Effect of Microwave-Assisted Synthesis and Sintering of Lead-Free KNL-NTS Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Powder Calcination
2.3. Sintering Processes
2.4. Characterization Methods
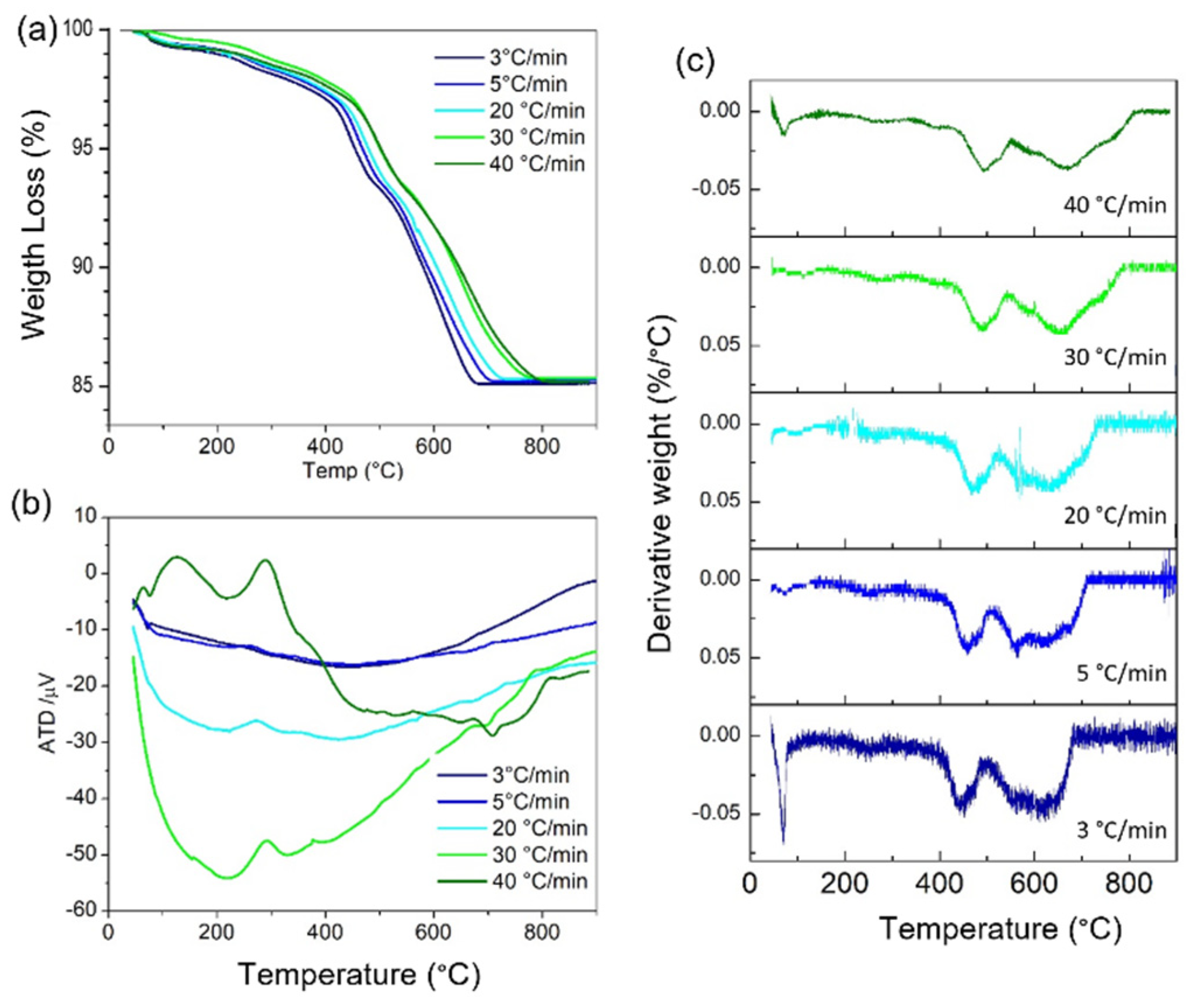
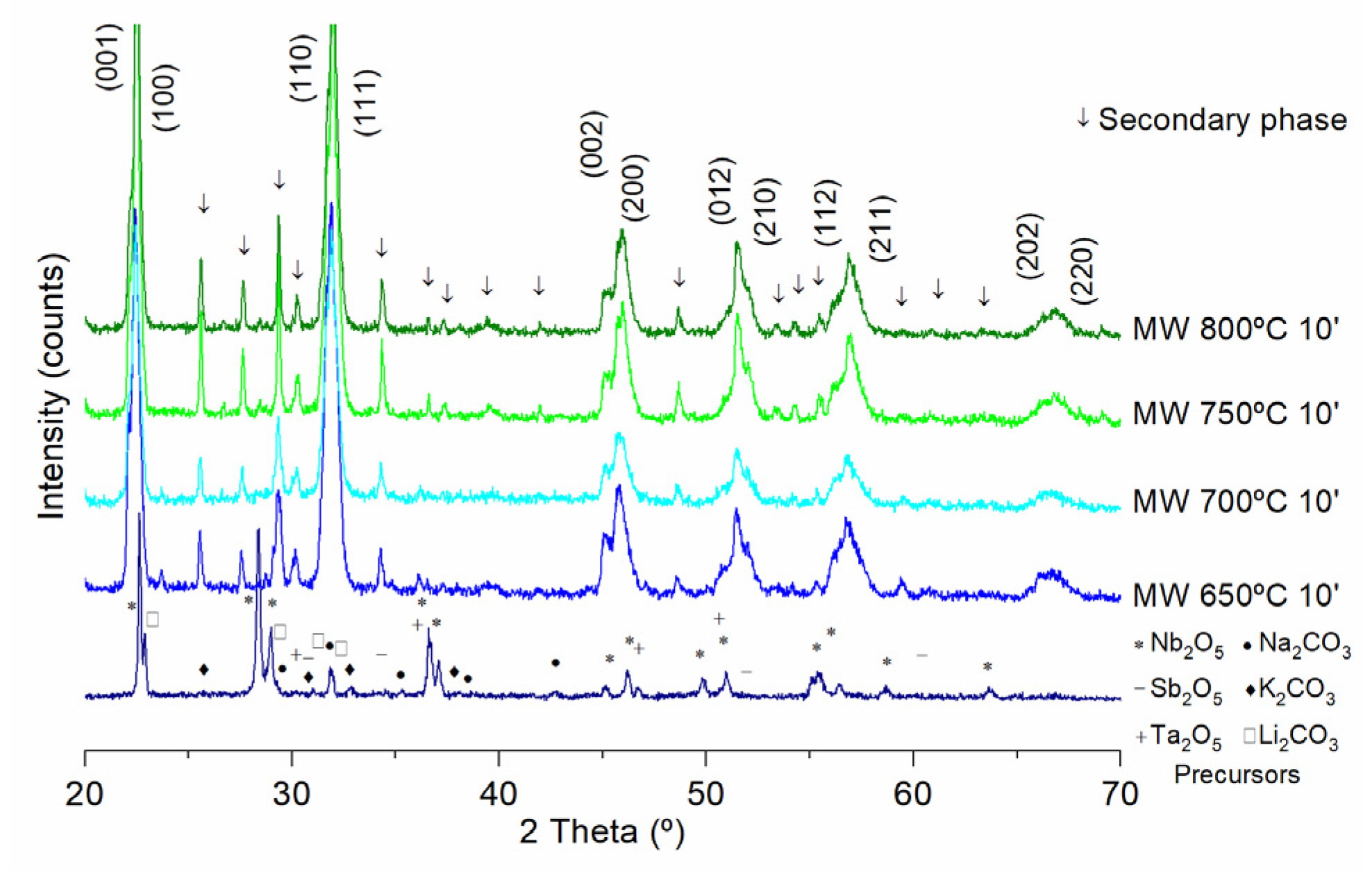
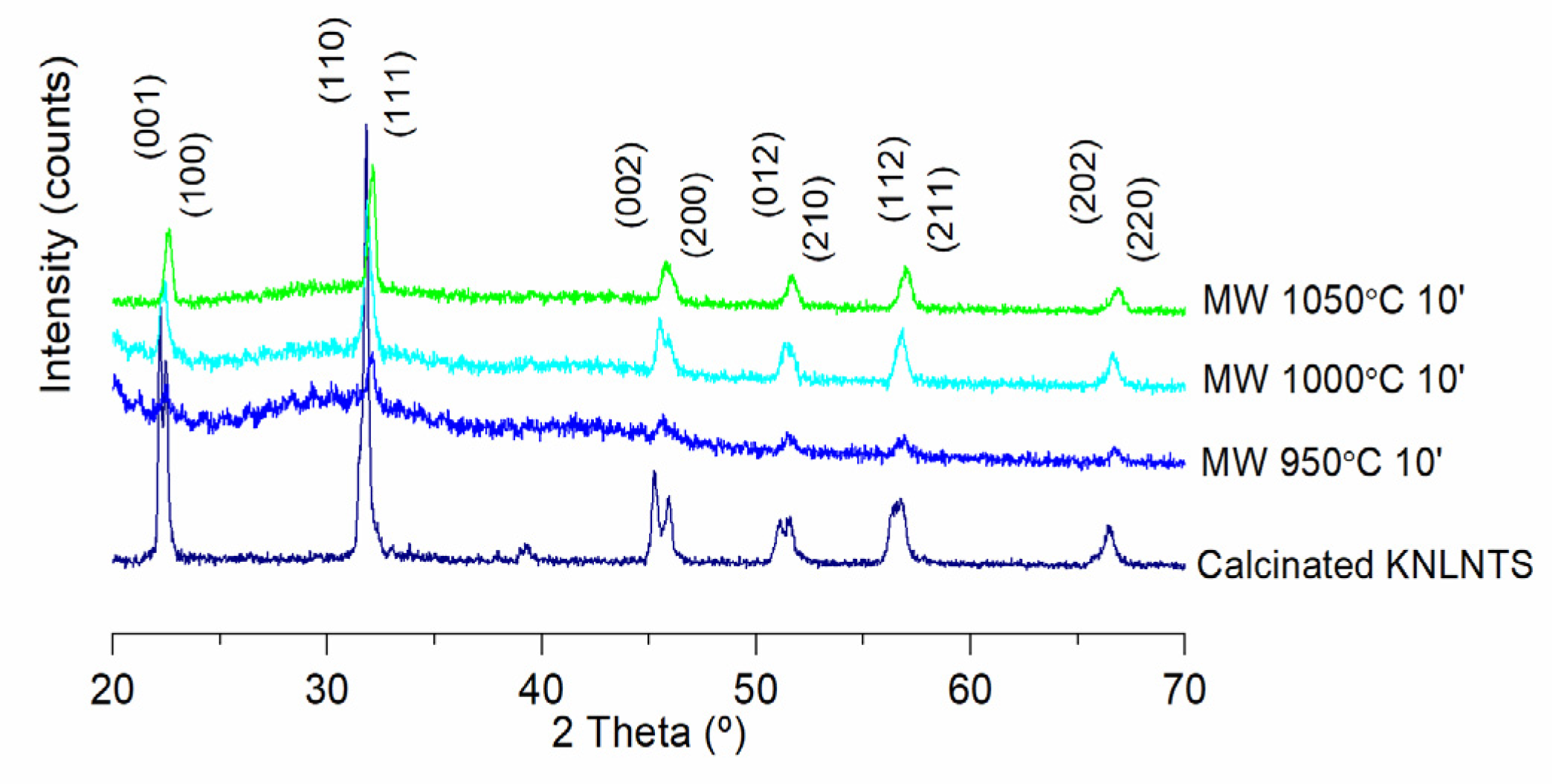
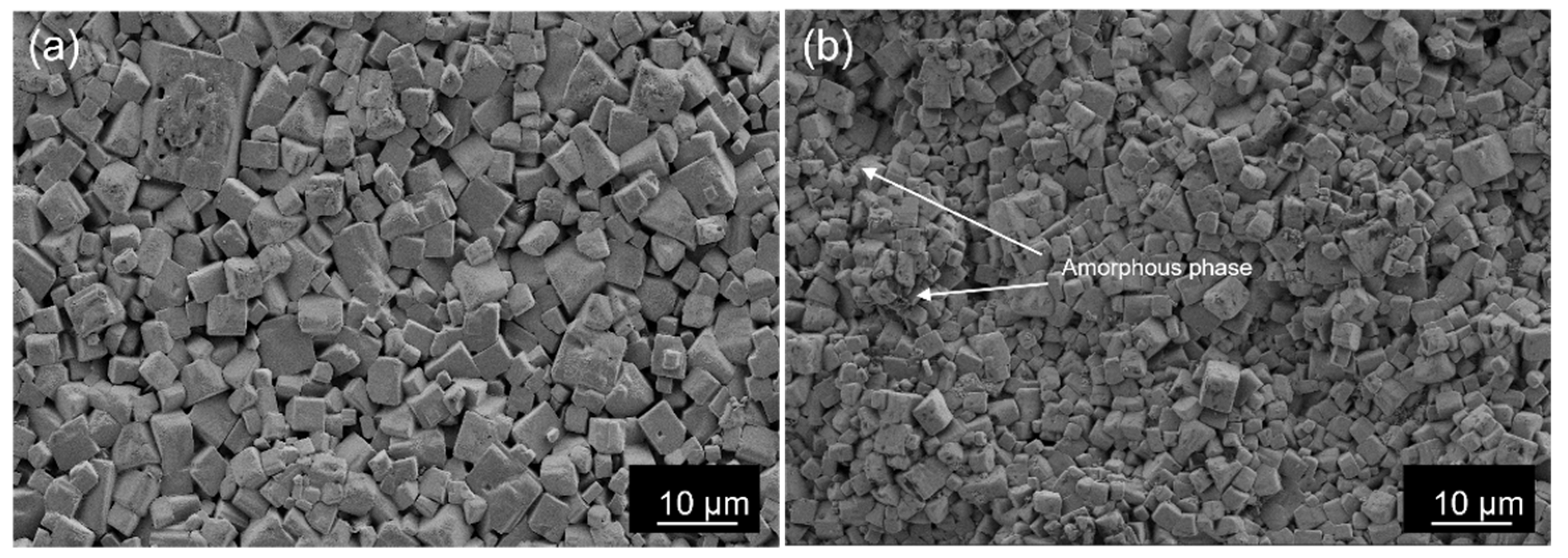
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naik, T.P.; Singh, I.; Sharma, A.K. Processing of polymer matrix composites using microwave energy: A review. Compos. Part A Appl. Sci. Manuf. 2022, 156, 106870. [Google Scholar] [CrossRef]
- El Khaled, D.; Novas, N.; Gazquez, J.A.; Manzano-Agugliaro, F. Microwave dielectric heating: Applications on metals processing. Renew. Sustain. Energy Rev. 2018, 82, 2880–2892. [Google Scholar] [CrossRef]
- Biesuz, M.; Saunders, T.; Ke, D.; Reece, M.J.; Hu, C.; Grasso, S. A review of electromagnetic processing of materials (EPM): Heating, sintering, joining and forming. J. Mater. Sci. Technol. 2021, 69, 239–272. [Google Scholar] [CrossRef]
- Curto, H.; Thuault, A.; Jean, F.; Violier, M.; Dupont, V.; Hornez, J.C.; Leriche, A. Coupling additive manufacturing and microwave sintering: A fast processing route of alumina ceramics. J. Eur. Ceram. Soc. 2020, 40, 2548–2554. [Google Scholar] [CrossRef]
- Ravi Teja, P.; Raja Annamalai, A.; Gecil Evangeline, T.; Srikanth, M.; Dinesh, K.A.; Chun-Ping, J. Effect of Heating Modes on Reactive Sintering of Ca3Co4O9 Ceramics. Materials 2021, 14, 273. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Huang, Z.; Qi, J.; Tang, Z.; Duan, J.; Wu, L.; Zhang, K.; Lu, T. Fast densification of dense nano-grained Gd2Zr2O7 ceramic prepared by two-step microwave sintering. J. Nucl. Mater. 2022, 558, 153353. [Google Scholar] [CrossRef]
- Benavente, R.; Pruna, A.; Borrell, A.; Salvador, M.D.; Pullini, D.; Peñaranda-Foix, F.; Busquets, D. Fast route to obtain Al2O3-based nanocomposites employing graphene oxide: Synthesis and sintering. Mater. Res. Bull. 2015, 64, 245–251. [Google Scholar] [CrossRef]
- Raja Annamalai, A.; Nagaraju, N.; Dinesh, K.A.; Muthuchamy, A. Effect of Heating Mode on Sinterability of YSZ+CeO2 Ceramics. Metals 2018, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Madhan, M.; Prabhakaran, G. Microwave versus conventional sintering: Microstructure and mechanical properties of Al2O3–SiC ceramic composites. Bol. Soc. Esp. Ceram. Vidr. 2019, 58, 14–22. [Google Scholar] [CrossRef]
- Benavente, R.; Borrell, A.; Salvador, M.D.; Garcia-Moreno, O.; Peñaranda-Foix, F.L.; Catala-Civera, J.M. Fabrication of near-zero thermal expansion of fully dense β-eucryptite ceramics by microwave sintering. Ceram. Int. 2014, 40, 935–941. [Google Scholar] [CrossRef]
- Meng, L.Y.; Wang, B.; Ma, M.G.; Lin, K.L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Lara-Cerón, J.A.; Vidyasagar, C.C.; Muñoz-Flores, B.M.; Pérez, V.M.J. Recent advances in microwave assisted syntheses of organometallic and coordination compounds. Handb. Greener Synth. Nanomater. Compd. 2021, 1, 543–584. [Google Scholar]
- Rybakov, K.I.; Buyanova, M.N. Microwave resonant sintering of powder metals. Scr. Mater. 2018, 149, 108–111. [Google Scholar] [CrossRef]
- Zhan, H.; Zhang, N.; Wu, D.; Wu, Z.; Bi, S.; Ma, B.; Liu, W. Controlled synthesis of β-SiC with a novel microwave sintering method. Mater. Lett. 2019, 255, 126586. [Google Scholar] [CrossRef]
- Safari, A.; Hejazi, M. Lead-Free KNN-Based Piezoelectric Materials. In Lead-Free Piezoelectrics; Springer Science & Business Media: New York, NY, USA, 2012; pp. 139–175. [Google Scholar]
- Ok, Y.-P.; Ji, H.-N.; Kim, K.-S.; Tai, W.-P.; Seol, J.-H.; Hong, I.-K.; Lee, J.-S. Sintering and piezoelectric properties of lead-free (K0.38Na0.58Li0.04)(Nb0.86Ta0.10Sb0.04)O3 ceramics doped with Fe2O3. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 92053. [Google Scholar] [CrossRef] [Green Version]
- Ramajo, L.; Castro, M.; Rubio-Marcos, F.; Fernandez-Lozano, J. Influence of MoO3 on electrical and microstructural properties of (K0.44Na0.52Li0.04)(Nb0.86Ta 0.10Sb0.04)O3. J. Mater. Sci. Mater. Electron. 2013, 24, 3587–3593. [Google Scholar] [CrossRef]
- Feizpour, M.; Barzegar-Bafrooei, H.; Hayati, R.; Ebadzadeh, T. Microwave-assisted synthesis and sintering of potassium sodium niobate lead-free piezoelectric ceramics. Ceram. Int. 2014, 40, 871–877. [Google Scholar] [CrossRef]
- Lagunas-Chavarría, A.; Navarro-Rojero, M.; Salvador, M.; Catala-Civera, J.; Borrell, A. Effect of synthesis and sintering temperatures on K0.5Na0.5NbO3 lead-free piezoelectric ceramics by microwave heating. J. Mater. Sci. Mater. Electron. 2021, 32, 15279–15290. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, J.; Lu, P.; Zhang, L.; Yao, H.; Xu, F.; Li, Y. Influence of secondary phase on polymorphic phase transition in Li-doped KNN lead-free ceramics. Ceram. Int. 2017, 43, 12893–12897. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Romero, J.J.; Navarro-Rojero, M.G.; Fernandez, J.F. Effect of ZnO on the structure, microstructure and electrical properties of KNN-modified piezoceramics. J. Eur. Ceram. Soc. 2009, 29, 3045–3052. [Google Scholar] [CrossRef]
- Mathrmool, K.; Udeye, T.; Bongkarn, T. Low temperature fabrication of lead-free piezoelectric KNLNTS ceramics by the solid state combustion technique. Ferroelectrics 2017, 518, 31–41. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.S.; Han, S.H.; Kang, H.W.; Lee, H.G.; Cheon, C.I. Low-temperature sintering and piezoelectric properties of CuO-doped (K,Na)NbO3 ceramics. Mater. Res. Bull. 2017, 96, 121–125. [Google Scholar] [CrossRef]
- Chao, X.; Wang, J.; Kang, C.; Li, Z.; Yang, Z. Microstructure, electrical properties, strain and temperature stability of (K, Na, Li)(Nb, Ta)O3 ceramics: The effect of BiFeO3 additive. Ceram. Int. 2015, 41, 12887–12895. [Google Scholar] [CrossRef]
- Feizpour, M.; Ebadzadeh, T.; Jenko, D. Synthesis and characterization of lead-free piezoelectric (K0.50Na0.50)NbO3 powder produced at lower calcination temperatures: A comparative study with a calcination temperature of 850 °C. J. Eur. Ceram. Soc. 2016, 36, 1595–1603. [Google Scholar] [CrossRef]
- Malic, B.; Jenko, D.; Holc, J.; Hrovat, M.; Kosec, M. Synthesis of Sodium Potassium Niobate: A Diffusion Couples Study. J. Am. Ceram. Soc. 2008, 91, 1916–1922. [Google Scholar] [CrossRef]
- Hoffmann, M.J.; Kungl, H.; Acker, J.; Elsässer, C.; Körbel, S.; Marton, P.; Eichel, R.-A.; Erünal, E.; Jakes, P. Influence of the A/B Stoichiometry on Defect Structure, Sintering, and Microstructure in Undoped and Cu-Doped KNN. In Lead-Free Piezoelectrics; Priya, S., Nahm, S., Eds.; Springer: New York, NY, USA, 2012; pp. 209–255. [Google Scholar]
- Yang, Z.; Chang, Y.; Liu, B.; Wei, L. Effects of composition on phase structure, microstructure and electrical properties of (K0.5Na0.5)NbO3–LiSbO3 ceramics. Mater. Sci. Eng. A 2006, 432, 292–298. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Marchet, P.; Merle-Méjean, T.; Fernandez, J.F. Role of sintering time, crystalline phases and symmetry in the piezoelectric properties of lead-free KNN-modified ceramics. Mater. Chem. Phys. 2010, 123, 91–97. [Google Scholar] [CrossRef]
- Yin, N.; Jalalian, A.; Gai, Z.G.; Zhao, L.L.; Wang, X.L. Effect of Doping Ions on Structure and Electrical Properties of Lead-Free KNN Ceramics. Adv. Mater. Res. 2014, 1058, 190–195. [Google Scholar]
- Coondoo, I.; Panwar, N.; Kholkin, A. Lead-free piezoelectrics: Current status and perspectives. J. Adv. Dielectr. 2013, 3, 1330002. [Google Scholar] [CrossRef]
- Fuentes, J.; Portelles, J.; Durruthy-Rodríguez, M.D.; H’Mok, H.; Raymond, O.; Heiras, J.; Cruz, M.P.; Siqueiros, J.M. Dielectric and piezoelectric properties of the KNN ceramic compound doped with Li, La and Ta. Appl. Phys. A 2015, 118, 709–715. [Google Scholar] [CrossRef]
- Singh, R.; Kambale, K.; Kulkarni, A.R.; Harendranath, C.S. Structure composition correlation in KNN–BT ceramics—An X-ray diffraction and Raman spectroscopic investigation. Mater. Chem. Phys. 2013, 138, 905–908. [Google Scholar] [CrossRef]
- Malic, B.; Bernard, J.; Holc, J.; Jenko, D.; Kosec, M. Alkaline-earth doping in (K,Na)NbO3 based piezoceramics. J. Eur. Ceram. Soc. 2005, 25, 2707–2711. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Romero, J.J.; Martín-Gonzalez, M.S.; Fernández, J.F. Effect of stoichiometry and milling processes in the synthesis and the piezoelectric properties of modified KNN nanoparticles by solid state reaction. J. Eur. Ceram. Soc. 2010, 30, 2763–2771. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Marcos, F.; Reinosa, J.J.; Vendrell, X.; Romero, J.J.; Mestres, L.; Leret, P.; Fernández, J.F.; Marchet, P. Structure, microstructure and electrical properties of Cu2+ doped (K,Na,Li)(Nb,Ta,Sb)O3 piezoelectric ceramics. Ceram. Int. 2013, 39, 4139–4149. [Google Scholar] [CrossRef]
- Alzamil, M.; Alfaramawi, K.; Abboudy, S.; Abulnasr, L. Electrical conduction hysteresis in carbon black–filled butyl rubber compounds. Int. J. Mod. Phys. B 2017, 32, 1850100. [Google Scholar] [CrossRef]
- Navarro-Rojero, M.G.; Romero, J.J.; Rubio-Marcos, F.; Fernandez, J.F. Intermediate phases formation during the synthesis of Bi4Ti3O12 by solid state reaction. Ceram. Int. 2010, 36, 1319–1325. [Google Scholar] [CrossRef]
- Swain, S.; Kumar, P.; Agrawal, D.K. Sonia Dielectric and ferroelectric study of KNN modified NBT ceramics synthesized by microwave processing technique. Ceram. Int. 2013, 39, 3205–3210. [Google Scholar] [CrossRef]
- Wattanawikkam, C.; Vittayakorn, N.; Bongkarn, T. Low temperature fabrication of lead-free KNN-LS-BS ceramics via the combustion method. Ceram. Int. 2013, 39, S399–S403. [Google Scholar] [CrossRef]





| Sintering Process | Temperature (°C) | Dwell Time (min) | Density (g·cm−1) | Relative Density (%) | d33 (pC·N−1) |
|---|---|---|---|---|---|
| Conventional | 1100 | 120 | 4.37 ± 0.02 | 92.6 ± 0.1 | 145 ± 0.2 |
| Conventional | 1100 | 960 | 4.41 ± 0.01 | 93.4 ± 0.1 | 168 ± 0.2 |
| Microwave | 950 | 10 | 4.34 ± 0.03 | 92.0 ± 0.1 | 11 ± 0.1 |
| Microwave | 1000 | 10 | 4.35 ± 0.01 | 92.2 ± 0.1 | 13 ± 0.1 |
| Microwave | 1050 | 10 | 4.36 ± 0.02 | 92.4 ± 0.1 | 15 ± 0.1 |
| Microwave | 1050 | 20 | 4.38 ± 0.02 | 92.8 ± 0.1 | 10 ± 0.1 |
| Microwave | 1050 | 30 | 4.38 ± 0.01 | 92.8 ± 0.1 | 12 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagunas-Chavarría, A.; Navarro-Rojero, M.G.; Salvador, M.D.; Benavente, R.; Catalá-Civera, J.M.; Borrell, A. Effect of Microwave-Assisted Synthesis and Sintering of Lead-Free KNL-NTS Ceramics. Materials 2022, 15, 3773. https://doi.org/10.3390/ma15113773
Lagunas-Chavarría A, Navarro-Rojero MG, Salvador MD, Benavente R, Catalá-Civera JM, Borrell A. Effect of Microwave-Assisted Synthesis and Sintering of Lead-Free KNL-NTS Ceramics. Materials. 2022; 15(11):3773. https://doi.org/10.3390/ma15113773
Chicago/Turabian StyleLagunas-Chavarría, Anggel, María Guadalupe Navarro-Rojero, María Dolores Salvador, Rut Benavente, Jose Manuel Catalá-Civera, and Amparo Borrell. 2022. "Effect of Microwave-Assisted Synthesis and Sintering of Lead-Free KNL-NTS Ceramics" Materials 15, no. 11: 3773. https://doi.org/10.3390/ma15113773






