Co-Utilization of Sewage Sludge and Rice Husk in Ceramsite Preparation with Selective Adsorption Capacity to Pb
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
- (SiO2 + Al2O3)/(Fe2O3 + RO + R2O) = 3.5–10;
- (2)
- SiO2: 48–70%; Al2O3: 8%–25%; and (Fe2O3 + CaO + MgO + K2O + Na2O): 4.5–31%.
2.2. Sample Preparation
- (1)
- Pellet: SS and RH were mixed sufficiently according to their corresponding proportions (0%RH, 35%RH, and 70%RH) using a pelletizer to produce SRC raw pellets with diameters ranging from 5 to 10 mm. SRC raw pellets were obtained after natural air drying at room temperature for 3 days [29].
- (2)
- Sintering: The SRC raw pellets were heated from the base temperature of 25 °C and then with a heating rate of 10 °C/min, the pellets were further heated to the preheating temperature (500 °C), and maintained for 20 min [30]. Finally, the SRC pellets were heated to the final temperatures (1050 °C, 1100 °C, and 1150 °C) at the same rate, and naturally cooled to room temperature after a period of sintering (10, 20, and 30 min) to obtain the final SRC products.
- (3)
- Adsorption: The maximum Pb2+ removal rate was taken as the evaluation index to optimize the preparation process. The adsorption conditions were a 2 g/L dosage of SRC and a 20 mg/L concentration of Pb(NO)3. The adsorption equation was fitted according to the experimental results, and the analysis of variance (ANOVA) was conducted to investigate the suitability of the regression model. The optimum preparation process was selected according to the fitted equation, and the experimental adsorption rate was compared with the predicted value of the equation.
2.3. SRC Characteristics
2.4. Adsorption Experiments
2.5. Adsorption Kinetics
2.6. Adsorption Isothermal
2.7. Adsorption Thermodynamics
2.8. Mineral Composition
2.9. Mineral Composition
3. Results and Discussion
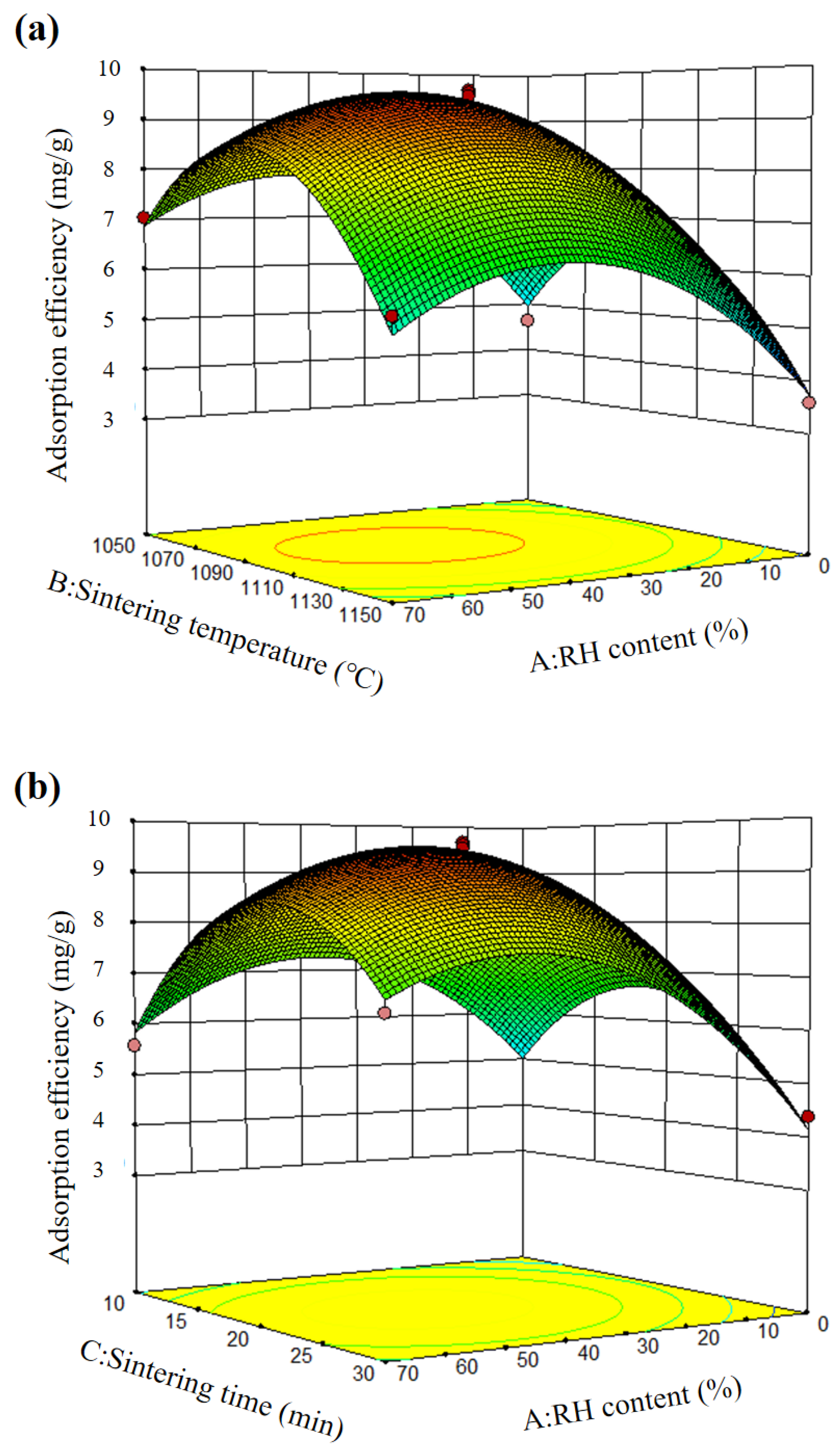
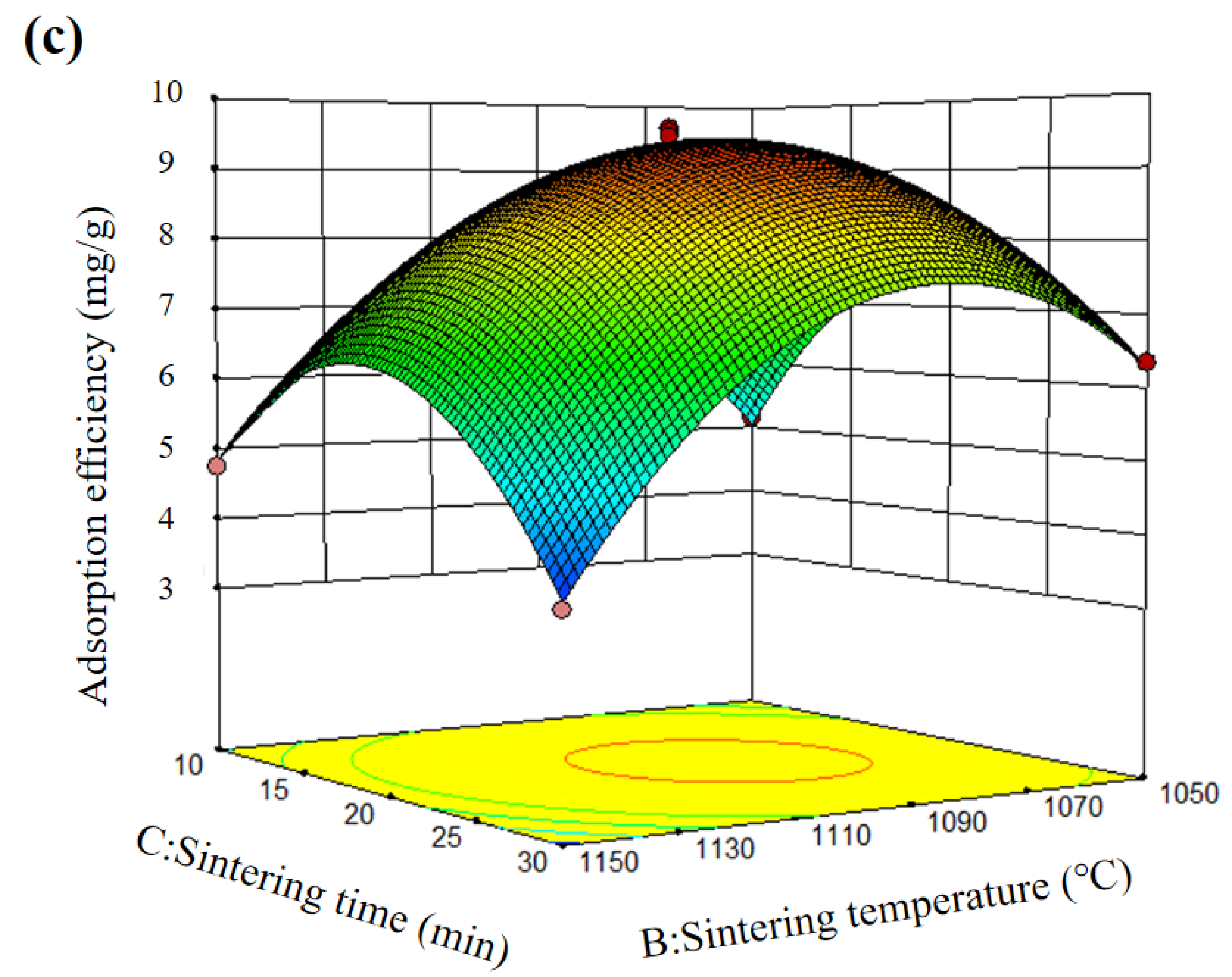
3.1. Response Surface Methodology
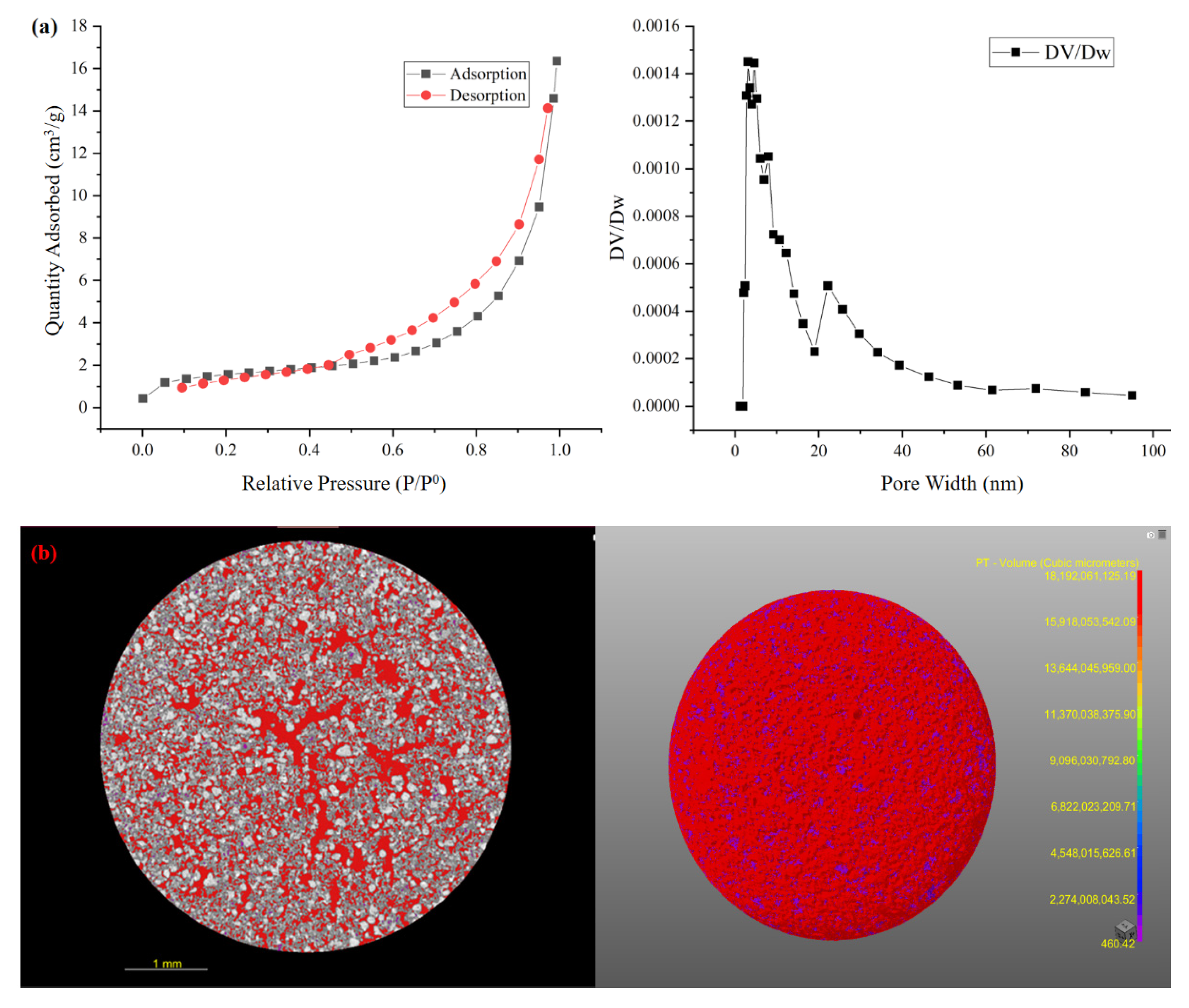
3.2. SRC Characteristics
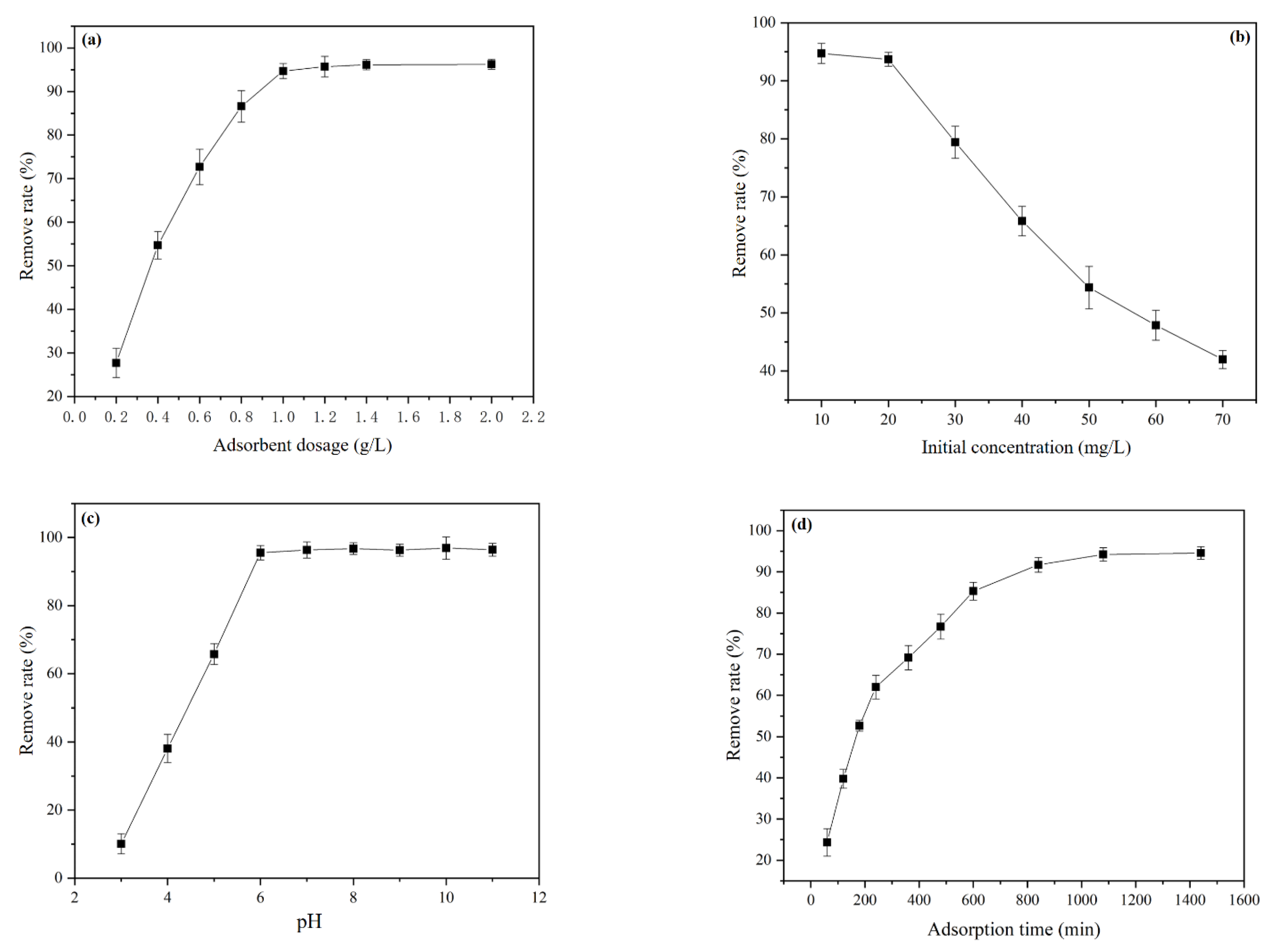
3.3. Static Adsorption of Pb
3.3.1. Adsorption Single-Factor Experiment
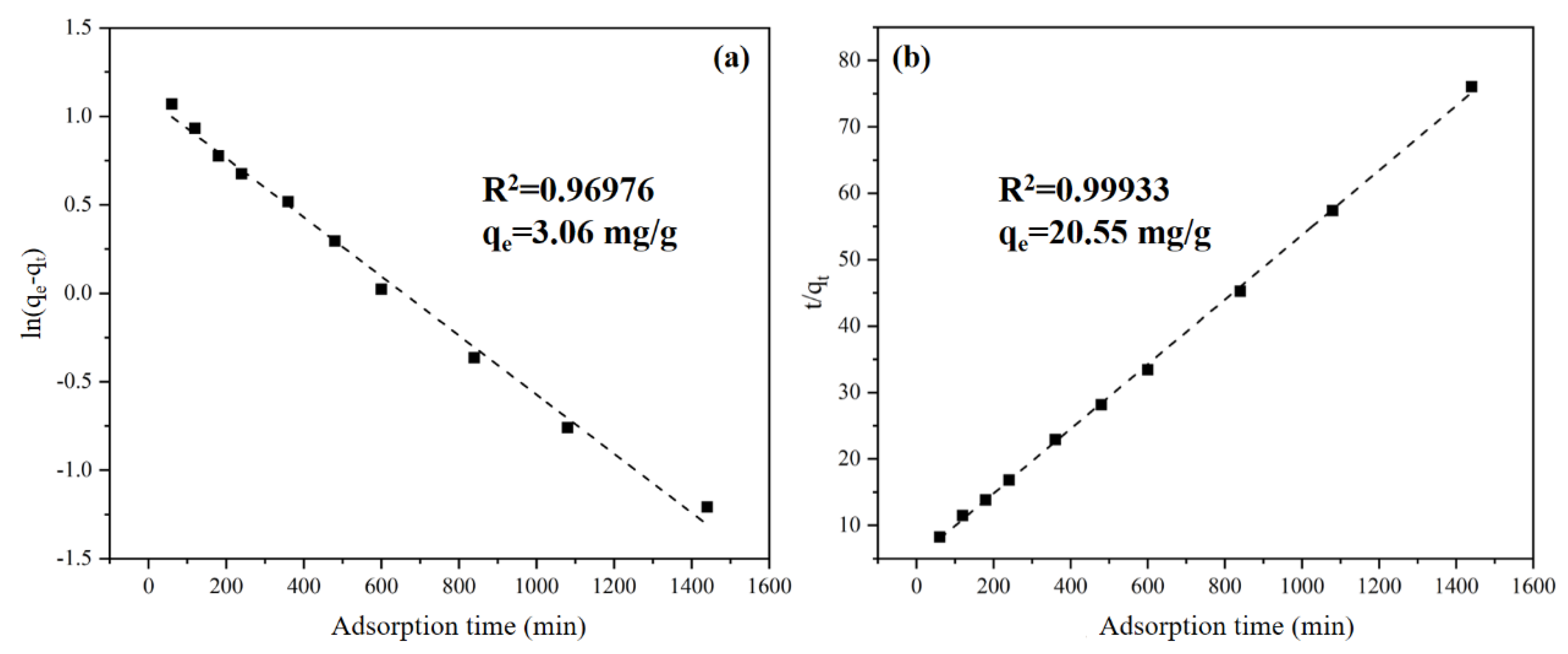
3.3.2. Adsorptive Kinetics
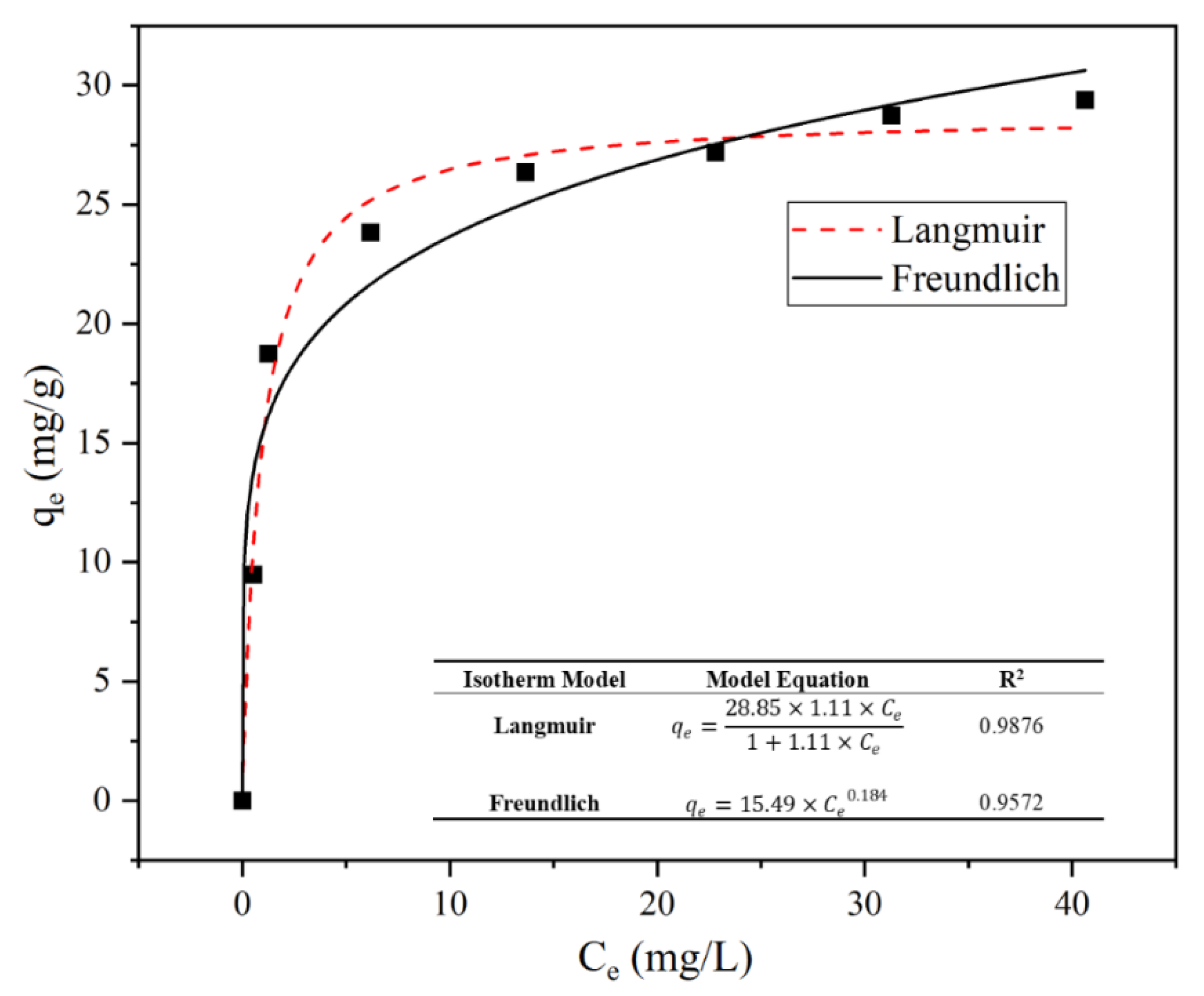
3.3.3. Adsorption Isothermal
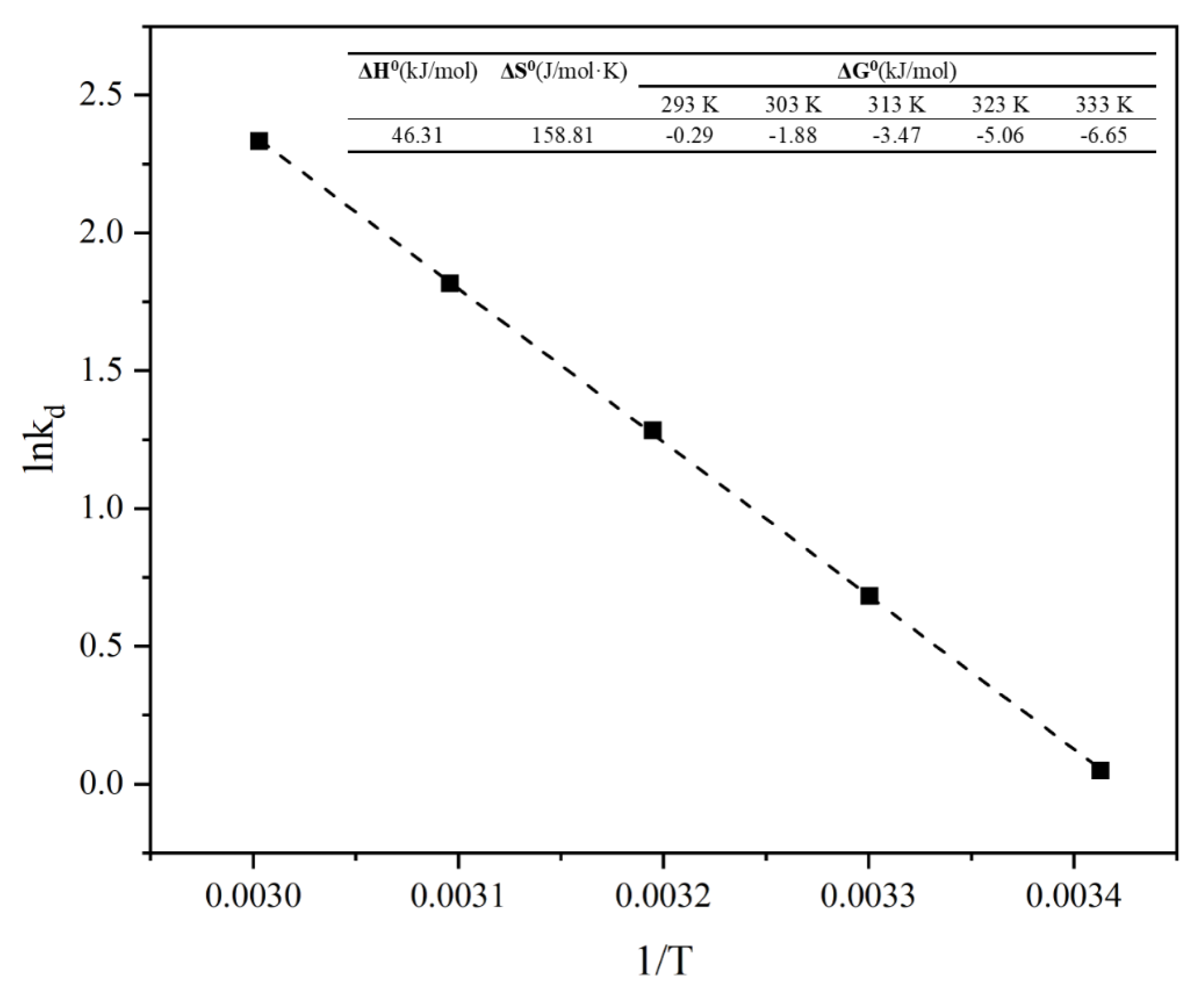
3.3.4. Adsorption Thermodynamics
3.4. Possible Adsorptive Mechanisms
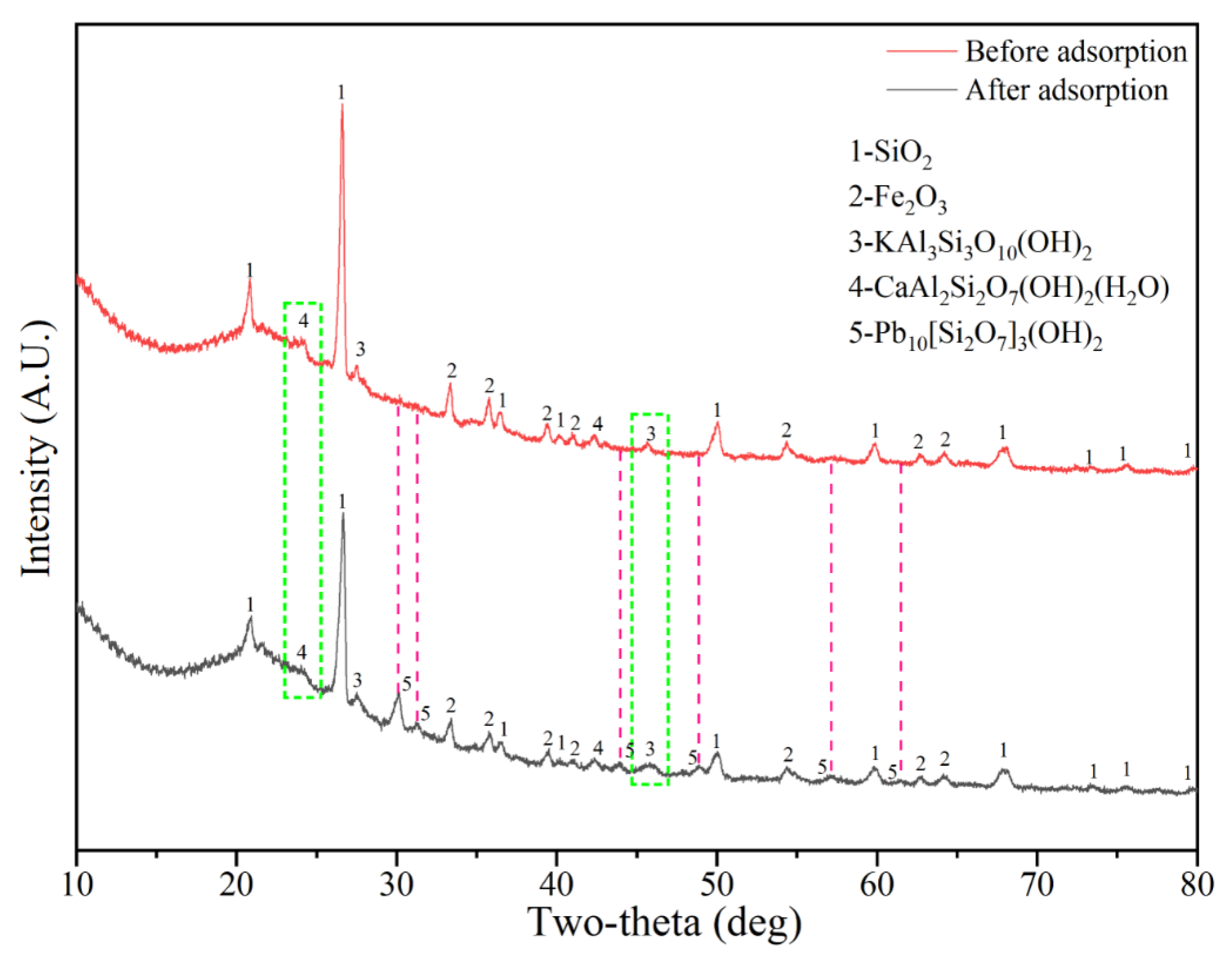
3.4.1. XRD Analysis
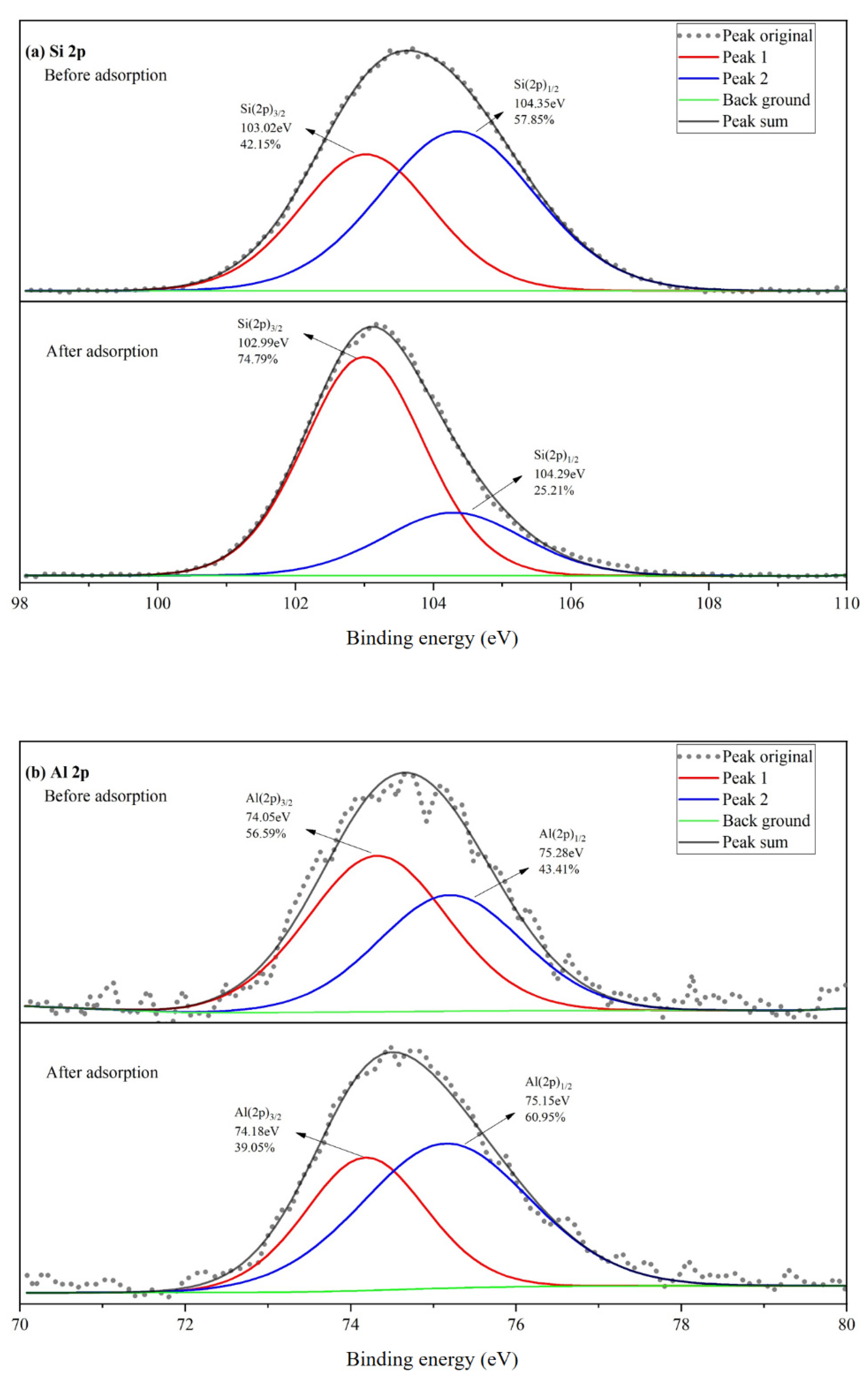
3.4.2. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation proces—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawanta, S.Y.; Pawar, R.R.; Lee, S.M.; Cho, M.H. Binder-free production of 3D N-doped porous carbon cubes for efficient Pb2+ removal through batch and fixed bed adsorption. J. Clean. Prod. 2017, 168, 290–301. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Hosseini-Bandegharaei, A.; Tsafrakidou, P.; Triantafyllidis, K.S.; Kornaros, M.; Anastopoulos, I. Aloe vera waste biomass-based adsorbents for the removal of aquatic pollutants: A review. J. Environ. Manag. 2018, 227, 354–364. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Li, J.; Xue, Y.; Liu, J.; Mei, M.; Hou, H.; Chen, S. Co-pyrolysis behavior of sewage sludge and rice husk by TG-MS and residue analysis. J. Clean. Prod. 2020, 250, 119557. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijalkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almas, A.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Envrion. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- He, B.; Wang, G. Is ceramsite the last straw for sewage sludge disposal: A review of sewage sludge disposal by producing ceramsite in China. Water Sci. Technol. 2019, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Shao, Y.; Zhang, W.; Zhu, Y.; Dou, T.; Chu, L.; Liu, Z. Preparation of municipal solid waste incineration fly ash-based ceramsite and its mechanisms of heavy metal immobilization. Waste Manag. 2022, 143, 54–60. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Zhou, M.; Hou, H.; Xue, Y.; Wang, H. Rice husk and sewage sludge co-combustion ash: Leaching behavior analysis and cementitious property. Constr. Build. Mater. 2018, 163, 63–72. [Google Scholar] [CrossRef]
- Cusido, J.A.; Soriano, C. Valorization of pellets from municipal WWTP sludge in lightweight clay ceramics. Waste Manag. 2011, 31, 1372–1380. [Google Scholar] [CrossRef]
- Czarnota, J.; Tomaszek, J.A.; Maslon, A.; Piech, A.; Lagod, G. Powdered Ceramsite and Powdered Limestone Use in Aerobic Granular Sludge Technology. Materials 2020, 13, 3894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Xu, H.; Liu, C.; Shen, Z.; Hu, K. Preparation of Ceramsite Based on Waterworks Sludge and Its Application as Matrix in Constructed Wetlands. Int. J. Environ. Res. Public Health 2019, 16, 2637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Sun, T.; Li, D. Preparation, sintering behavior, and expansion performance of ceramsite filter media from dewatered sewage sludge, coal fly ash, and river sediment. J. Mater. Cycles Waste Manag. 2016, 20, 71–79. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, Y.; Ji, B.; Wei, T.; Shen, C. Granulation of Drinking Water Treatment Residues: Recent Advances and Prospects. Water 2020, 12, 1400. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Q.; Su, Z.; Sheng, S.; Fu, J. Preparation, optimization, and application of sustainable ceramsite substrate from coal fly ash/waterworks sludge/oyster shell for phosphorus immobilization in constructed wetlands. J. Clean. Prod. 2018, 175, 572–581. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Zhang, P.; Yang, L.; Xu, H.A.; Xi, G. Adsorption characteristics of a novel ceramsite for heavy metal removal from stormwater runoff. Chin. J. Chem. Eng. 2018, 26, 96–103. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Dong, X.; Zhang, S.; Zhao, Y.; Cen, Q.; Zhang, K. The effect and mechanism of Si/Al ratio on microstructure of zeolite modified ceramsite derived from industrial wastes. Microporous Mesoporous Mater. 2021, 311, 110667. [Google Scholar] [CrossRef]
- June Choi, H.; Jo, D.; Bong Hong, S. Effect of framework Si/Al ratio on the adsorption mechanism of CO2 on small-pore zeolites: II. Merlinoite. Chem. Eng. J. 2022, 446, 137100. [Google Scholar] [CrossRef]
- Alvarado-Perea, L.; Colín-Luna, J.A.; López-Gaona, A.; Wolff, T.; Pacheco-Sosa, J.G.; García-Martínez, J.C. Simultaneous adsorption of quinoline and dibenzothiophene over Ni-based mesoporous materials at different Si/Al ratio. Catal. Today 2020, 353, 26–38. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Q.; Gao, S.; Poon, C.S.; Zhou, Y.; Li, J.S. Novel recycling of incinerated sewage sludge ash (ISSA) and waste bentonite as ceramsite for Pb-containing wastewater treatment: Performance and mechanism. J. Environ. Manag. 2021, 288, 112382. [Google Scholar] [CrossRef]
- Wang, C.; Huang, C.; Xu, H.; Yuan, N.; Liu, X.; Bai, L.; He, X.; Liu, R. Ceramsite production using water treatment residue as main ingredient: The key affecting factors identification. J. Environ. Manag. 2022, 308, 114611. [Google Scholar] [CrossRef] [PubMed]
- Aprianti, E.; Shafigh, P.; Bahri, S.; Farahani, J.N. Supplementary cementitious materials origin from agricultural wastes—A review. Constr. Build. Mater. 2015, 74, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Geraldo, R.H.; Fernandes, L.F.R.; Camarini, G. Water treatment sludge and rice husk ash to sustainable geopolymer production. J. Clean. Prod. 2017, 149, 146–155. [Google Scholar] [CrossRef]
- Xu, G.R.; Zou, J.L.; Li, G.B. Ceramsite obtained from water and wastewater sludge and its characteristics affected by (Fe(2)O(3)+CaO+MgO)/(SiO(2)+Al(2)O(3)). Water Res. 2009, 43, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Qin, J.; Liu, Y.; Qu, Y.; Liu, J.; Cao, R.; Zhang, Y. Mechanical properties of sintered ceramsite from iron ore tailings affected by two-region structure. Constr. Build. Mater. 2020, 240, 117919. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, Y.; Sheng, L. Preparation of ceramsite from municipal sludge and its application in water treatment: A review. J. Environ. Manag. 2021, 287, 112374. [Google Scholar] [CrossRef]
- Peyne, J.; Gautron, J.; Doudeau, J.; Rossignol, S. Development of low temperature lightweight geopolymer aggregate, from industrial Waste, in comparison with high temperature processed aggregates. J. Clean. Prod. 2018, 189, 47–58. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, S.; Wang, Q.; Gao, M.; Ma, H. Ceramsite production from sediment in Beian River: Characterization and parameter optimization. R. Soc. Open Sci. 2019, 6, 190197. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Liu, X.; Wang, W.; Guo, J.; Zhang, L.; Zhang, H. Effect of SiO2 and Al2O3 on characteristics of lightweight aggregate made from sewage sludge and river sediment. Ceram. Int. 2018, 44, 4313–4319. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Xie, S.; Pan, L.; Li, C.; You, F.; Wang, Y. Immobilization of heavy metals in ceramsite produced from sewage sludge biochar. Sci. Total Environ. 2018, 628–629, 131–140. [Google Scholar] [CrossRef]
- Ghahremani, A.; Manteghian, M.; Kazemzadeh, H. Removing lead from aqueous solution by activated carbon nanoparticle impregnated on lightweight expanded clay aggregate. J. Environ. Chem. Eng. 2020, 9, 104478. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Z.; Shan, W.; Mei, Z.; Wang, W. Adsorption behavior of phosphate on lanthanum(III)-coordinated diamino-functionalized 3D hybrid mesoporous silicates material. J. Hazard. Mater. 2011, 186, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kousha, M.; Daneshvar, E.; Sohrabi, M.S.; Jokar, M.; Bhatnagar, A. Adsorption of acid orange II dye by raw and chemically modified brown macroalga Stoechospermum marginatum. Chem. Eng. J. 2012, 192, 67–76. [Google Scholar] [CrossRef]
- Isah, A.U.; Abdulraheem, G.; Bala, S.; Muhammad, S.; Abdullahi, M. Kinetics, equilibrium and thermodynamics studies of C.I. Reactive Blue 19 dye adsorption on coconut shell based activated carbon. Int. Biodeterior. Biodegrad. 2015, 102, 265–273. [Google Scholar] [CrossRef]
- Wang, T.; Xue, Y.; Hao, R.; Hou, H.; Liu, J.; Li, J. Mechanism investigations into the effect of rice husk and wood sawdust conditioning on sewage sludge thermal drying. J. Environ. Manag. 2019, 239, 316–323. [Google Scholar] [CrossRef]
- Kumar, S.J.; Kumar, V. Effect of Immobilized Enzymes Naringinase and Tannase Produced from Aspergillus Sp. Isolate Mk156394 Isolated from Rotten Pomelo on Quality Characteristics of Citrus Limetta Juice and Process Optimization by Using Response Surface Methodology. Biointerface Res. Appl. Chem. 2020, 11, 9646–9657. [Google Scholar]
- Ghobadi, N.; Mohammadi, T.; Kasiri, N.; Kazemimoghadam, M. Modified poly(vinyl alcohol)/chitosan blended membranes for isopropanol dehydration via pervaporation: Synthesis optimization and modeling by response surface methodology. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Bao, T.; Chen, T.; Tan, J.; Wille, M.-L.; Zhu, D.; Chen, D.; Xi, Y. Synthesis and performance of iron oxide-based porous ceramsite in a biological aerated filter for the simultaneous removal of nitrogen and phosphorus from domestic wastewater. Sep. Purif. Technol. 2016, 167, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Cai, C.; Xue, Y.; Xiao, Y.; Chen, S.; Liu, J.; Mei, M.; Li, J. Regulation of ash slagging behavior for sewage sludge by rice husk addition: Focusing on control mechanisms. J. Clean. Prod. 2021, 284, 124677. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Jelle, B.P.; Wang, Y.; Gustavsen, A. Hygrothermal properties of compressed earthen bricks. Constr. Build. Mater. 2018, 162, 576–583. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Rong, H.; Zhou, X.; Chen, F.; Li, X.; Wang, T.; Hou, H. Adsorption mechanism of lead ions on porous ceramsite prepared by co-combustion ash of sewage sludge and biomass. Sci. Total Environ. 2020, 702, 135017. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Ding, P.; Jia, X.; Hu, G.; Li, Z. Study of the properties and mechanism of deep reduction and efficient adsorption of Cr(VI) by low-cost Fe3O4-modified ceramsite. Sci. Total Environ. 2019, 688, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z.; Bagherpour, A. Synthesis of zeolite NaY and its nanocomposites with chitosan as adsorbents for lead(II) removal from aqueous solution. Powder Technol. 2018, 338, 744–763. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.-M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Sun, S.; Huang, X.; Lin, J.; Ma, R.; Fang, L.; Zhang, P.; Qu, J.; Zhang, X.; Liu, Y. Study on the effects of catalysts on the immobilization efficiency and mechanism of heavy metals during the microwave pyrolysis of sludge. Waste Manag. 2018, 77, 131–139. [Google Scholar] [CrossRef]
- de Almeida, A.S.V.; Mastelaro, V.R.; da Silva, M.G.C.; Prediger, P.; Vieira, M.G.A. Adsorption of 17α-ethinylestradiol onto a novel nanocomposite based on graphene oxide, magnetic chitosan and organoclay (GO/mCS/OC): Kinetics, equilibrium, thermodynamics and selectivity studies. J. Water Process Eng. 2022, 47, 102729. [Google Scholar] [CrossRef]
- Wu, C.; Lou, X.; Huang, A.; Zhang, M.; Ma, L. Thermodynamics and kinetics of pretilachlor adsorption: Implication to controlled release from organobentonites. Appl. Clay Sci. 2020, 190, 105566. [Google Scholar] [CrossRef]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Jain, M.; Khan, S.A.; Sahoo, A.; Dubey, P.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.T. Statistical evaluation of cow-dung derived activated biochar for phenol adsorption: Adsorption isotherms, kinetics, and thermodynamic studies. Bioresour. Technol. 2022, 352, 127030. [Google Scholar] [CrossRef]
- Fang, Z.; Suhua, H.; Xu, L.; Jian, F.; Qi, L.; Zhiwei, W.; Chuanchang, L.; Yuanlai, X. Adsorption kinetics and thermodynamics of rare earth on Montmorillonite modified by sulfuric acid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127063. [Google Scholar] [CrossRef]
- López, A.; Frost, R.L.; Scholz, R.; Gobac, Ž.Ž.; Xi, Y. Vibrational spectroscopy of the silicate mineral plumbotsumite Pb5(OH)10Si4O8—An assessment of the molecular structure. J. Mol. Struct. 2013, 1054–1055, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Di, Y.; Jiang, A.; Huang, H.; Luo, Q.; Wei, W.; Wang, R.; Chen, S. Molecular dynamics simulations of adsorption behavior of DDAH, NaOL and mixed DDAH/NaOL surfactants on muscovite (001) surface in aqueous solution. J. Mol. Graph. Model 2022, 113, 108161. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, S. Influence of slag as additive on compressive strength of fly ash-based geopolymer. J. Mater. Civ. Eng. 2007, 19, 470–474. [Google Scholar] [CrossRef]
- Zhang, N.; Ejtemaei, M.; Nguyen, A.V.; Zhou, C. XPS analysis of the surface chemistry of sulfuric acid-treated kaolinite and diaspore minerals with flotation reagents. Miner. Eng. 2019, 136, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, F.; Zhou, F.; Lu, M.; Hou, H.; Li, J.; Liu, D.; Wang, T. Early solidification/stabilization mechanism of heavy metals (Pb, Cr and Zn) in Shell coal gasification fly ash based geopolymer. Sci. Total Environ. 2022, 802, 149905. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Ai, Z.; Li, M.; Li, H.; Peng, W.; Zhao, Y.; Song, S. Adsorption toward Pb(II) occurring on three-dimensional reticular-structured montmorillonite hydrogel surface. Appl. Clay Sci. 2021, 210, 106153. [Google Scholar] [CrossRef]
- Guo, B.; Pan, D.A.; Liu, B.; Volinsky, A.A.; Fincan, M.; Du, J.; Zhang, S. Immobilization mechanism of Pb in fly ash-based geopolymer. Constr. Build. Mater. 2017, 134, 123–130. [Google Scholar] [CrossRef]
- Hu, S.; Zhong, L.; Yang, X.; Bai, H.; Ren, B.; Zhao, Y.; Zhang, W.; Ju, X.; Wen, H.; Mao, S.; et al. Synthesis of rare earth tailing-based geopolymer for efficiently immobilizing heavy metals. Constr. Build. Mater. 2020, 254, 119273. [Google Scholar] [CrossRef]


| Component | RH (%) | SS (%) | |
|---|---|---|---|
| Proximate analysis | Ash | 14.17 ± 0.10 | 71.51 ± 0.74 |
| Volatile matter | 71.41 ± 0.29 | 27.61 ± 0.31 | |
| Fixed carbon | 14.42 ± 0.21 | 0.88 ± 0.05 | |
| Ultimate analysis | C | 41.68 ± 0.74 | 12.04 ± 0.11 |
| H | 5.76 ± 0.08 | 1.31 ± 0.01 | |
| O | 37.68 ± 1.89 | 13.38 ± 1.55 | |
| N | 0.71 ± 0.03 | 1.76 ± 0.15 | |
| Ash analysis | Al2O3 | 0.64 ± 0.03 | 16.49 ± 0.43 |
| SiO2 | 83.81 ± 1.79 | 50.55 ± 0.89 | |
| Fe2O3 | 2.63 ± 0.06 | 7.78 ± 0.17 | |
| Na2O | 0.18 ± 0.01 | 0.53 ± 0.04 | |
| K2O | 5.62 ± 0.14 | 3.00 ± 0.01 | |
| CaO | 1.63 ± 0.12 | 4.60 ± 0.17 | |
| P2O5 | 0.48 ± 0.02 | 11.78 ± 0.91 | |
| MgO | 0.71 ± 0.04 | 2.65 ± 0.05 |
| Source | Sum of Squares | Df | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 73.90 | 9 | 8.21 | 84.62 | <0.0001 |
| A | 5.92 | 1 | 5.92 | 61.03 | 0.0001 |
| B | 4.08 | 1 | 4.08 | 42.03 | 0.0003 |
| C | 0.0014 | 1 | 0.0014 | 0.014 | 0.9085 |
| AB | 0.042 | 1 | 0.042 | 0.44 | 0.5296 |
| AC | 0.85 | 1 | 0.85 | 8.73 | 0.0213 |
| BC | 1.43 | 1 | 1.43 | 14.47 | 0.0064 |
| A2 | 14.13 | 1 | 14.13 | 145.62 | <0.0001 |
| B2 | 22.69 | 1 | 22.69 | 233.79 | <0.0001 |
| C2 | 18.36 | 1 | 18.36 | 189.16 | <0.0001 |
| Lack of Fit | 0.52 | 3 | 0.17 | 4.27 | 0.0972 |
| Std. Dev. | 0.31 | R2 | 0.9909 | Adj R2 | 0.9792 |
| Mean | 6.42 | Adeq Precision | 23.892 |
| Parameters | Ceramsite | Criterion |
|---|---|---|
| Breaking and wear rate (%) | 3.9 ± 0.5 | ≤6 |
| Silt carrying capacity (%) | N.d | ≤1 |
| Solubility in hydrochloric acid (%) | 0.31 ± 0.06 | ≤2 |
| Void fraction (%) | 56.4 ± 1.4 | ≥40 |
| Water absorption (%) | 61 ± 4 | \ |
| Specific surface area (×104, cm2/g) | 4.68 ± 0.18 | ≥0.5 |
| Total pore volume (cm3/g) | 0.024 ± 0.004 | \ |
| Average pore diameter (nm) | 20.7 ± 1.6 | \ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Lu, M.; Wang, J. Co-Utilization of Sewage Sludge and Rice Husk in Ceramsite Preparation with Selective Adsorption Capacity to Pb. Materials 2022, 15, 4310. https://doi.org/10.3390/ma15124310
Wang R, Lu M, Wang J. Co-Utilization of Sewage Sludge and Rice Husk in Ceramsite Preparation with Selective Adsorption Capacity to Pb. Materials. 2022; 15(12):4310. https://doi.org/10.3390/ma15124310
Chicago/Turabian StyleWang, Rui, Meng Lu, and Junxing Wang. 2022. "Co-Utilization of Sewage Sludge and Rice Husk in Ceramsite Preparation with Selective Adsorption Capacity to Pb" Materials 15, no. 12: 4310. https://doi.org/10.3390/ma15124310





