Magnetic Properties and Washability of Roasted Suspended Siderite Ores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Suspension State Magnetization Roasting Test Method
2.2.2. Characterization Method of Roasted Ore
2.2.3. Magnetic Separation Test
3. Results
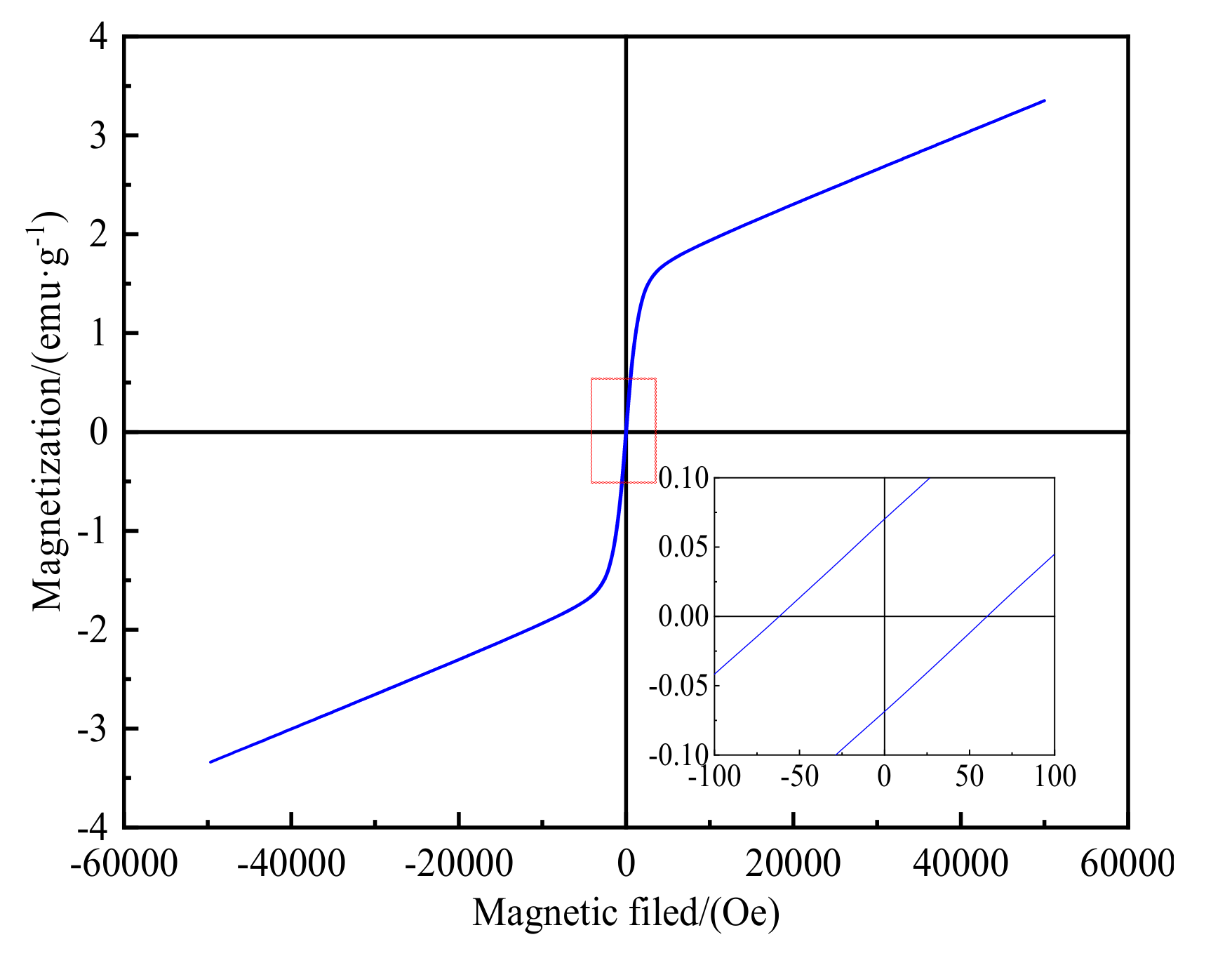
3.1. Magnetic Properties of Roasted Ores
3.2. Physical Property Analysis of Roasted Ore
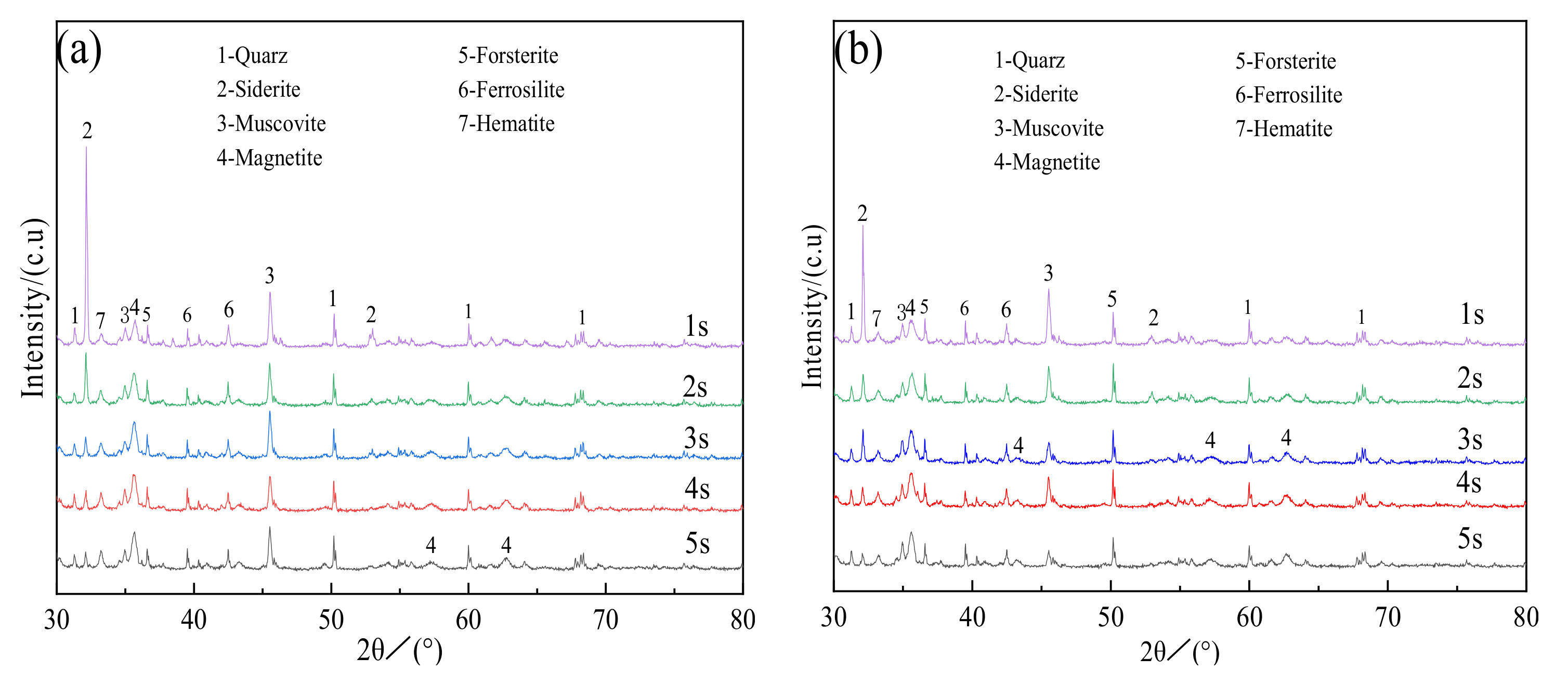
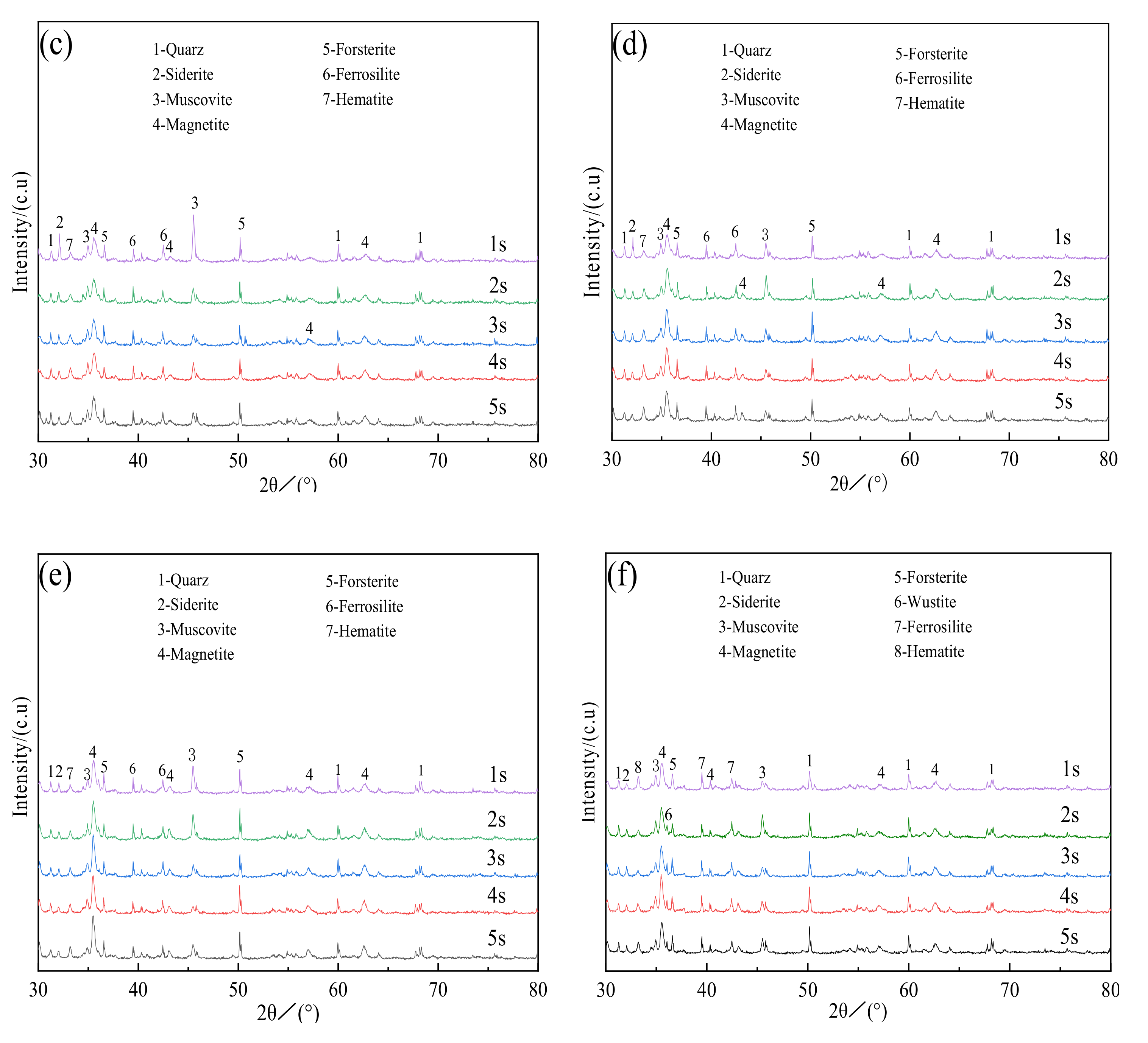
3.2.1. X-ray Diffraction Analysis
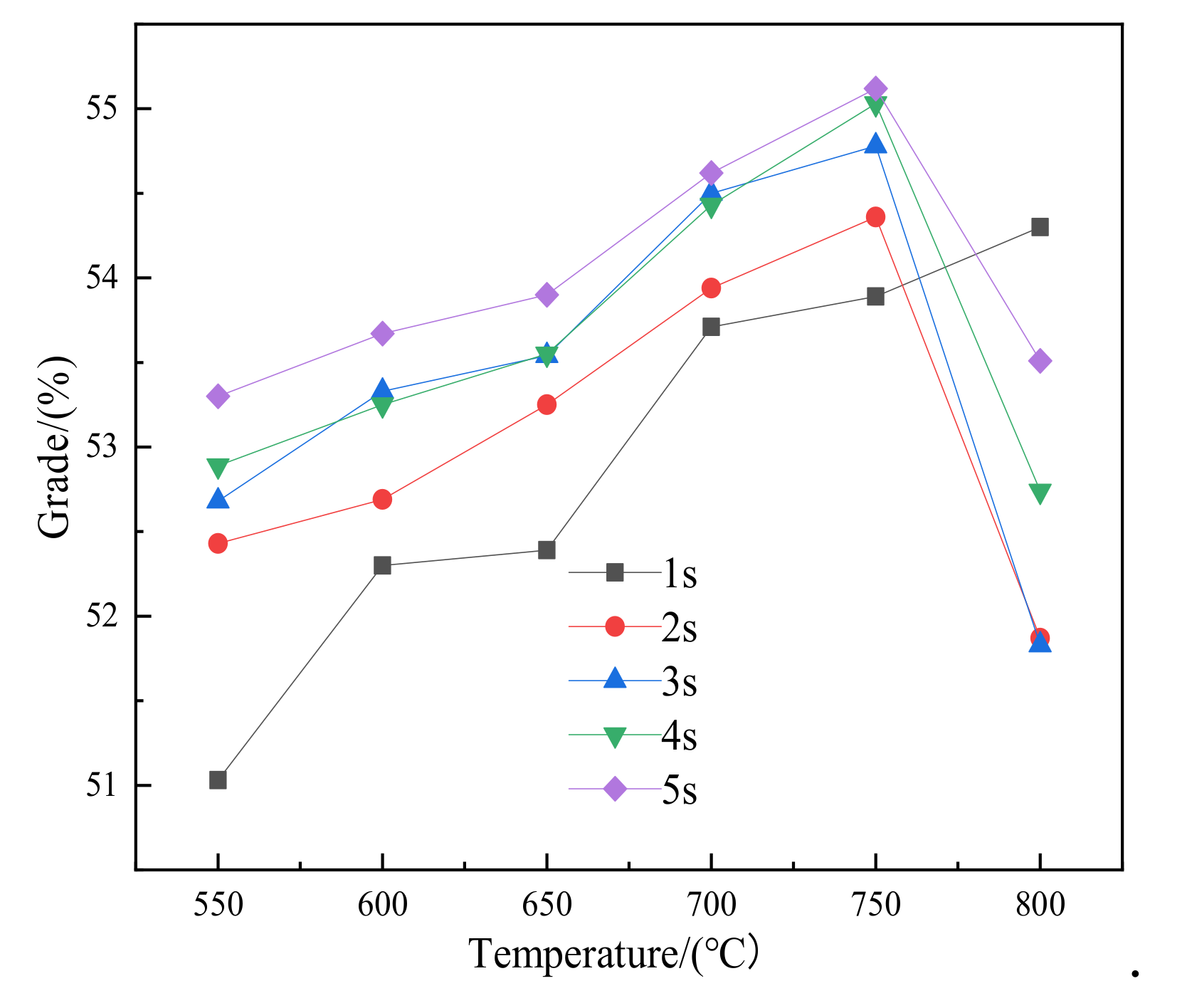
3.3. Low-Intensity Magnetic Separation
4. Conclusions
- (1)
- Daxigou siderite ores contain paramagnetic and ferromagnetic materials, with weak magnetism making direct beneficiation problematic. Before magnetization roasting using a suspension roasting device, the saturation magnetization of the raw ore was only 3.35 emu·g–1, but after roasting, the maximum magnetization reached 21.23 emu·g–1. Roasting can obviously enhance the magnetism of siderite ores.
- (2)
- When the siderite ore was roasted at 550–750 °C, the saturation magnetization and remanence increased as the roasting time increased. The saturation magnetization of the roasted ore was 20.76 emu·g–1, the coercivity was 146.51 Oe, and the remanence was 3.34 emu·g–1 after 5 s of roasting at 750 °C.
- (3)
- Siderite ores displayed an evident iron phase transformation during magnetization roasting, in which FeCO3 was transformed into Fe3O4, however, the other minerals, such as quartz and muscovite, hardly reacted. When roasting at 800 ℃ for 2 s, Fe3O4 in the roasted ore was excessively restored by CO to FeO, and FeO still existed when the time was 3–5 s, which was unfavorable for magnetic separation.
- (4)
- Before 800 °C, the iron grade and recovery rate of the concentrate obtained by magnetic separation basically increased with the increase in roasting time and temperature. The iron concentrate with an iron grade of 55.12% and recovery rate of 90.34% could be obtained via magnetic separation of the roasted ore obtained after roasting at 750 °C for 5 s. If the temperature continues to rise, the iron grade and recovery rate of the concentrate after magnetic separation will decrease to some extent. On the whole, the best roasting condition of siderite is 750 °C for 5 s.
- (5)
- The vibrating sample magnetometer can provide the basis for magnetic separation. When the minerals reach the saturation magnetization state, the magnetic field required for magnetic separation can be judged by measuring the external magnetic field applied at this time, thus reducing unnecessary energy consumption and improving mineral processing efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Zhang, Q.; Han, Y.; Gao, P.; Li, G. Comprehensive utilization of iron and phosphorus from high-phosphorus refractory iron ore. JOM 2017, 70, 144–149. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Gao, P.; Li, Y. Thermal decomposition kinetics of siderite ore during magnetization roasting. Mining Metall. Explor. 2021, 38, 1497–1508. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Han, Y.; Li, Y. A new approach for recovering iron from iron ore tailings using suspension magnetization roasting: A pilot-scale study. Powder Technol. 2019, 361, 571–580. [Google Scholar] [CrossRef]
- Feilmayr, C.; Thurnhofer, A.; Winter, F.; Mali, H.; Schenk, J. Reduction Behavior of Hematite to Magnetite under Fluidized Bed Conditions. ISIJ Int. 2004, 44, 1125–1133. [Google Scholar] [CrossRef]
- Fan, J.F.; Li, W.G.; Zhou, Y.S.; Li, Z.Y. An Introduction to Processes for Fine Iron Ore Treating in Fluidized Bed. Iron Steel 2007, 11, 17–20. [Google Scholar]
- Zhang, B.; Zhi, W.; Gong, X.; Guo, Z. Comparative study of influence of fluidized conditions on sticking time during reduction of FeO particles with CO. Powder Technol. 2012, 225, 1–6. [Google Scholar] [CrossRef]
- Tang, Z.; Han, Y.; Gao, P.; Li, E. Fluidization characteristics of a U-type reduction chamber in a suspension roaster. Powder Technol. 2019, 345, 64–73. [Google Scholar] [CrossRef]
- Song, S.; Lu, S.; Lopez-Valdivieso, A. Magnetic separation of hematite and limonite fines as hydrophobic flocs from iron ores. Miner. Eng. 2002, 15, 415–422. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, J.; Chen, W. Upgrading of Iron Concentrate by Fluidized-Bed Magnetizing Roasting of Siderite to Magnetite in CO–H2–N2 Atmosphere. Trans. Indian Inst. Met. 2019, 72, 1381–1391. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, T. Recovery of low grade haematite via fluidised bed magnetising roasting: Investigation of magnetic properties and liberation characteristics. Ironmak. Steelmak. 2012, 39, 112–120. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Bai, J.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings: Part 1. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. J. Hazard. Mater. 2010, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, Q.; Etsell, T.H. Magnetic properties of ilmenite, hematite and oilsand minerals after roasting. Miner. Eng. 2002, 15, 1121–1129. [Google Scholar] [CrossRef]
- Khedr, M. Reduction kinetics, magnetic behavior and morphological changes during reduction of magnetite single crystal. Mater. Sci. Eng. B 2007, 138, 251–258. [Google Scholar]
- Florek, I.; Lovás, M.; Murova, I. The Effect of Microwave Radiation on Magnetic Properties of Grained Iron Containing Minerals. In Proceedings of the 31st International Microwave Power Symposium, Boston, MA, USA, 28–31 July 1996. [Google Scholar]
- ZnamenáKová, I.; Lovás, M.; MockovIaková, A.; Jakabský, S.; Briancin, J. Modification of magnetic properties of siderite ore by microwave energy. Sep. Purif. Technol. 2005, 43, 169–174. [Google Scholar] [CrossRef]
- Waters, K.E.; Rowson, N.A.; Greenwood, R.W.; Williams, A. The effect of heat treatment on the magnetic properties of pyrite. Miner. Eng. 2008, 21, 679–682. [Google Scholar] [CrossRef]
- Wu, X.; Xu, P.; Duan, Y.; Hu, C.; Li, G. Surface magnetization of siderite mineral. Int. J. Min. Sci. Technol. 2012, 22, 816–821. [Google Scholar] [CrossRef]
- Waters, K.E.; Rowson, N.A.; Greenwood, R.W.; Williams, A. Characterising the effect of microwave radiation on the magnetic properties of pyrite. Sep. Purif. Technol. 2007, 56, 9–17. [Google Scholar] [CrossRef]
- Zhang, X.L.; Han, Y.X.; Sun, Y.S.; Li, Y.J. Innovative utilization of refractory iron ore via suspension magnetization roasting: A pilot-scale study. Powder Technol. 2019, 352, 16–24. [Google Scholar] [CrossRef]
- Jang, K.O.; Nunna, V.; Hapugoda, S.; Nguyen, A.V.; Bruckard, W.J. Chemical and mineral transformation of a low grade goethite ore by dehydroxylation, reduction roasting and magnetic separation. Miner. Eng. 2014, 60, 14–22. [Google Scholar] [CrossRef]




| Element | TFe | FeO | SiO2 | MgO | CaO | Al2O3 | Mn | S | P |
|---|---|---|---|---|---|---|---|---|---|
| Content | 22.53 | 37.76 | 40.05 | 1.22 | 0.31 | 13.17 | 0.71 | 0.14 | 0.04 |
| Iron Phase | Fe in Magnetite | Fe in Goethite and Hematite | Fe in Siderite | Fe in Silicate | TOTAL iron |
|---|---|---|---|---|---|
| Content | 1.33 | 4.41 | 11.47 | 5.32 | 22.53 |
| Percentage | 5.90 | 19.58 | 50.91 | 23.61 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, C.; Jiu, S.; Zhao, B.; Song, Q. Magnetic Properties and Washability of Roasted Suspended Siderite Ores. Materials 2022, 15, 3582. https://doi.org/10.3390/ma15103582
Chen Y, Yang C, Jiu S, Zhao B, Song Q. Magnetic Properties and Washability of Roasted Suspended Siderite Ores. Materials. 2022; 15(10):3582. https://doi.org/10.3390/ma15103582
Chicago/Turabian StyleChen, Yanxin, Chao Yang, Shaowu Jiu, Bo Zhao, and Qiang Song. 2022. "Magnetic Properties and Washability of Roasted Suspended Siderite Ores" Materials 15, no. 10: 3582. https://doi.org/10.3390/ma15103582






