Synthesis of a Superhydrophobic Fluorinated Nano-Emulsion and Its Modification on the Wettability of Tight Sandstone
Abstract
:1. Introduction
2. Experiments
2.1. Experimental Materials
2.2. Experimental Methods
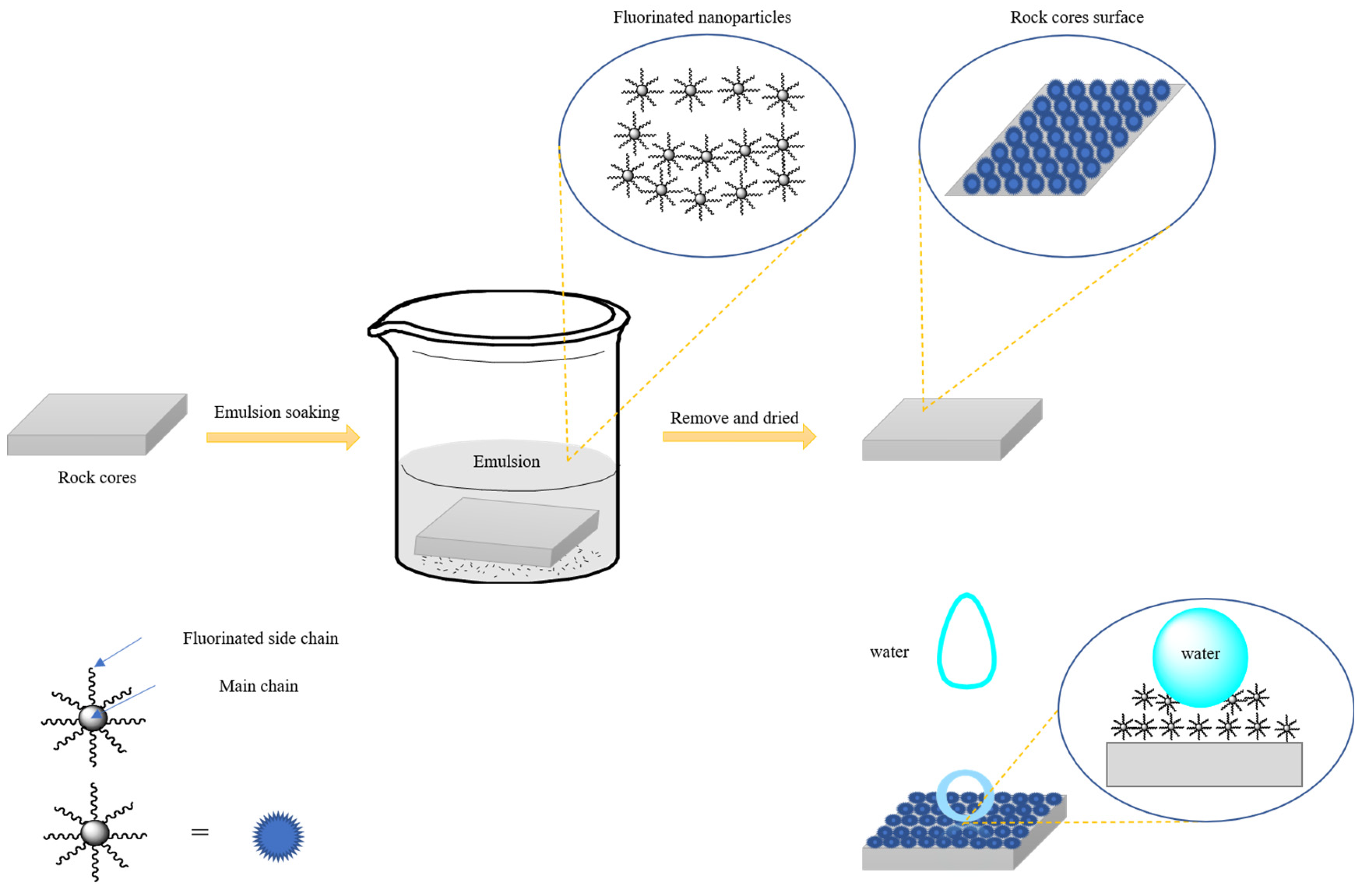
2.2.1. Synthesis of the Water-Soluble Superhydrophobic Fluorinated Nanofluids
2.2.2. Measurement of the Molecular Structure of the Nano-Emulsion
2.2.3. Characterization of the Nanoparticle Size and Morphology
2.2.4. Contact Angle Measurement
2.2.5. Measurement of Water Spontaneous Imbibition Volume in Core Samples
3. Results and Discussion
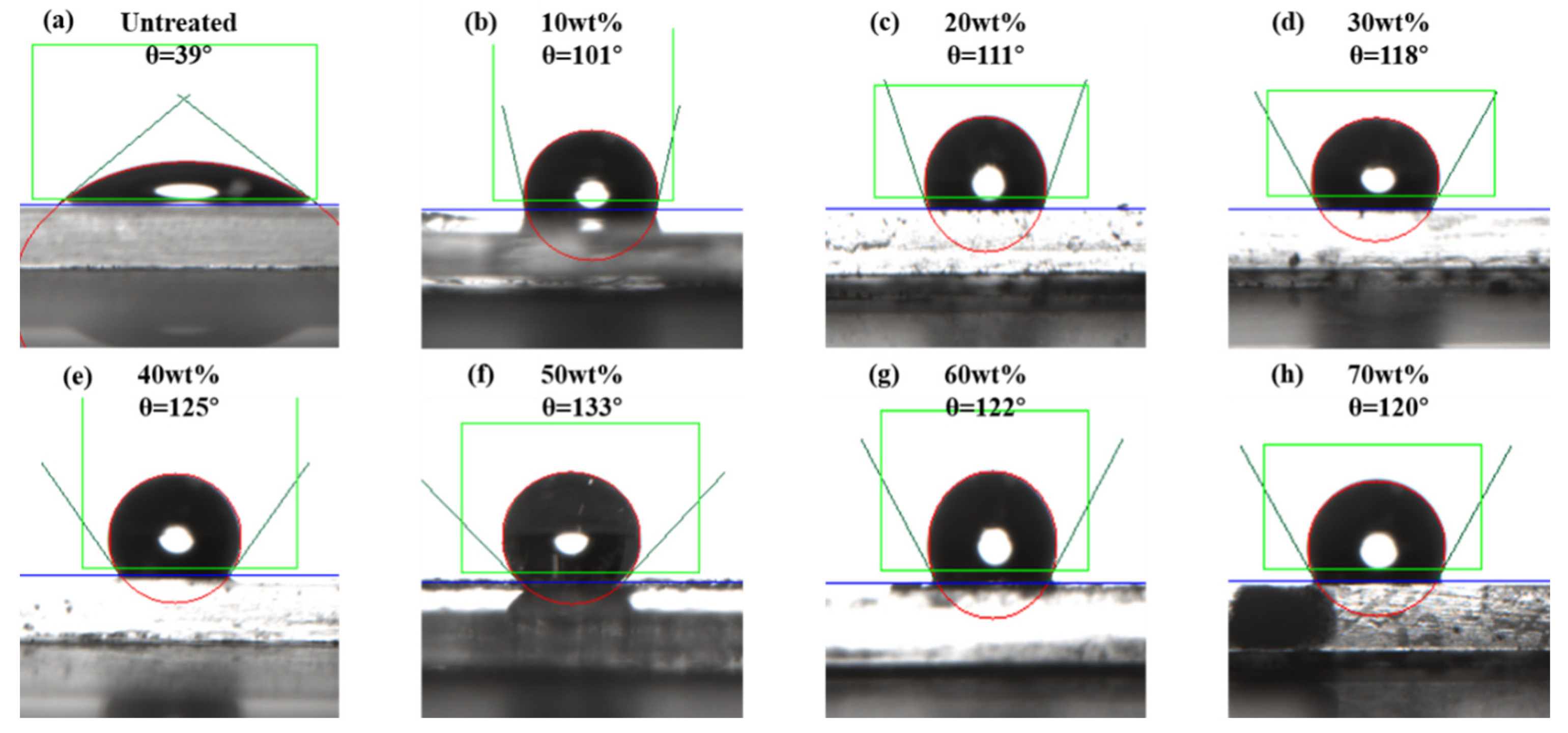
3.1. Effect of Fluorinated Monomer Concentration on the Wettability of Glass Sheets
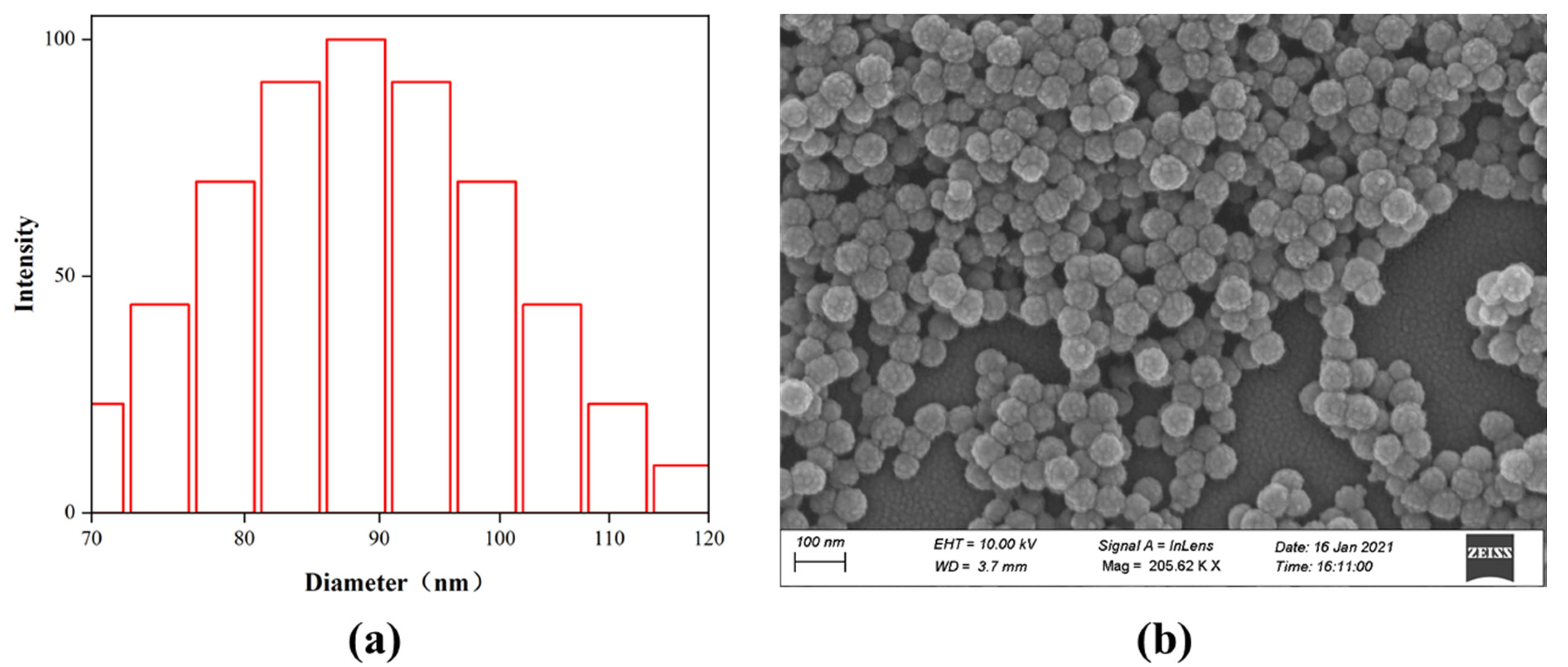
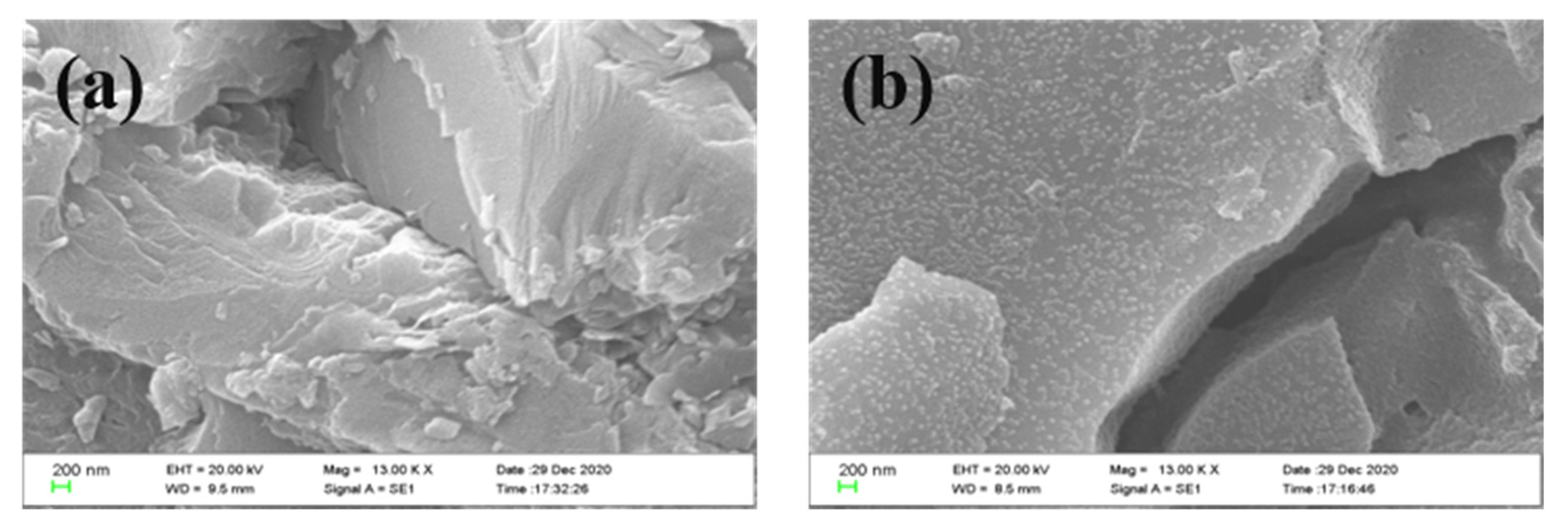
3.2. Characterization of Structure and Properties of Fluorinated Nanoparticles
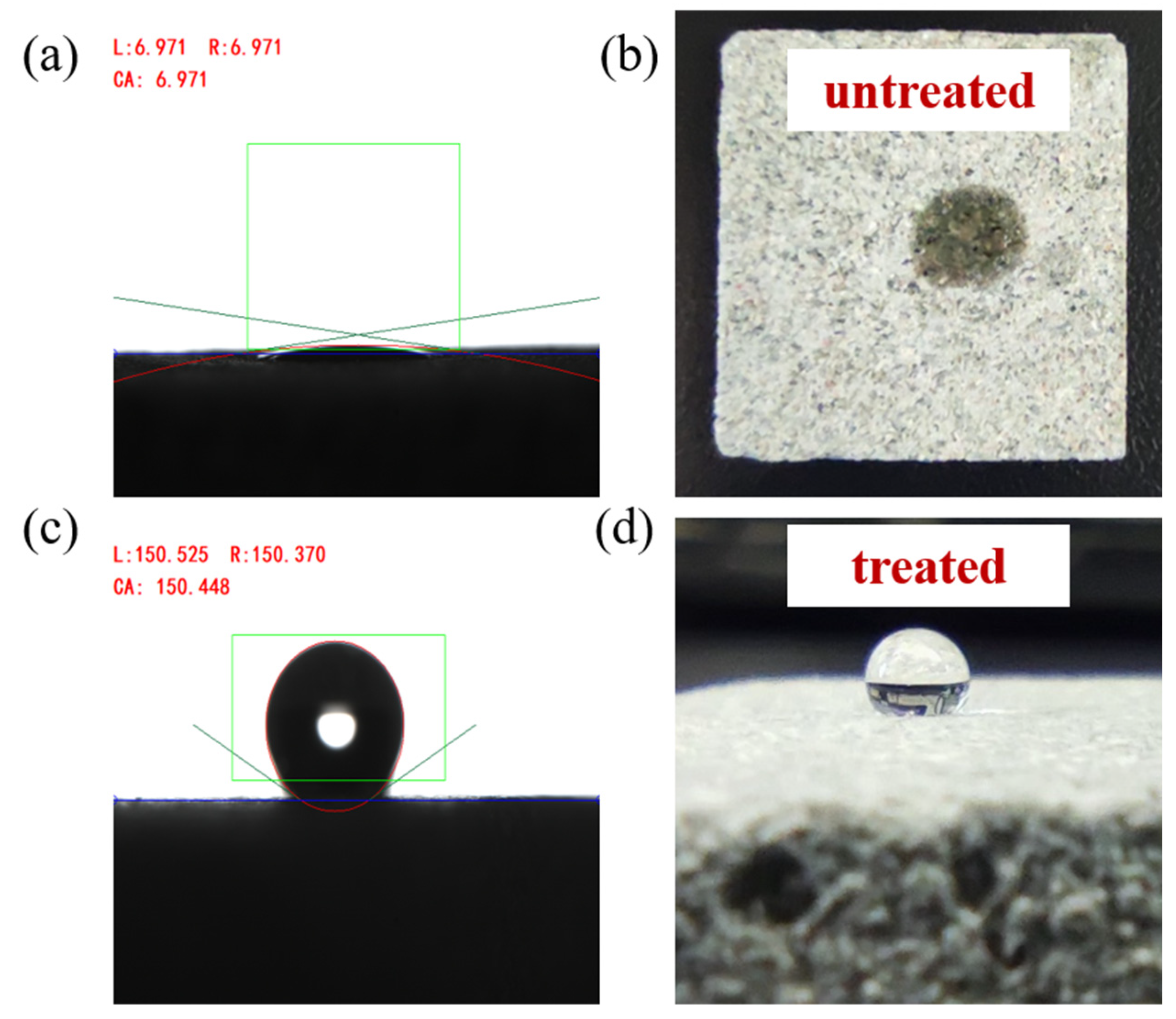
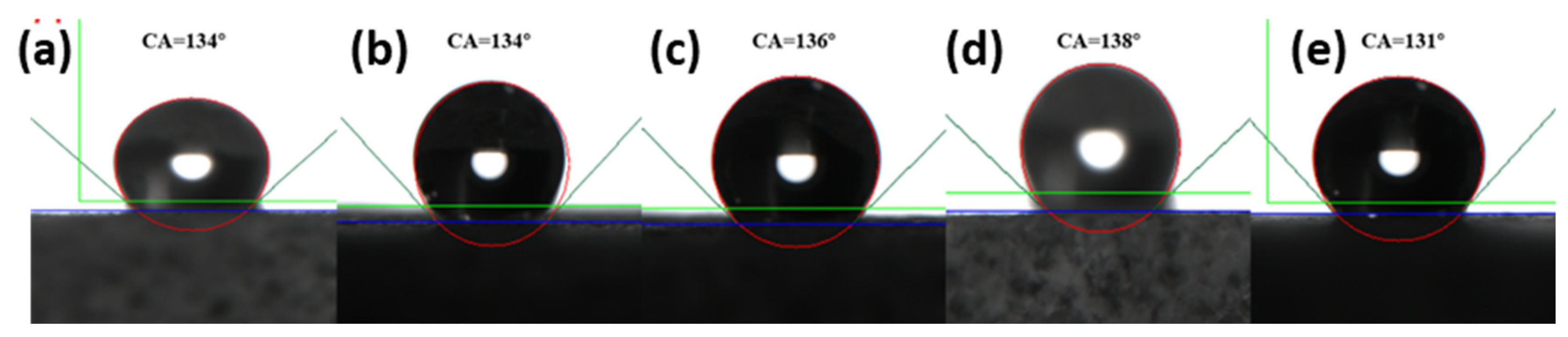
3.3. Effect of Nanoparticles on the Wettability of Natural Tight Sand Cores
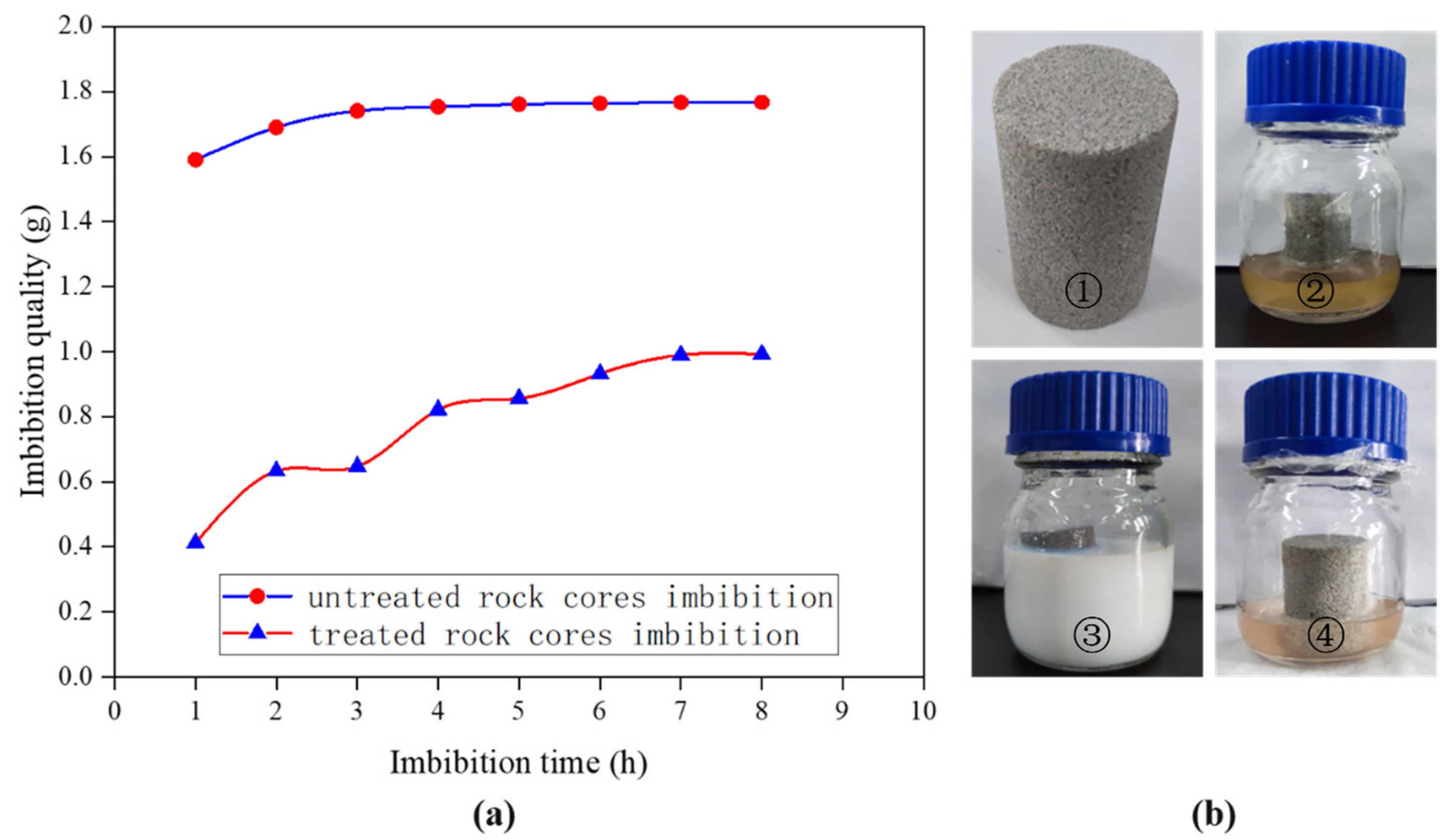
3.4. Spontaneous Imbibition of Natural Tight Sand Cores
3.5. Hydrophobic Mechanism of the Nano-Emulsion
4. Conclusions
- (1)
- A fluorinated nano-emulsion with a wettability alteration function was prepared through the soap-free emulsion polymerization method using cationic functional monomers, fluorinated monomers, and hydrophobic monomers as the main raw materials.
- (2)
- The effect of the fluorinated monomer concentration on the hydrophobicity of the nano-emulsion was evaluated by measuring the contact angles on glass slides. Within a certain concentration range, the hydrophobicity of the nano-emulsion increased with the increase in fluorinated monomer concentration, and the optimum mass ratio of the fluorinated monomer was 50 wt%.
- (3)
- The nano-emulsion’s wettability modification effect on the natural tight sandstone core was studied through the contact angle and spontaneous imbibition experiments. The contact angle increased from 7° to 150°, and the water absorption quality decreased from 1.75 g to 0.98 g, indicating that the wettability of the core changed from hydrophilic to superhydrophobic.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bahrami, H.; Rezaee, R.; Clennell, B. Water blocking damage in hydraulically fractured tight sand gas reservoirs: An example from Perth Basin, Western Australia. J. Pet. Sci. Eng. 2012, 88, 100–106. [Google Scholar] [CrossRef]
- Bahrami, H.; Rezaee, M.R.; Nazhat, D.; Ostojic, J.; Clennell, B.; Jamili, A. Effect of water blocking damage on flow efficiency and productivity in tight gas reservoirs. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 27–29 March 2011. [Google Scholar]
- Lai, F.; Li, Z.; Wang, Y. Impact of water blocking in fractures on the performance of hydraulically fractured horizontal wells in the tight gas reservoir. J. Pet. Sci. Eng. 2017, 156, 134–141. [Google Scholar] [CrossRef]
- Lufeng, Z.; Fujian, Z.; Shicheng, Z.; Jin, W.; Yuechun, W. Evaluation of permeability damage caused by drilling and fracturing fluids in tight low permeability sandstone reservoirs. J. Pet. Sci. Eng. 2019, 175, 1122–1135. [Google Scholar] [CrossRef]
- Mirzaei-Paiaman, A.; Dalvand, K.; Oraki Kohshour, I.; Masihi, M.; Moghadasi, J. A study on the key influential factors of a gas reservoir’s potential for aqueous phase trapping. Energy Sources Part A Recovery Util. Environ. Eff. 2012, 34, 1541–1549. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.; An, F.; Lin, L.; Lai, Y. The water-blocking effect caused by the use of hydraulic methods for permeability enhancement in coal seams and methods for its removal. Int. J. Min. Sci. Technol. 2016, 26, 615–621. [Google Scholar] [CrossRef]
- Tang, H.; Lv, J.J.; Lv, D.L.; Wu, X.Q.; PENG, D.B. Study on influencing factors of water blocking effect on the tight gas reservoir. J. Southwest Pet. Univ. Sci. Technol. Ed. 2009, 31, 91–94. [Google Scholar]
- Kamath, J.; Laroche, C. Laboratory-based evaluation of gas well deliverability loss caused by water blocking. SPE J. 2003, 8, 71–80. [Google Scholar] [CrossRef]
- Bennion, D.B.; Thomas, F.B.; Bietz, R.F.; Bennion, D.W. Water and hydrocarbon phase trapping in porous media-diagnosis, prevention and treatment. J. Can. Pet. Technol. 1996, 35, 10. [Google Scholar] [CrossRef]
- Li, K.; Firoozabadi, A. Experimental study of wettability alteration to preferential gas-wetting in porous media and its effects. SPE Reserv. Eval. Eng. 2000, 3, 139–149. [Google Scholar] [CrossRef]
- Ni, G.; Li, Z.; Xie, H. The mechanism and relief method of the coal seam water blocking effect (WBE) is based on the surfactants. Powder Technol. 2018, 323, 60–68. [Google Scholar] [CrossRef]
- Ni, G.; Cheng, W.; Lin, B.; Zhai, C. Experimental study on removing water blocking effect (WBE) from two aspects of the pore negative pressure and surfactants. J. Nat. Gas Sci. Eng. 2016, 31, 596–602. [Google Scholar] [CrossRef]
- Para, G.; Jarek, E.; Warszynski, P. The Hofmeister series effect in adsorption of cationic surfactants—Theoretical description and experimental results. Adv. Colloid Interface Sci. 2006, 122, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Jones, D.R.; Alexander, S.; Barron, A.R. New insights into the interactions between asphaltene and a low surface energy anionic surfactant under low and high brine salinity. J. Colloid Interface Sci. 2020, 571, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, J.H.; Zhang, W.; Zhuang, B.; Qi, H. Cloud point of nonionic surfactant Triton X-45 in aqueous solution. Colloids Surf. B Biointerfaces 2008, 61, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Firoozabadi, A. Phenomenological modeling of critical condensate saturation and relative permeabilities in gas/condensate systems. SPE J. 2000, 5, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.Q.; Firoozabadi, A. Relative permeability modification in gas/liquid systems through wettability alteration to intermediate gas wetting. SPE Reserv. Eval. Eng. 2002, 5, 427–436. [Google Scholar] [CrossRef]
- Fahes, M.M.; Firoozabadi, A. Wettability alteration to intermediate gas-wetting in gas-condensate reservoirs at high temperatures. SPE J. 2007, 12, 397–407. [Google Scholar] [CrossRef]
- Shaker, M.; Salahinejad, E. A combined criterion of surface free energy and roughness to predict the wettability of non-ideal low-energy surfaces. Prog. Org. Coat. 2018, 119, 123–126. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Islamova, A.G.; Orlova, E.G.; Ivashutenko, A.S.; Shanenkov, I.I.; Zykov, I.Y.; Feoktistov, D.V. Influence of roughness on polar and dispersed components of surface free energy and wettability properties of copper and steel surfaces. Surf. Coat. Technol. 2021, 422, 127518. [Google Scholar] [CrossRef]
- Duca, M.D.; Plosceanu, C.L.; Pop, T. Surface modifications of polyvinylidene fluoride (PVDF) under rf Ar plasma. Polym. Degrad. Stab. 1998, 61, 65–72. [Google Scholar] [CrossRef]
- Lazar, P.; Otyepková, E.; Karlický, F.; Čépe, K.; Otyepka, M. The surface and structural properties of graphite fluoride. Carbon 2015, 94, 804–809. [Google Scholar] [CrossRef]
- Liu, X.; Kang, Y.; Luo, P.; You, L.; Tang, Y.; Kong, L. Wettability modification by fluoride and its application in aqueous phase trapping damage removal in tight sandstone reservoirs. J. Pet. Sci. Eng. 2015, 133, 201–207. [Google Scholar] [CrossRef]
- Adibhatla, B.; Mohanty, K.K.; Berger, P.; Lee, C. Effect of surfactants on wettability of near-wellbore regions of gas reservoirs. J. Pet. Sci. Eng. 2006, 52, 227–236. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Chen, J.M.; Kuo, R.R.; Lin, T.S.; Wu, C.F. Influence of surface roughness on water-and oil-repellent surfaces coated with nanoparticles. Appl. Surf. Sci. 2005, 240, 318–326. [Google Scholar] [CrossRef]
- Alan, B.O.; Barisik, M.; Ozcelik, H.G. Roughness effects on the surface charge properties of silica nanoparticles. J. Phys. Chem. C 2020, 124, 7274–7286. [Google Scholar] [CrossRef]
- Niu, Y.; Yu, M.; Meka, A.; Liu, Y.; Zhang, J.; Yang, Y.; Yu, C. Understanding the contribution of surface roughness and hydrophobic modification of silica nanoparticles to enhanced therapeutic protein delivery. J. Mater. Chem. B 2016, 4, 212–219. [Google Scholar] [CrossRef]
- Aminnaji, M.; Fazeli, H.; Bahramian, A.; Gerami, S.; Ghojavand, H. Wettability alteration of reservoir rocks from liquid wetting to gas wetting using nanofluid. Transp. Porous Media 2015, 109, 201–216. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progress in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Naik, S.; You, Z.; Bedrikovetsky, P. Effect of wettability alteration on productivity enhancement in unconventional gas reservoirs: Application of nanotechnology. In Proceedings of the SPE Asia Pacific Unconventional Resources Conference and Exhibition, Brisbane, Australia, 9–11 November 2015. [Google Scholar]
- Mousavi, M.A.; Hassanajili, S.; Rahimpour, M.R. Synthesis of fluorinated nano-silica and its application in wettability alteration near-wellbore region in gas condensate reservoirs. Appl. Surf. Sci. 2013, 273, 205–214. [Google Scholar] [CrossRef]
- Esmaeilzadeh, P.; Sadeghi, M.T.; Fakhroueian, Z.; Bahramian, A.; Norouzbeigi, R. Wettability alteration of carbonate rocks from liquid-wetting to ultra gas-wetting using TiO2, SiO2 and CNT nanofluids containing fluorochemicals, for enhanced gas recovery. J. Nat. Gas Sci. Eng. 2015, 26, 1294–1305. [Google Scholar] [CrossRef]
- Seipenbusch, M.; Rothenbacher, S.; Kirchhoff, M.; Schmid, H.J.; Kasper, G.; Weber, A.P. Interparticle forces in silica nanoparticle agglomerates. J. Nanopart. Res. 2010, 12, 2037–2044. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Wong, C.P. Study on mono-dispersed nano-size silica by surface modification for underfill applications. J. Colloid Interface Sci. 2005, 292, 436–444. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Fan, Z.; Liu, Q.; Ma, W.; Li, J.; Li, N.; Ma, P.; Zhang, H. Synthesis of a Superhydrophobic Fluorinated Nano-Emulsion and Its Modification on the Wettability of Tight Sandstone. Materials 2022, 15, 4015. https://doi.org/10.3390/ma15114015
Li Q, Fan Z, Liu Q, Ma W, Li J, Li N, Ma P, Zhang H. Synthesis of a Superhydrophobic Fluorinated Nano-Emulsion and Its Modification on the Wettability of Tight Sandstone. Materials. 2022; 15(11):4015. https://doi.org/10.3390/ma15114015
Chicago/Turabian StyleLi, Qiang, Zhenzhong Fan, Qingwang Liu, Wenhai Ma, Junliang Li, Nan Li, Pingang Ma, and Hongtao Zhang. 2022. "Synthesis of a Superhydrophobic Fluorinated Nano-Emulsion and Its Modification on the Wettability of Tight Sandstone" Materials 15, no. 11: 4015. https://doi.org/10.3390/ma15114015




