Reusing Construction and Demolition Waste to Prepare Alkali-Activated Cement
Abstract
:1. Introduction
2. Experimental Programme
2.1. C&DW Conditioning and Characterisation
2.2. Other Materials
2.3. Experimental Procedure
3. Results and Discussion
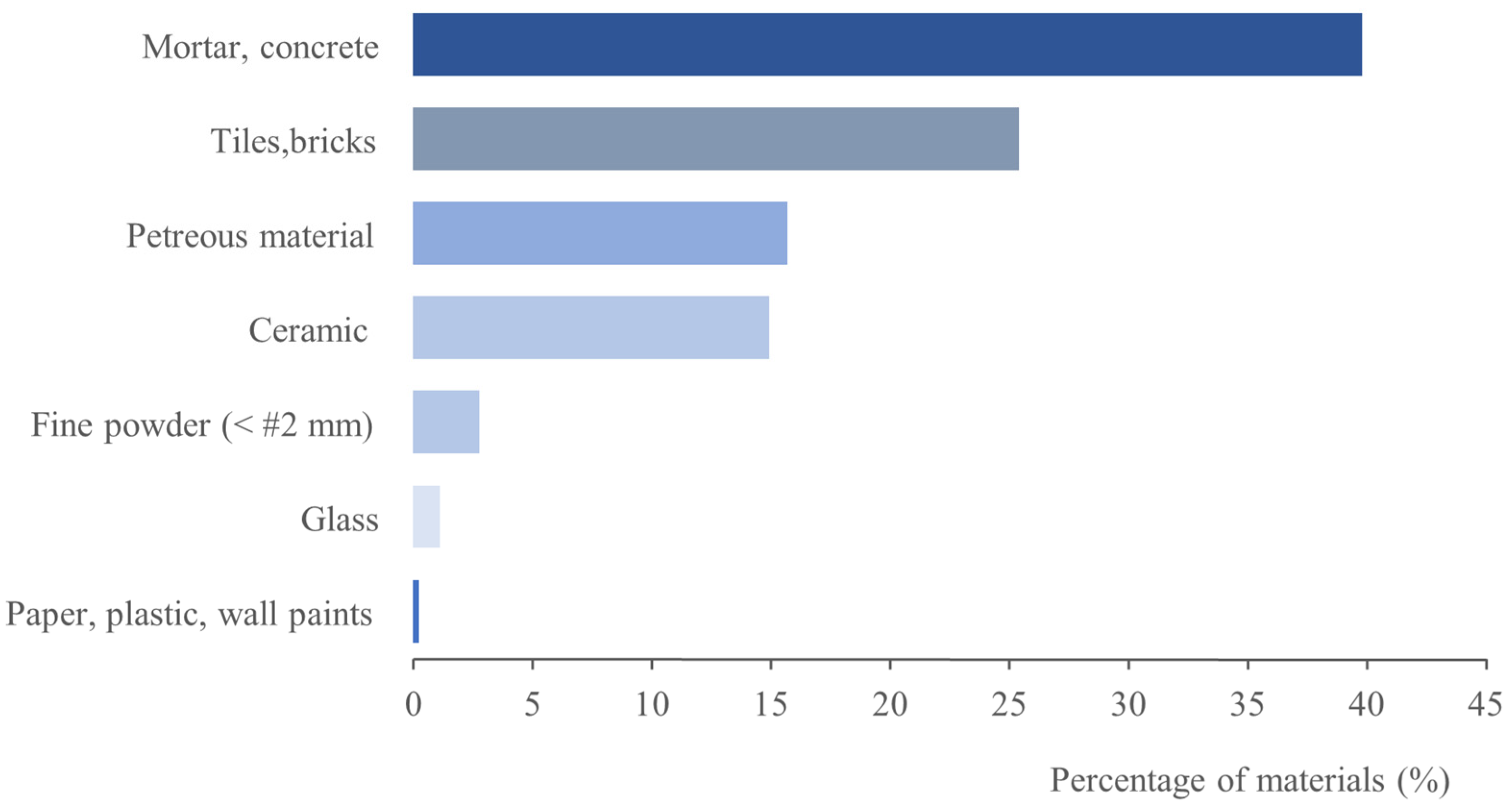
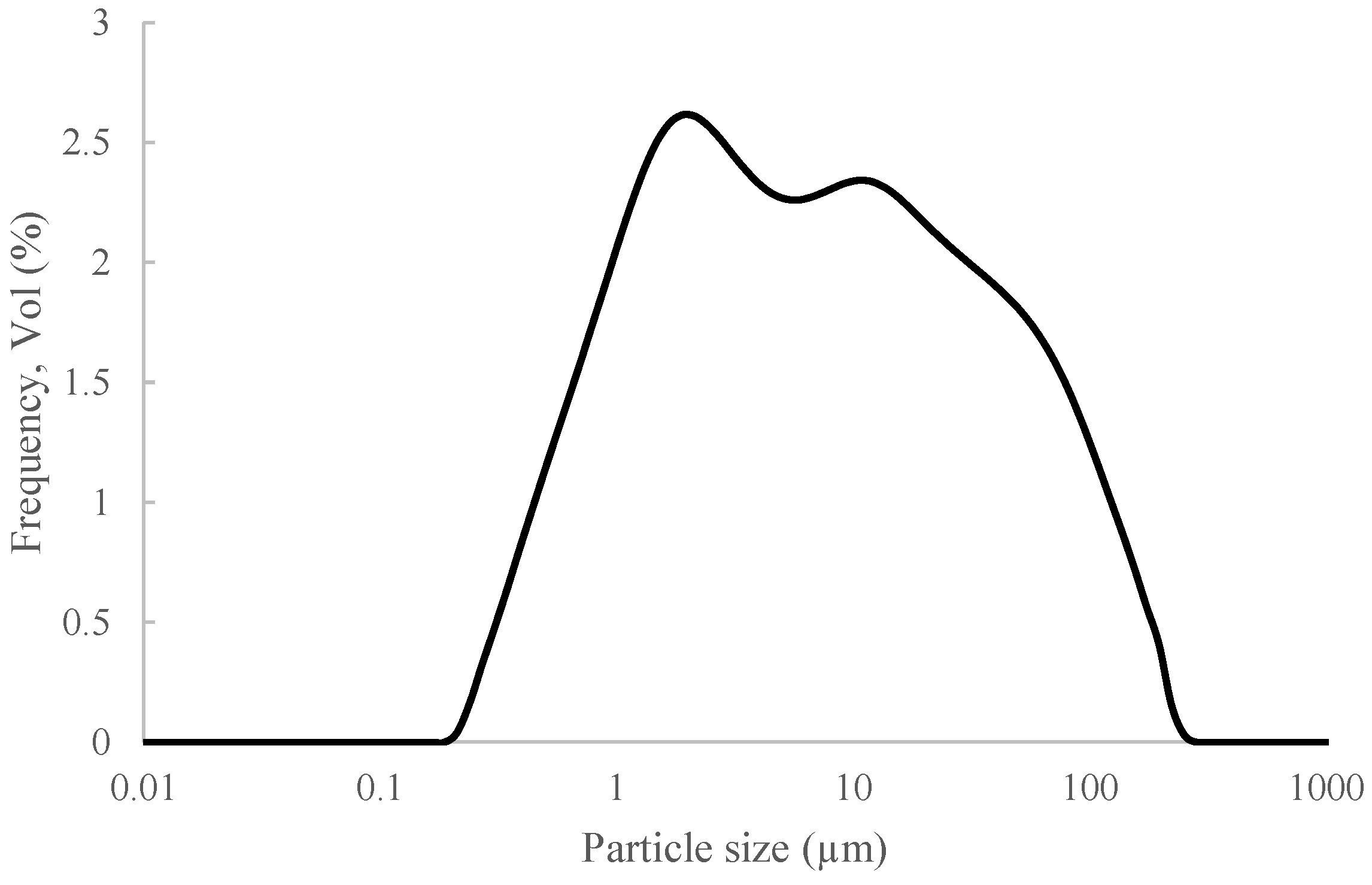
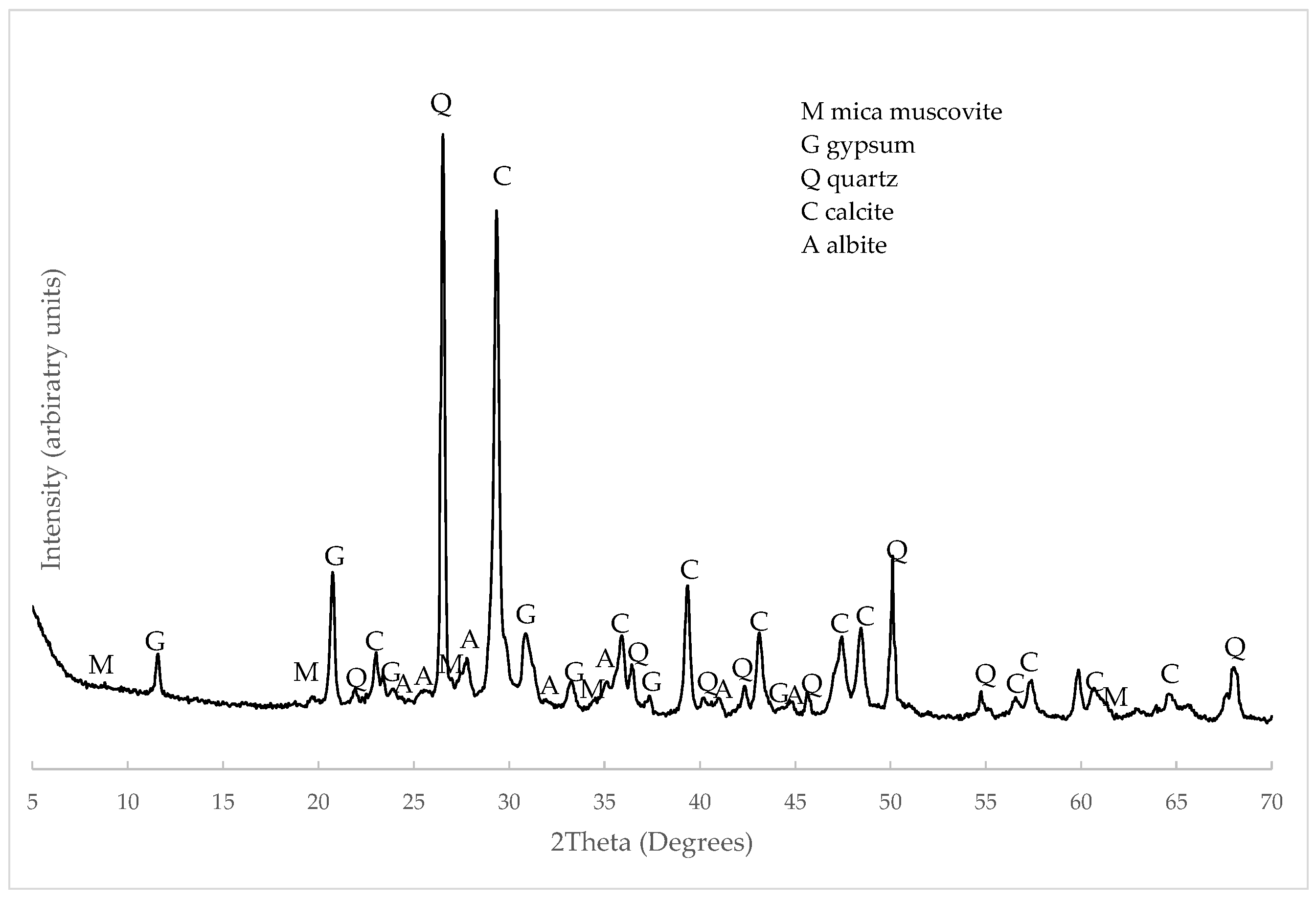
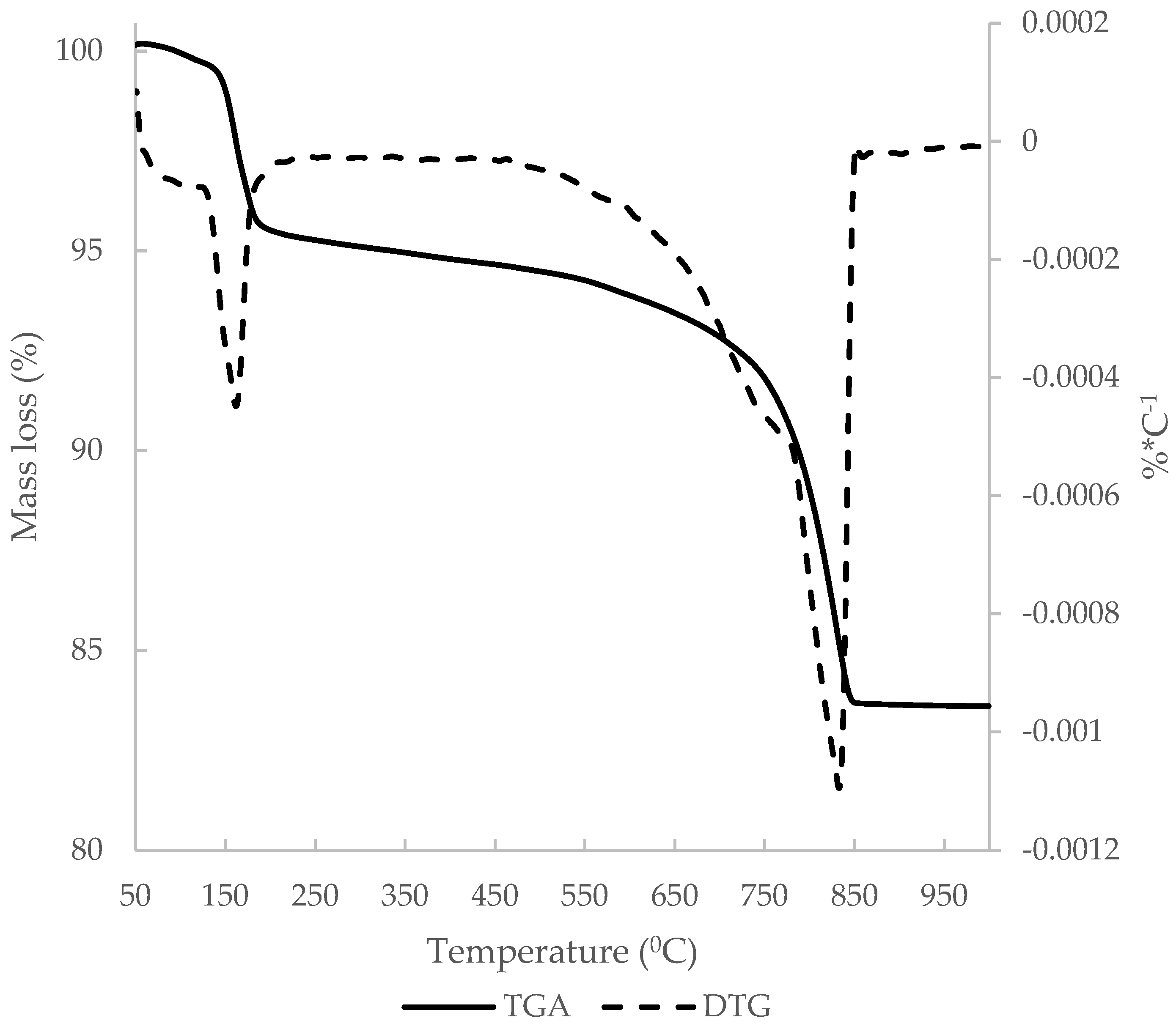
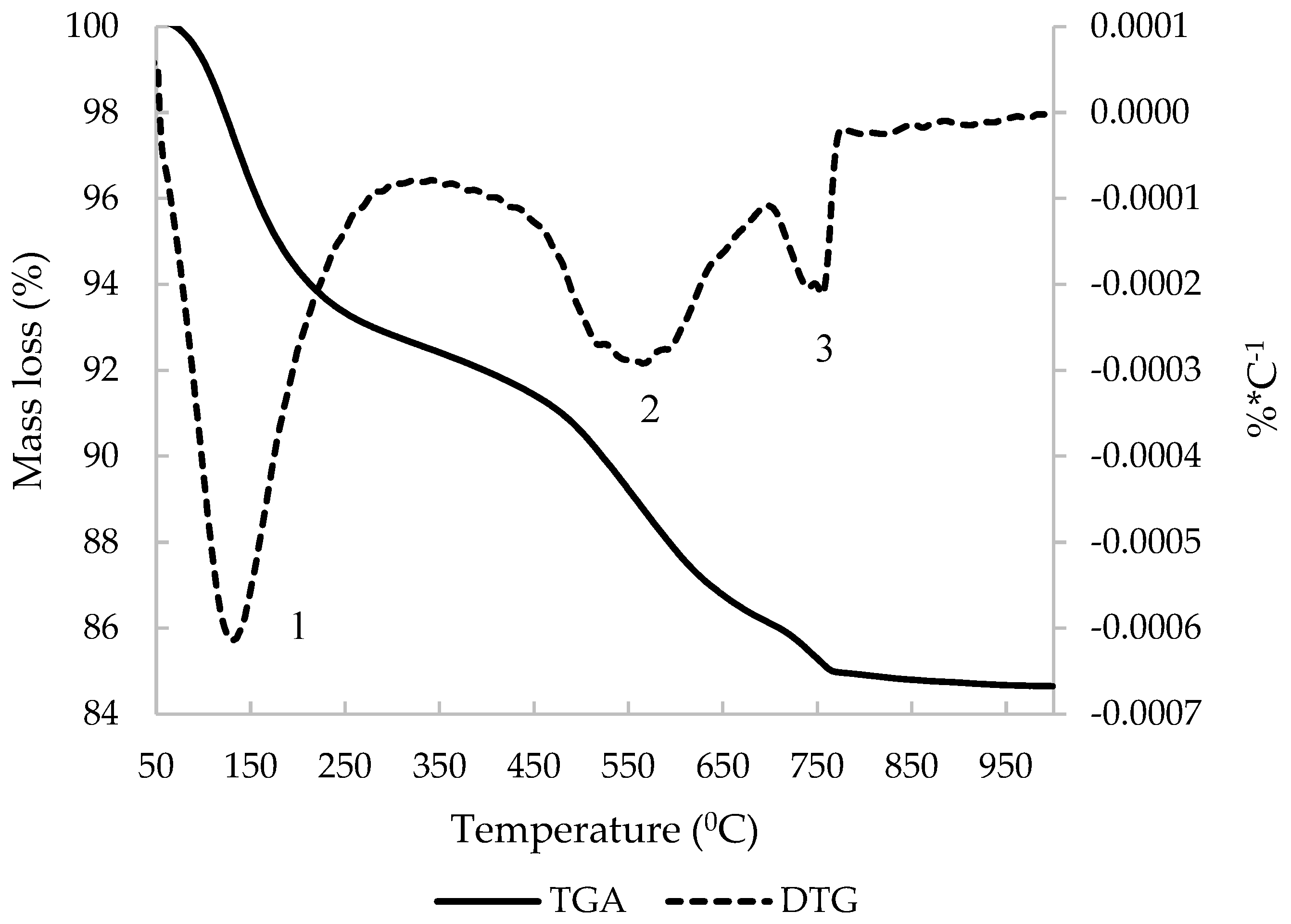
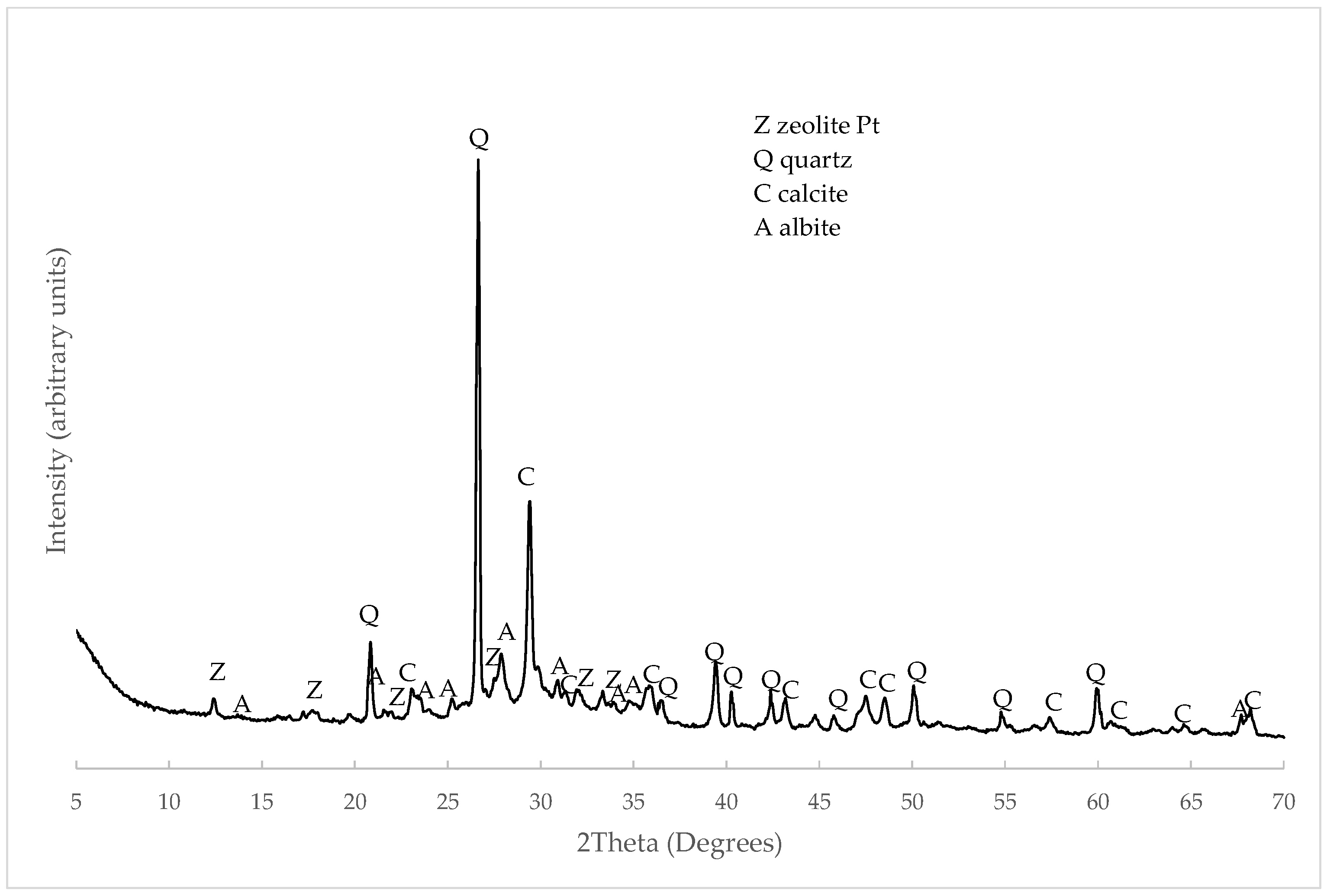
3.1. C&DW Characterisation
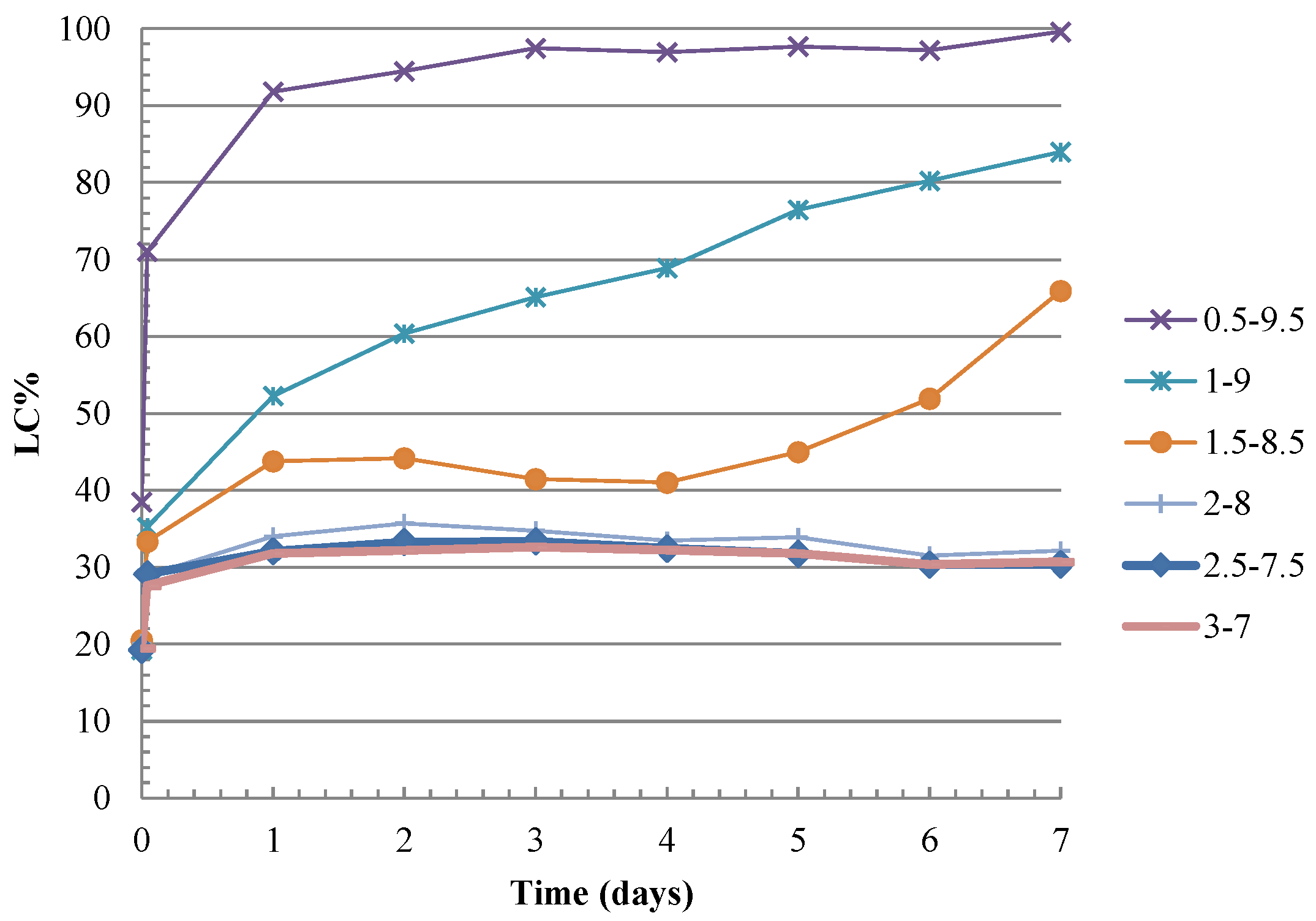
3.2. C&DW Reactivity
3.2.1. C&DW Reactivity as Pozzolanic Material
3.2.2. C&DW Reactivity as Precursor Material in Alkaline Activated Pastes
3.3. Properties of the Alkali-Activated Mortars Based on the C&DW/BFS System
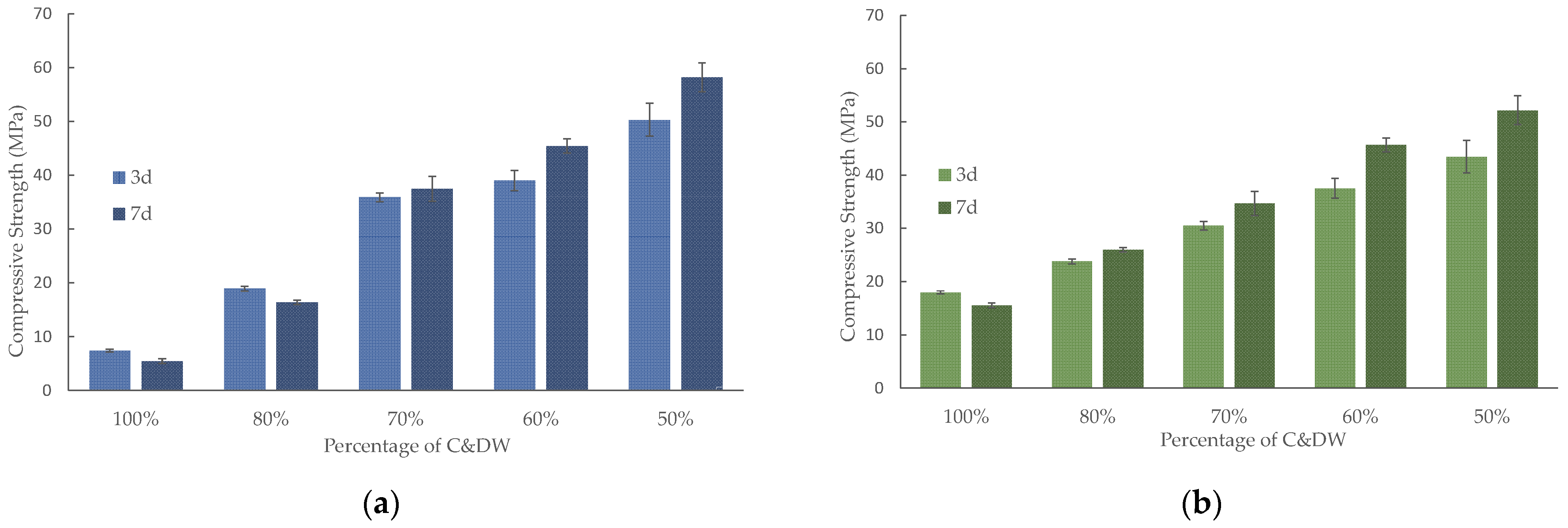
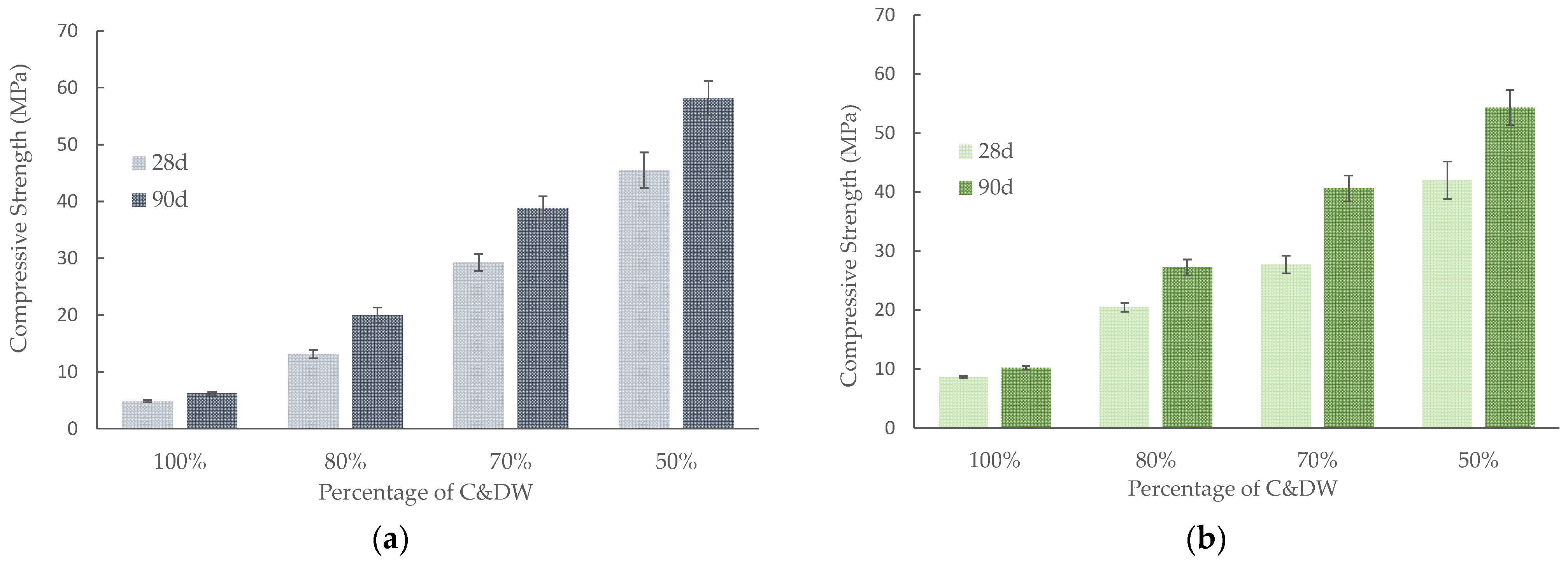
3.3.1. Mechanical Strength
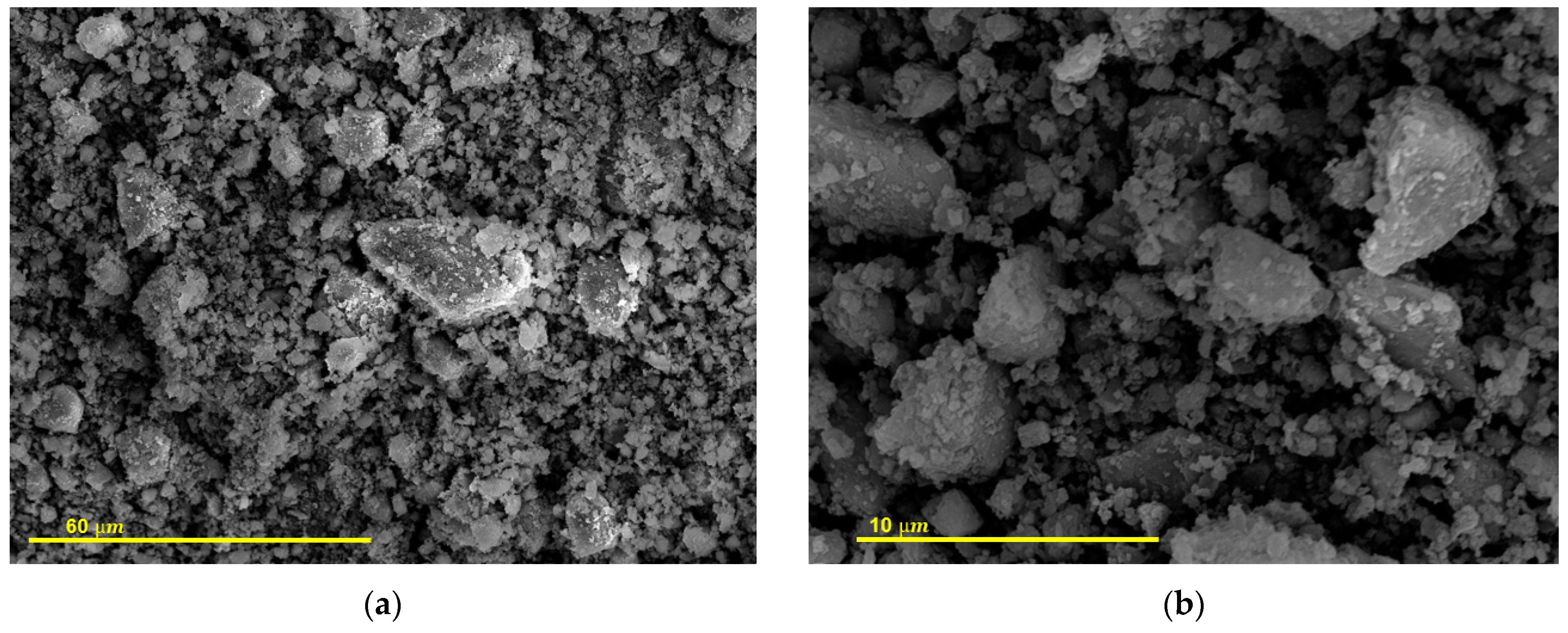
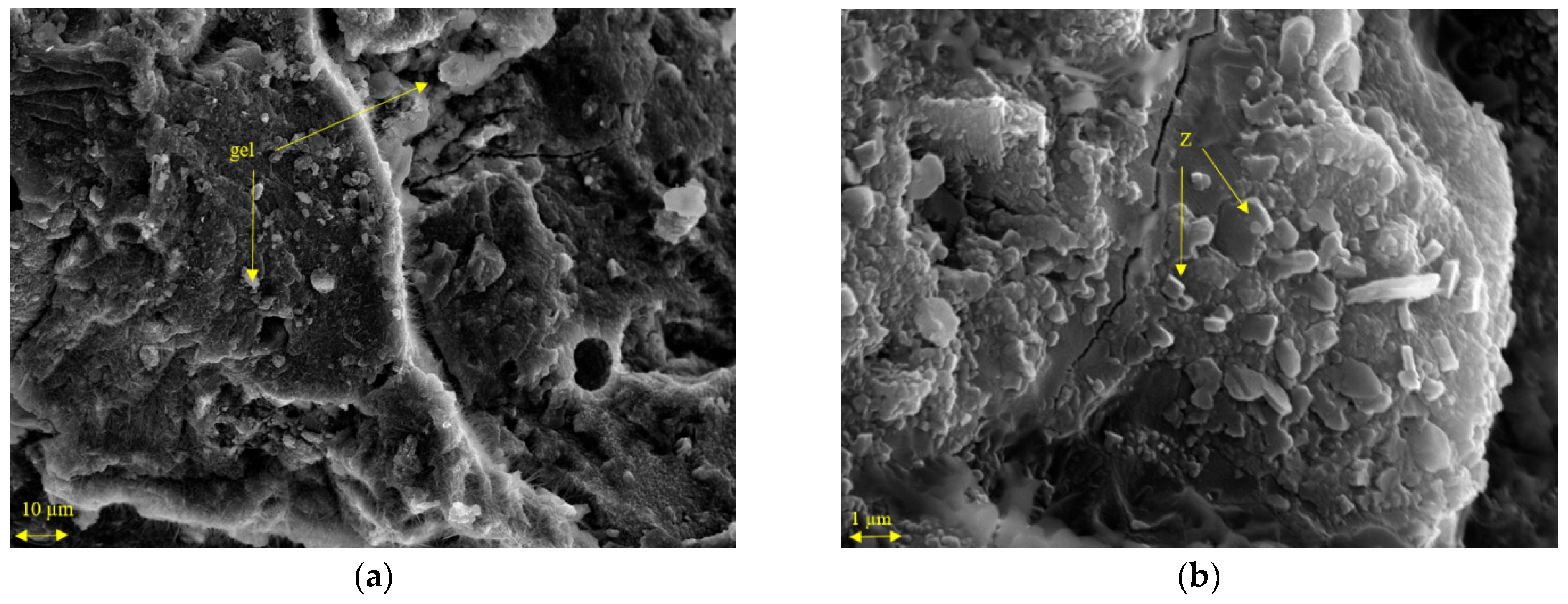
3.3.2. FESEM Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://ec.europa.eu/environment/topics/waste-and-recycling/waste-fr0amework-directive_en (accessed on 10 April 2022).
- Ginga, C.P.; Ongpeng, J.M.C.; Daly, M.K.M. Circular Economy on construction and demolition waste: A literature review on material recovery and production. Materials 2020, 13, 2970. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yan, L.; Fu, Q.; Kasal, B. A comprehensive review on recycled aggregate and recycled aggregate concrete. Resour. Conserv. Recycl. 2021, 171, 105565. [Google Scholar] [CrossRef]
- Akhtar, A.; Sarmah, A.K. Construction and demolition waste generation and properties of recycled aggregate concrete: A global perspective. J. Clean. Prod. 2018, 186, 262–281. [Google Scholar] [CrossRef]
- López, L.A.; Roca, R.; Gassó, S. The circular economy in the construction and demolition waste sector-A review and an integrative model approach. J. Clean. Prod. 2020, 248, 119238. [Google Scholar] [CrossRef]
- Ahmari, S.; Ren, X.; Toufigh, V.; Zhang, L. Production of geoplymeric binder from blended waste concrete powder and fly ash. Constr. Build. Mater. 2012, 35, 718–729. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Luukkonen, T.; Mastali, M.; Giosue, C.; Favoni, O.; Ruello, M.L.; Kinnunen, P.; Illikainen, M. Microstructural analysis and strength development of one-part alkali-activated slag/ceramic binders under different curing regimes. Waste Biomass Valor 2020, 11, 3081–3096. [Google Scholar] [CrossRef] [Green Version]
- Cosa, J.; Soriano, L.; Borrachero, M.V.; Reig, L.; Payá, J.; Monzó, J.M. Influence of addition of fluid catalytic cracking residue (FCC) and the SiO2 concentration in alkali-activated ceramic sanitary ware (CSW) binders. Minerals 2018, 8, 123. [Google Scholar] [CrossRef] [Green Version]
- Ulugöl, H.; Kul, A.; Yildirim, G.; Sahmaran, M.; Aldemir, A.; Figueria, D.; Ashour, A. Mechanical and microstructural characterisation of geopolymers from assorted construction and demoliton waste-based masonry and glass. J. Clean. Prod. 2021, 280, 124358. [Google Scholar] [CrossRef]
- Kioupis, D.; Skaropoulou, A.; Tsivilis, S.; Kakali, G. Valorization of brick and glass CDWs for the development of geopolymers containing more than 80% of wastes. Minerals 2020, 10, 672. [Google Scholar] [CrossRef]
- Vásquez, A.; Cárdenas, V.; Robayo, R.A.; Mejía de Gutiérrez, R. Geopolymer based on concrete demolition waste. Adv. Powder Technol. 2016, 27, 1173–1179. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Valencia-Saavedra, W.; Mejía de Gutiérrez, R. Construction and demolition waste (CDW) recycling-as both binder and aggregates-in alkali-activated materials: A novel re-use concept. Sustainability 2020, 12, 5775. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Valencia-Saavedra, W.; Ramírez-Benavides, S.; Mejía de Gutiérrez, R.; Orobio, A. Eco-House prototype constructed with alkali-activated blocks: Material production, characterization, design, construction and environmental impact. Materials 2021, 14, 1275. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K. Co-valorization of marine sediments and construction & demolition wastes trough alkali activation. J. Environ. Chem. Eng. 2016, 4, 4661–4669. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Shariati, M.; Shariati, A.; Trung, N.T.; Shoaei, P.; Ameri, F.; Bahrami, N.; Zamanabadi, S.N. Alkali-activated slag (AAS) paste: Correlation between durability and microstructural characteristics. Constr. Build. Mater. 2021, 267, 120886. [Google Scholar] [CrossRef]
- Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Akasaki, J.L.; Payá, J. New method to assess the pozzolanic reactivity of mineral admixtures by means of pH and conductivity measurements in lime:Pozzolan suspensions. Mater. Constr. 2014, 64, e032. [Google Scholar] [CrossRef] [Green Version]
- UNE-EN 196-1; Methods of Testing Cement-Part 1: Determination of Strength. AENOR: Madrid, Spain, 2005.
- Mucsi, G.; Papné, N.H.; Ulsen, C.; Oliveira, P.; Kristály, F. Mechanical activation of construction and demolition waste in order to improve its pozzolanic reactivity. ACS Sustain. Chem. Eng. 2021, 9, 3416–3427. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.A.; Mejía de Gutiérrez, R. Comparison of different activators for alkaline activation of construction and demolition wastes. Constr. Build. Mater. 2021, 281, 122599. [Google Scholar] [CrossRef]
- Frías, M.; de la Villa, V.R.; Martínez-Ramírez, S.; Fernández-Carrasco, L.; Villar-Cociña, E.; García-Giménez, R. Multi-Technique characterization of a fine fraction of CDW and assessment of reactivity in a CDW/Lime system. Minerals 2020, 10, 590. [Google Scholar] [CrossRef]
- Ortega-Zavala, D.E.; Santana-Carrillo, J.L.; Burciaga-Diaz, O.; Escalante-García, J.I. An initial study on alkali activated limestone binders. Cem. Concr. Res. 2019, 120, 267–278. [Google Scholar] [CrossRef]
- Deboucha, W.; Sebaibi, N.; El Mendili, Y.; Fabien, A.; Alengaram, U.J.; Leklou, N.; Hamdadou, M.N.; Bourdout, A.; Gascoin, S. Reactivity effect of calcium carbonate on the formation of carboaluminate phases in ground granulated blast furnace slag blended cements. Sustainability 2021, 13, 6504. [Google Scholar] [CrossRef]
- De Souza Oliveira, A.; De Moraes Rego, E.; Da Fonseca, O.; Dias, R. Crystalline admixture effects on crystal formation phenomena during cement pastes hydration. J. Therm. Anal. Calorim. 2020, 139, 3361–3375. [Google Scholar] [CrossRef]
- Ipavec, A.; Gabrowšek, R.; Vuk, T.; Kaučič, V.; Maček, J.; Meden, A. Carboaluminate phases formation during the hydration of calcite-containing Porland cement. J. Am. Ceram. Soc. 2011, 94, 1238–1242. [Google Scholar] [CrossRef]
- Payá, J.; Borrachero, M.V.; Monzó, J.; Soriano, L.; Tashima, M.M. A new geopolymeric binder from hydrated-carbonated cement. Mater. Lett. 2012, 74, 223–225. [Google Scholar] [CrossRef]
- Cosa, J.; Soriano, L.; Borrachero, M.V.; Reig, L.; Payá, J.; Monzó, J. The compressive strength and microstructure of alkali-activated binary cements developed by combining ceramic sanitaryware with fly ash or blast furnace slag. Minerals 2018, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Segura, Y.P.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Preliminary studies on hydrated cement for its reuse in geopolymers. DYNA 2016, 83, 229–238. [Google Scholar] [CrossRef]
- Heikal, M.; Nassar, M.Y.; El-Sayed, G.; Ibrahim, S.M. Physico-chemical, mechanical, microstructure of alkali activated Egyptian slag. Constr. Build. Mater. 2014, 69, 60–72. [Google Scholar] [CrossRef]
- Moraes, J.C.B.; Tashima, M.M.; Akasaki, J.L.; Monzó, J.; Borrachero, M.V.; Soriano, L.; Payá, J. Effect of sugar cane straw ash (SCSA) as solid precursor and the alkaline activator composition on alkali-activated binders based on blast furnace slag (BFS). Constr. Build. Mater. 2017, 144, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, Y.; Lin, C. Microstructure analysis and effects of single and mixed activator on setting time and strength of coal-gangue based geopolymers. Gels 2022, 8, 195. [Google Scholar] [CrossRef]




| Al2O3 | SiO2 | CaO | Fe2O3 | K2O | Na2O | MgO | SO3 | Other | LOI * |
|---|---|---|---|---|---|---|---|---|---|
| 8.43 | 38.76 | 23.41 | 3.67 | 1.71 | 0.22 | 1.29 | 3.80 | 1.24 | 17.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrachero, M.V.; Payá, J.; Brito, S.; Segura, Y.P.; Soriano, L.; Tashima, M.M.; Monzó, J.M. Reusing Construction and Demolition Waste to Prepare Alkali-Activated Cement. Materials 2022, 15, 3437. https://doi.org/10.3390/ma15103437
Borrachero MV, Payá J, Brito S, Segura YP, Soriano L, Tashima MM, Monzó JM. Reusing Construction and Demolition Waste to Prepare Alkali-Activated Cement. Materials. 2022; 15(10):3437. https://doi.org/10.3390/ma15103437
Chicago/Turabian StyleBorrachero, María V., Jordi Payá, Santiago Brito, Yasna Pamela Segura, Lourdes Soriano, Mauro M. Tashima, and Jose María Monzó. 2022. "Reusing Construction and Demolition Waste to Prepare Alkali-Activated Cement" Materials 15, no. 10: 3437. https://doi.org/10.3390/ma15103437









