 5, 57820–57827. [Google Scholar] [CrossRef]
- Zhang, W.L.; Choi, H.J. Fast and facile fabrication of a graphene oxide/titania nanocomposite and its electro-responsive characteristics. Chem. Commun. 2011, 47, 12286–12288. [Google Scholar] [CrossRef]
- Zhang, W.L.; Tian, Y.; Liu, Y.D.; Song, Z.Q.; Liu, J.Q.; Choi, H.J. Large scale and facile sonochemical synthesis of magnetic graphene oxide nanocomposites and their dual electro/magneto-stimuli responses. RSC Adv. 2016, 6, 77925–77930. [Google Scholar] [CrossRef]
- Mrlík, M.; Ilčíková, M.; Plachý, T.; Moučka, R.; Pavlínek, V.; Mosnáček, J. Tunable electrorheological performance of silicone oil suspensions based on controllably reduced graphene oxide by surface initiated atom transfer radical polymerization of poly(glycidyl methacrylate). J. Ind. Eng. Chem. 2018, 57, 104–112. [Google Scholar] [CrossRef]
- Mrlik, M.; Ilcikova, M.; Osicka, J.; Kutalkova, E.; Minarik, A.; Vesel, A.; Mosnacek, J. Electrorheology of SI-ATRP-modified graphene oxide particles with poly(butyl methacrylate): Effect of reduction and compatibility with silicone oil. RSC Adv. 2019, 9, 1187–1198. [Google Scholar] [CrossRef] [Green Version]
- Kutalkova, E.; Mrlik, M.; Ilcikova, M.; Osicka, J.; Sedlacik, M.; Mosnacek, J. Enhanced and Tunable Electrorheological Capability using Surface Initiated Atom Transfer Radical Polymerization Modification with Simultaneous Reduction of the Graphene Oxide by Silyl-Based Polymer Grafting. Nanomaterials 2019, 9, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zygo, M.; Mrlik, M.; Ilcikova, M.; Hrabalikova, M.; Osicka, J.; Cvek, M.; Sedlacik, M.; Hanulikova, B.; Munster, L.; Skoda, D.; et al. Effect of Structure of Polymers Grafted from Graphene Oxide on the Compatibility of Particles with a Silicone-Based Environment and the Stimuli-Responsive Capabilities of Their Composites. Nanomaterials 2020, 10, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.Y.; Kim, M.H.; Jin, H.J.; Choi, H.J. Synthesis and Electrorheological Response of Graphene Oxide/Polydiphenylamine Microsheet Composite Particles. Polymers 2020, 12, 1984. [Google Scholar] [CrossRef]
- Yin, J.; Wang, X.; Chang, R.; Zhao, X. Polyaniline decorated graphene sheet suspension with enhanced electrorheology. Soft Matter 2012, 8, 294–297. [Google Scholar] [CrossRef]
- Yin, J.; Chang, R.; Shui, Y.; Zhao, X. Preparation and enhanced electro-responsive characteristic of reduced graphene oxide/polypyrrole composite sheet suspensions. Soft Matter 2013, 9, 7468. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Xiang, L.; Zhao, X.; Yin, J. Understanding the enhanced electrorheological effect of reduced graphene oxide-supported polyaniline dielectric nanoplates by a comparative study with graphene oxide as the support core. IET Nanodielectr. 2021, 4, 143–154. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Chen, H.; Zhao, X.; Yin, J. Dielectric Polarization and Electrorheological Response of Poly(ethylaniline)-Coated Reduced Graphene Oxide Nanoflakes with Different Reduction Degrees. Polymers 2020, 12, 2528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Lei, Q.; Zhao, J.; Zhao, X.; Yin, J. The Effect of Dielectric Polarization Rate Difference of Filler and Matrix on the Electrorheological Responses of Poly(ionic liquid)/Polyaniline Composite Particles. Polymers 2020, 12, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Wang, X.; Wang, J.; Liu, F.; Zhang, M.; Xu, C. Microwave-assisted covalent modification of graphene nanosheets with chitosan and its electrorheological characteristics. Appl. Surf. Sci. 2011, 257, 2637–2642. [Google Scholar] [CrossRef]
- Yin, J.; Shui, Y.; Chang, R.; Zhao, X. Graphene-supported carbonaceous dielectric sheets and their electrorheology. Carbon 2012, 50, 5247–5255. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y.; Yin, J.; Zhao, X. Preparation and enhanced electro-responsive characteristic of graphene/layered double-hydroxide composite dielectric nanoplates. J. Mater. Chem. C 2014, 2, 10386–10394. [Google Scholar] [CrossRef]
- Cerda, C.M.; Foister, R.T.; Mason, S.G. Experimental observation of electrooptical phenomena in fibrated suspensions. J. Colloid Interface Sci. 1981, 82, 577–579. [Google Scholar] [CrossRef]
- Cho, M.S.; Choi, H.J.; Jhon, M.S. Shear stress analysis of a semiconducting polymer based electrorheological fluid system. Polymer 2005, 46, 11484–11488. [Google Scholar] [CrossRef]
- Zhang, W.L.; Choi, H.J. Silica-graphene oxide hybrid composite particles and their electroresponsive characteristics. Langmuir 2012, 28, 7055–7062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J. Fabrication of semiconducting graphene oxide/polyaniline composite particles and their electrorheological response under an applied electric field. Carbon 2012, 50, 290–296. [Google Scholar] [CrossRef]
- Gamota, D.R.; Filisko, F.E. High frequency dynamic mechanical study of an aluminosilicate electrorheological material. J. Rheol. 1991, 35, 1411–1425. [Google Scholar] [CrossRef]
- Gamota, D.R.; Filisko, F.E. Linear/nonlinear mechanical properties of electroeheologic materials. Int. J. Mod. Phys. B 1992, 6, 2595–2607. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A Complex Plane Representation of Dielectric and Mechanical Relaxation Processes in Some Polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Dong, Y.; He, F.; Zhao, X.; Yin, J. Low-Temperature Interfacial Polymerization and Enhanced Electro-Responsive Characteristic of Poly(ionic liquid)s@polyaniline Core-shell Microspheres. Macromol. Rapid Commun. 2019, 40, 1800351. [Google Scholar] [CrossRef]
- Wübbenhorst, M.; Turnhout, J. Analysis of Complex Dielectric Spectra. I. One-Dimensional Derivative Techniques and Three-Dimensional Modelling. J. Non-Cryst. Solids 2002, 305, 40–49. [Google Scholar] [CrossRef]
- Ikazaki, F.; Kawai, A.; Uchida, K.; Kawakami, T.; Edamura, K.; Sakurai, K.; Anzai, K.; Asako, Y. Mechanisms of Electrorheology: The Effect of the Dielectric Property. J. Phys. D Appl. Phys. 1998, 31, 336–347. [Google Scholar] [CrossRef]
- He, K.; Wen, Q.; Wang, C.; Wang, B.; Yu, S.; Hao, C.; Chen, K. A facile synthesis of hierarchical flower-like TiO2 wrapped with MoS2 sheets nanostructure for enhanced electrorheological activity. Chem. Eng. J. 2018, 349, 416–427. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Dong, Y.; Zhao, X.; Yin, J. Distinctly Different Electroresponsive Electrorheological Effect in Low-Molecular-Weight and Polymerized Ionic Liquids: Rheological and Dielectric Relaxation Studies. J. Phys. Chem B 2018, 122, 12184–12193. [Google Scholar] [CrossRef]



| Material Category | Material | Method | Size | Particle Concentration | Yield Stress (Pa) | (s) | (S/cm) | Sedimentation Ratio | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GO-based composite | Pure GO | GO [70] | Direct dispersion | ~5 µm | 5 wt% | ~180 (2.5 kV/mm) | 2.54 | 0.45 | 1.3 × 10−5 | - | - |
| GO [72] | Solvent exchange | - | 5 wt% | ~270 (5 kV/mm) | - | - | - | 1.0 × 10−7 | ~95% | ||
| Molecular/GO | POSS/GO [74] | Electrostatic adsorption | Thickness ~3.5 nm | - | ~600 (3 kV/mm) | - | - | - | - | One year without settlement | |
| Betaine/GO [75] | Silanization and thiolene click reaction | - | 1 wt% | ~97 (3 kV/mm) | 4.98 | 1.73 | 0.04 | 6.22 × 10−8 | - | ||
| Nanoparticle/GO | TiO2/GO [76] | Electrostatic adsorption | - | 15 wt% | - | - | - | - | 5.5 × 10−10 | - | |
| Fe3O4/GO [77] | Electrostatic adsorption | - | 15 wt% | - | - | - | - | 10−7 | - | ||
| Organic/ GO | PGMA/GO [78] | SI-ATRP | - | - | ~100 (2 kV/mm) | 5.39 | 1.81 | 0.009 | 6.1 × 10−7 | 90% | |
| PBMA/GO [79] | SI-ATRP | - | 5 wt% | ~110 (2.5 kV/mm) | 4.35 | 1.37 | 0.005 | 6.0 × 10−7 | 60% | ||
| PHEMATMS/GO [80] | SI-ATRP | - | 5 wt% | 200 (3 kV/mm) | - | - | 0.002 | 6.0 × 10−6 | 90% | ||
| PMMA/GO [81] | SI-ATRP | - | 5 wt% | ~68 (2.5 kV/mm) | - | - | - | 6.3 × 10−8 | 85% | ||
| PDPA/GO [82] | In situ polymerization | - | - | 76 (1.8 kV/mm) | 4.45 | 1.54 | 6.0 × 10−6 | 8.0 × 10−8 | - | ||
| Graphene/rGO-based composite | Graphene/Organic | PANI/rGO [83] | In situ polymerization | Thickness 40–75 nm | 10 vol% | 1220 (3 kV/mm) | 82.9 | - | - | 4.0 × 10−9 | - |
| Ppy/GO [84] | In situ polymerization | Thickness ~25–70 nm | 5 vol% | ~600 (1.5 kV/mm) | ~5.1 | - | - | 9.6 × 10−9 | - | ||
| PEANI/GO [85] | In situ polymerization | 3–10 μm, Thickness ~200 nm | 4.5 vol% | ~420 (1.5 kV/mm) | - | 5.02 | 3.0 × 10−5 | 1.09 × 10−9 | - | ||
| Chitosan/graphene [88] | Electrostatic adsorption assisted by microwave | - | 0.33 wt% | - | - | - | - | - | 24h without settlement | ||
| Graphene/SiO2 | SiO2/rGO [68] | Wet chemical method | ~1.5 μm, Thickness ~30 nm | 3 vol% | 578 (2 kV/mm) | 3.83 | 0.92 | 4.5 × 10−7 | 1.7 × 10−9 | - | |
| Graphene/mSiO2 [69] | Sol-gel | ~2 µm, Thickness ~20 nm | 5 vol% | 850 (1.5 kV/mm) | ~6.5 | - | 3.2 × 10−7 | 2.8 × 10−9 | ~95% | ||
| Graphene/C | Graphene/C [89] | Vacuum annealing | Thickness ~240 nm | 10 vol% | 1100 (2.5 kV/mm) | ~5.6 | - | 4.0 × 10−5 | 3.3 × 10−8 | - | |
| Graphene/LDH | Graphene/ LDH [90] | One-pot hydrothermal method | 3–5 µm, Thickness 50–100 nm | 10 wt% | 1000 (2.0 kV/mm) | 5.85 | 2.55 | 1.2 × 10−7 | 8.0 × 10−9 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yuan, J.; Zhao, X.; Yin, J. Electrorheological Fluids of GO/Graphene-Based Nanoplates. Materials 2022, 15, 311. https://doi.org/10.3390/ma15010311
Wang Y, Yuan J, Zhao X, Yin J. Electrorheological Fluids of GO/Graphene-Based Nanoplates. Materials. 2022; 15(1):311. https://doi.org/10.3390/ma15010311
Chicago/Turabian StyleWang, Yudong, Jinhua Yuan, Xiaopeng Zhao, and Jianbo Yin. 2022. "Electrorheological Fluids of GO/Graphene-Based Nanoplates" Materials 15, no. 1: 311. https://doi.org/10.3390/ma15010311






