Improvement of Pitting-Corrosion Resistance of Ultrafine-Grained 7475 Al Alloy by Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- CG_T6—coarse-grained, precipitation-strengthened: coarse-grained sample after solution annealing at 470 °C for 2 h, water quenching, and peak aging;
- HE—ultrafine-grained, naturally aged: ultrafine-grained sample after solution annealing at 470 °C for 2 h, water quenching, and hydrostatic extrusion in three consecutive passes from the initial diameter of 20 mm to the final diameter of 3 mm, which corresponds to a total true strain of about 4;
2.2. Microstructural Observations
2.3. Electrochemical Testing
3. Results
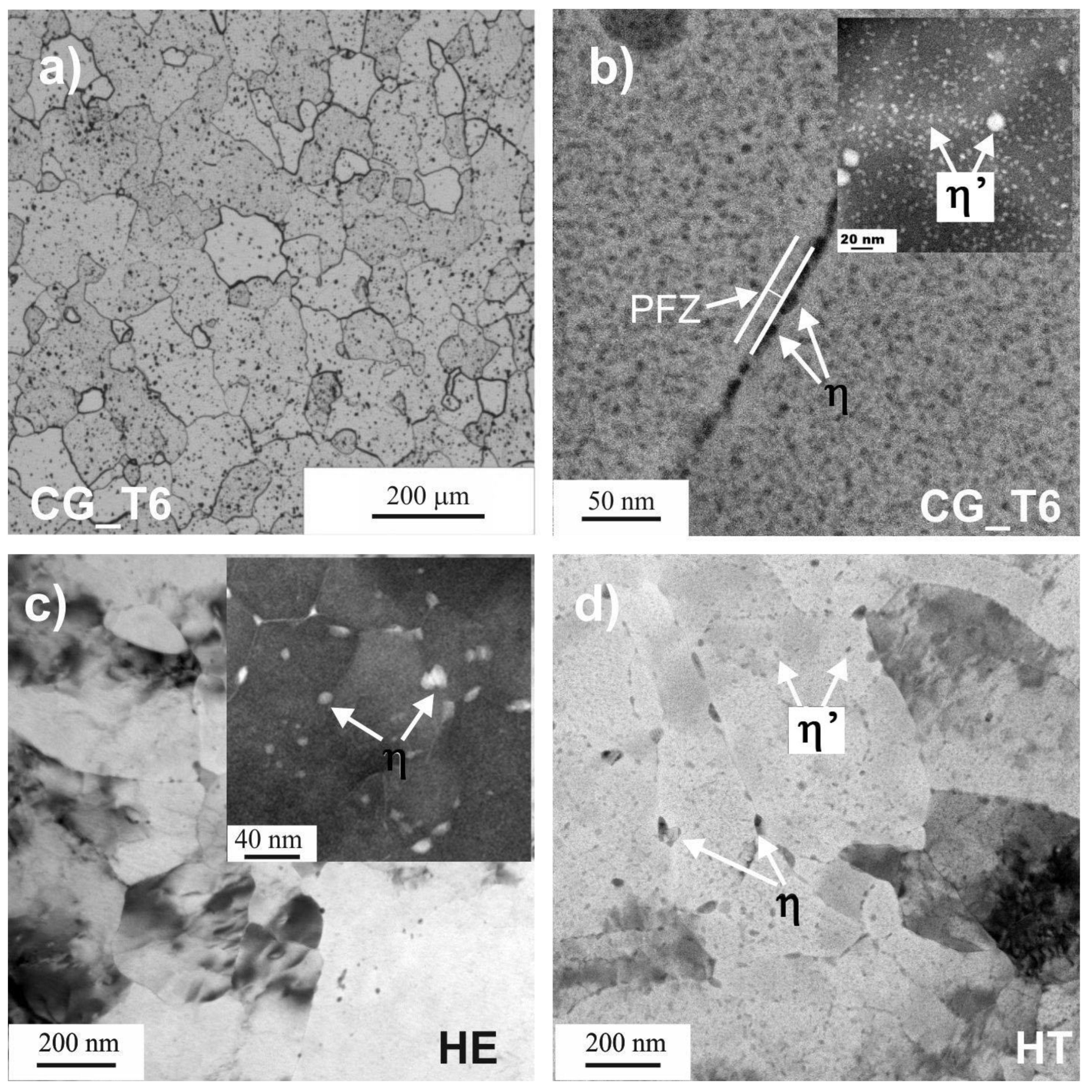
3.1. Microstructure Characterization
3.1.1. Grain Size and Strengthening Precipitates
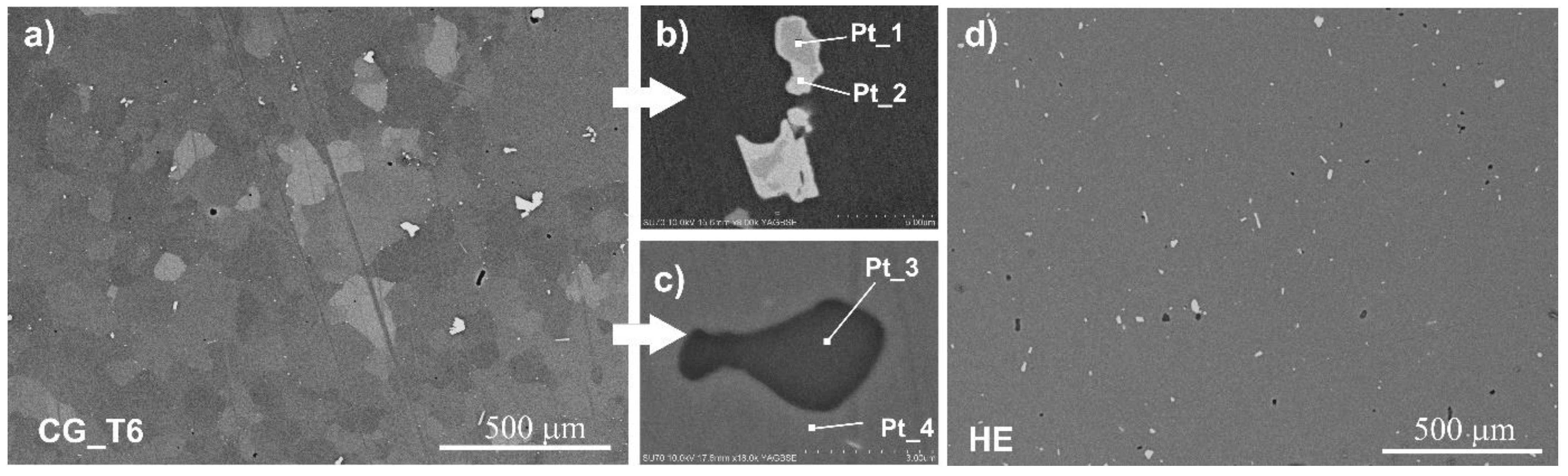
3.1.2. Intermetallic Particles
3.2. Corrosion Resistance
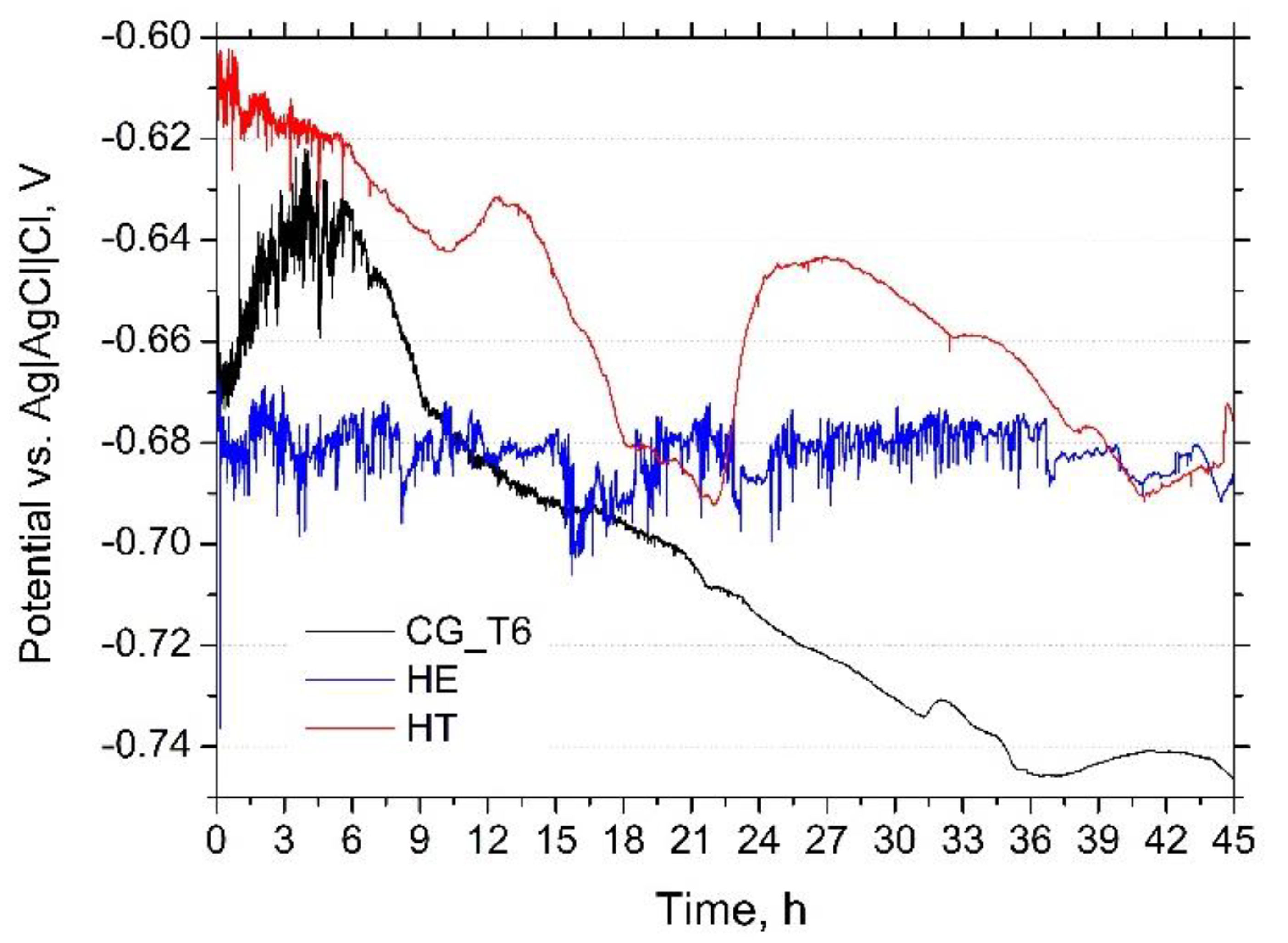
3.2.1. Corrosion under Open-Circuit Conditions
3.2.2. Potentiodynamic Polarization
3.3. Post-Corrosion Morphology
4. Discussion
Pitting Susceptibility
5. Conclusions
- The HE7475 Al alloy developed an ultrafine-grained structure with inhomogeneous grain size, coarse intermetallic particles, and MgZn2 strengthening precipitates at grain boundaries.
- Subsequent aging resulted in coarsening of stable intergranular MgZn2 precipitates and formation of metastable MgZn2 in grain interiors. The lower number of strengthening particles shifted EOCP to less noble values as a higher amount of Mg and Zn was dissolved in the matrix. The aging improved the corrosion resistance as the EOCP was shifted to more noble values and the icorr was lower.
- The resulting HE microstructural changes significantly altered the morphology of the corrosion attack. The IGC was no longer observed for ultrafine-grained materials. The subsequent aging limited the propagation of the corrosion attack towards the depth of the bulk material.
- The subsequent aging seems to have had an impact on the pitting susceptibility of the ultrafine-grained 7475 Al alloy.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk nanostructured materials from severe plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Hansen, N. Hall-petch relation and boundary strengthening. Scr. Mater. 2004, 51, 801–806. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Zhilyaev, A.P.; Langdon, T.G. Using high-pressure torsion for metal processing: Fundamentals and applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwak, B.J.; Kong, T.; Park, S.H.; Lee, T. Improved tensile properties of AZ31 Mg alloy subjected to various caliber-rolling strains. J. Magnes. Alloy. 2019, 7, 381–387. [Google Scholar] [CrossRef]
- Lewandowska, M.; Kurzydlowski, K.J. Recent development in grain refinement by hydrostatic extrusion. J. Mater. Sci. 2008, 43, 7299–7306. [Google Scholar] [CrossRef]
- Lewandowska, M. Mechanism of Grain Refinement in Aluminium in the Process of Hydrostatic Extrusion. Solid State Phenom. 2006, 114, 109–116. [Google Scholar] [CrossRef]
- MacKenzie, D.S. Metallurgy of heat treatable aluminum alloys. In ASM Handbook Volume 4E: Heat Treating of Nonferrous Alloys; Anderson, K., Weritz, J., Kaufman, J.G., Mackenzie, D.S., Eds.; ASM International: Almere, The Netherlands, 2018; pp. 65–113. [Google Scholar] [CrossRef]
- Heat Treatment Practice of Age-Hardenable Aluminium Alloys. In ASM Handbook Volume 4E: Heat Treating of Nonferrous Alloys; Totten, G.E. (Ed.) ASM International: Almere, The Netherlands, 2016; pp. 245–273. [Google Scholar] [CrossRef]
- Emmanuel, A.O.; Fayomi, O.S.I.; Akande, I.G. Aluminium Alloys as Advanced Materials: A short communication. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1107, 012024. [Google Scholar] [CrossRef]
- Palumbo, G.; Aust, K.T.; Erb, U. Triple line defects in nanostructured materials. Mater. Sci. Forum. 1996, 225–227, 281–286. [Google Scholar] [CrossRef]
- Palumbo, G.; Thorpe, S.J.; Aust, K.T. On the contribution of triple junctions to the structure and properties of nanocrystalline materials. Scr. Metall. Mater. 1990, 24, 1347–1350. [Google Scholar] [CrossRef]
- Liao, J.; Hotta, M.; Yamamoto, N. Corrosion behavior of fine-grained AZ31B magnesium alloy. Corros. Sci. 2012, 61, 208–214. [Google Scholar] [CrossRef]
- Abdulstaar, M.; Mhaede, M.; Wagner, L.; Wollmann, M. Corrosion behaviour of Al 1050 severely deformed by rotary swaging. Mater. Des. 2014, 57, 325–329. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, F.; Frankel, G.S. Effect of Altered Surface Layer on Localized Corrosion of Aluminum Alloy 2024. J. Electrochem. Soc. 2017, 164, C317–C323. [Google Scholar] [CrossRef] [Green Version]
- Dobkowska, A.; Castillo, M.D.H.; Turnbull, J.P.; Ramamurthy, S.; Zagidulin, D.; Moser, D.E.; Behazin, M.; Keech, P.G.; Shoesmith, D.W.; Noël, J.J. A comparison of the corrosion behaviour of copper materials in dilute nitric acid. Corros. Sci. 2021, 192, 109778. [Google Scholar] [CrossRef]
- Ralston, K.D.; Fabijanic, D.; Birbilis, N. Effect of grain size on corrosion of high purity aluminium. Electrochim. Acta 2011, 56, 1729–1736. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Orlov, D.; Ralston, K.D.; Birbilis, N.; Estrin, Y. Enhanced corrosion resistance of Mg alloy ZK60 after processing by integrated extrusion and equal channel angular pressing. Acta Mater. 2011, 59, 6176–6186. [Google Scholar] [CrossRef]
- Adamczyk-Cieślak, B.; Mizera, J.; Kurzydłowski, K.J. Microstructures in the 6060 aluminium alloy after various severe plastic deformation treatments. Mater. Charact. 2011, 62, 327–332. [Google Scholar] [CrossRef]
- Crump, J.; Qiao, X.G.; Starink, M.J. The effect of high-pressure torsion on the behaviour of intermetallic particles present in Al-1Mg and Al-3Mg. J. Mater. Sci. 2012, 47, 1751–1757. [Google Scholar] [CrossRef] [Green Version]
- Shaterani, P.; Zarei-Hanzaki, A.; Fatemi-Varzaneh, S.M.; Hassas-Irani, S.B. The second phase particles and mechanical properties of 2124 aluminum alloy processed by accumulative back extrusion. Mater. Des. 2014, 58, 535–542. [Google Scholar] [CrossRef]
- Hughes, A.E.; Birbilis, N.; Mol, J.M.C.; Garcia, S.J.; Zhou, X.; Thompson, G.E. High Strength Al-Alloys: Microstructure, Corrosion and Principles of Protection. In Recent Trends in Processing and Degradation of Aluminium Alloys; BoD—Books on Demand: Norderstedt, Germany, 2011; pp. 223–262. [Google Scholar] [CrossRef] [Green Version]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 2005, 152, B140. [Google Scholar] [CrossRef] [Green Version]
- Jilani, O.; Njah, N.; Ponthiaux, P. Transition from intergranular to pitting corrosion in fine grained aluminum processed by equal channel angular pressing. Corros. Sci. 2014, 87, 259–264. [Google Scholar] [CrossRef]
- Sikora, E.; Wei, X.J.; Shaw, B.A. Corrosion behavior of nanocrystalline bulk Al-Mg-based alloys. Corrosion 2004, 60, 387–398. [Google Scholar] [CrossRef]
- Chung, M.K.; Choi, Y.S.; Kim, J.G.; Kim, Y.M.; Lee, J.C. Effect of the number of ECAP pass time on the electrochemical properties of 1050 Al alloys. Mater. Sci. Eng. A 2004, 366, 282–291. [Google Scholar] [CrossRef]
- Akiyama, E.; Zhang, Z.; Watanabe, Y.; Tsuzaki, K. Effects of severe plastic deformation on the corrosion behavior of aluminum alloys. J. Solid State Electrochem. 2009, 13, 277–282. [Google Scholar] [CrossRef]
- Korchef, A.; Kahoul, A. Corrosion Behavior of Commercial Aluminum Alloy Processed by Equal Channel Angular Pressing. Int. J. Corros. 2013, 2013, 983261. [Google Scholar] [CrossRef] [Green Version]
- Laurino, A.; Andrieu, E.; Harouard, J.-P.; Lacaze, J.; Lafont, M.-C.; Odemer, G.; Blanc, C. Corrosion Behavior of 6101 Aluminum Alloy Strands for Automotive Wires. J. Electrochem. Soc. 2013, 160, C569–C575. [Google Scholar] [CrossRef] [Green Version]
- Pisarek, M.; Kędzierzawski, P.; Janik-Czachor, M.; Kurzydłowski, K.J. Effect of hydrostatic extrusion on passivity breakdown on 303 austenitic stainless steel in chloride solution. J. Solid State Electrochem. 2009, 13, 283–291. [Google Scholar] [CrossRef]
- Nickel, D.; Dietrich, D.; Mehner, T.; Frint, P.; Spieler, D.; Lampke, T. Effect of strain localization on pitting corrosion of an AlMgSi0.5 alloy. Metals 2015, 5, 172–191. [Google Scholar] [CrossRef] [Green Version]
- Ly, R.; Hartwig, K.T.; Castaneda, H. Influence of dynamic recrystallization and shear banding on the localized corrosion of severely deformed Al–Mg–Si alloy. Materialia 2018, 4, 457–465. [Google Scholar] [CrossRef]
- Quartiermeister, M.V.; Magalhães, D.C.C.; Vacchi, G.S.; Braga, D.P.; Silva, R.; Kliauga, A.M.; Sordi, V.L.; Rovere, C.A.D. On the pitting corrosion behavior of ultrafine-grained aluminum processed by ECAP: A statistical analysis. Mater. Corros. 2020, 71, 1244–1256. [Google Scholar] [CrossRef]
- Ramgopal, T.; Gouma, P.I.; Frankel, G.S. Role of Grain-Boundary Precipitates and Solute-Depleted Zone on the Intergranular Corrosion of Aluminum Alloy 7150. Corrosion 2002, 58, 687–697. [Google Scholar] [CrossRef]
- Xu, D.K.; Birbilis, N.; Lashansky, D.; Rometsch, P.A.; Muddle, B.C. Effect of solution treatment on the corrosion behaviour of aluminium alloy AA7150: Optimisation for corrosion resistance. Corros. Sci. 2011, 53, 217–225. [Google Scholar] [CrossRef]
- Lewandowska, M.; Wawer, K.; Kozikowski, P.; Ohnuma, M.; Kurzydlowski, K.J. Precipitation in a nanograined 7475 aluminium alloy—Processing, properties and nanoanalysis. Adv. Eng. Mater. 2014, 16, 482–485. [Google Scholar] [CrossRef]
- Chrominski, W.; Wenner, S.; Marioara, C.D.; Holmestad, R.; Lewandowska, M. Strengthening mechanisms in ultrafine grained Al-Mg-Si alloy processed by hydrostatic extrusion—Influence of ageing temperature. Mater. Sci. Eng. A 2016, 669, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Chrominski, W.; Lewandowska, M. Precipitation phenomena in ultrafine grained Al-Mg-Si alloy with heterogeneous microstructure. Acta Mater. 2016, 103, 547–557. [Google Scholar] [CrossRef]
- Deschamps, A.; De Geuser, F.; Horita, Z.; Lee, S.; Renou, G. Precipitation kinetics in a severely plastically deformed 7075 aluminium alloy. Acta Mater. 2014, 66, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Gopala Krishna, K.; Sivaprasad, K.; Sankara Narayanan, T.S.N.; Hari Kumar, K.C. Localized corrosion of an ultrafine grained Al-4Zn-2Mg alloy produced by cryorolling. Corros. Sci. 2012, 60, 82–89. [Google Scholar] [CrossRef]
- Wawer, K.; Lewandowska, M.; Kurzydlowski, K.J. Precipitate strengthening of nanostructured aluminium alloy. J. Nanosci. Nanotechnol. 2012, 12, 8783–8786. [Google Scholar] [CrossRef]
- Wawer, K.; Lewandowska, M.; Kurzydlowski, K.J. The influence of hydrostatic extrusion on size, shape, and spatial distribution of intermetallic particles in 7475 aluminium alloy. Mater. Eng. 2007, 28, 486–490. (In Polish) [Google Scholar]
- Ayer, R.; Koo, J.Y.; Steeds, J.W.; Park, B.K. Microanalytical study of the heterogeneous phases in commercial Al-Zn-Mg-Cu alloys. Metall. Trans. A 1985, 16, 1925–1936. [Google Scholar] [CrossRef]
- Meng, G.; Wei, L.; Zhang, T.; Shao, Y.; Wang, F.; Dong, C.; Li, X. Effect of microcrystallization on pitting corrosion of pure aluminium. Corros. Sci. 2009, 51, 2151–2157. [Google Scholar] [CrossRef]
- Dobkowska, A.; Sotniczuk, A.; Bazarnik, P.; Mizera, J.; Garbacz, H. Corrosion behavior of cold-formed aa5754 alloy sheets. Materials 2021, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Sykes, J.M.; Hogg, S.C.; Grant, P.S. Pitting corrosion of spray formed Al-Li-Mg alloys. Corros. Sci. 2008, 50, 3221–3226. [Google Scholar] [CrossRef]
- Benedetti, A.; Cirisano, F.; Delucchi, M.; Faimali, M.; Ferrari, M. Potentiodynamic study of Al-Mg alloy with superhydrophobic coating in photobiologically active/not active natural seawater. Colloids Surf. B Biointerfaces 2016, 137, 167–175. [Google Scholar] [CrossRef]
- Puiggali, M.; Zielinski, A.; Olive, J.M.; Renauld, E.; Desjardins, D.; Cid, M. Effect of microstructure on stress corrosion cracking of an Al-Zn-Mg-Cu alloy. Corros. Sci. 1998, 40, 805–819. [Google Scholar] [CrossRef]
- Knight, S.P.; Birbilis, N.; Muddle, B.C.; Trueman, A.R.; Lynch, S.P. Correlations between intergranular stress corrosion cracking, grain-boundary microchemistry, and grain-boundary electrochemistry for Al–Zn–Mg–Cu alloys. Corros. Sci. 2010, 52, 4073–4080. [Google Scholar] [CrossRef]
- Kairy, S.K.; Turk, S.; Birbilis, N.; Shekhter, A. The role of microstructure and microchemistry on intergranular corrosion of aluminium alloy AA7085-T7452. Corros. Sci. 2018, 143, 414–427. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; De Wit, J.H.W. Corrosion behaviour of different tempers of AA7075 aluminium alloy. Electrochim. Acta 2004, 49, 2851–2862. [Google Scholar] [CrossRef]
- Chromiński, W. Microstructural Heterogeneities and Their Influence on Precipitation Phenomena in a Severely Deformed 6082 Aluminium Alloy. Ph.D. Thesis, Warsaw University of Technology, Warsaw, Poland, 2016. [Google Scholar]


| Alloy | Zn | Mg | Cu | Zr | Fe | Si | Ti | Mn |
|---|---|---|---|---|---|---|---|---|
| 7475 | 6.00 | 2.49 | 1.66 | 0.12 | 0.12 | 0.094 | 0.015 | 0.01 |
| % at. | Mg-K | Al-K | Si-K | Fe-L | Cu-L | Zn-L |
|---|---|---|---|---|---|---|
| Pt_1 | 0.4 ± 0.1 | 66.4 ± 0.2 | 2.5 ± 0.1 | 22.2 ± 0.1 | 8.5 ± 0.1 | - |
| Pt_2 | - | 61.0 ± 0.2 | - | 15.0 ± 0.2 | 24.0 ± 0.2 | - |
| Pt_3 | 60.2 ± 0.3 | 2.1 ± 0.1 | 37.8 ± 0.3 | - | - | - |
| Pt_4 | 2.8 ± 0.1 | 92.5 ± 0.4 | - | 0.2 ± 0.1 | 0.7 ± 0.1 | 3.8 ± 0.1 |
| Sample | Ecorr vs. Ag|AgCl|Cl−, V | Epit vs. Ag|AgCl|Cl−, V | icorr, µA/cm2 |
|---|---|---|---|
| CG_T6 | −0.629 ± 0.02 | −0.626 ± 0.2 | 1.0 ± 0.2 |
| HE | −0.672 ± 0.01 | −0.669 ± 0.1 | 3.4 ± 0.2 |
| HT | −0.610 ± 0.01 | −0.604 ± 0.1 | 1.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ura-Bińczyk, E. Improvement of Pitting-Corrosion Resistance of Ultrafine-Grained 7475 Al Alloy by Aging. Materials 2022, 15, 360. https://doi.org/10.3390/ma15010360
Ura-Bińczyk E. Improvement of Pitting-Corrosion Resistance of Ultrafine-Grained 7475 Al Alloy by Aging. Materials. 2022; 15(1):360. https://doi.org/10.3390/ma15010360
Chicago/Turabian StyleUra-Bińczyk, Ewa. 2022. "Improvement of Pitting-Corrosion Resistance of Ultrafine-Grained 7475 Al Alloy by Aging" Materials 15, no. 1: 360. https://doi.org/10.3390/ma15010360






