Internal Sulphate Attack in Masonry Mortars with Thaumasite Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Experimental
- The in situ visual inspection of the property allowed the most affected areas to be identified. As has been pointed out, these correspond to the mortar of the roof tiles and paving, from which samples were taken for subsequent analysis in the laboratories and scientific–technical services of the University of Alicante. The analyses conducted were:
- Firstly, the samples have been visualised under an optical microscope to select the areas to be analysed later by Scanning Electron Microscopy (SEM-EDX) and X-Ray Diffraction (XRD), both in the Research Support Services of the University of Alicante (Spain).
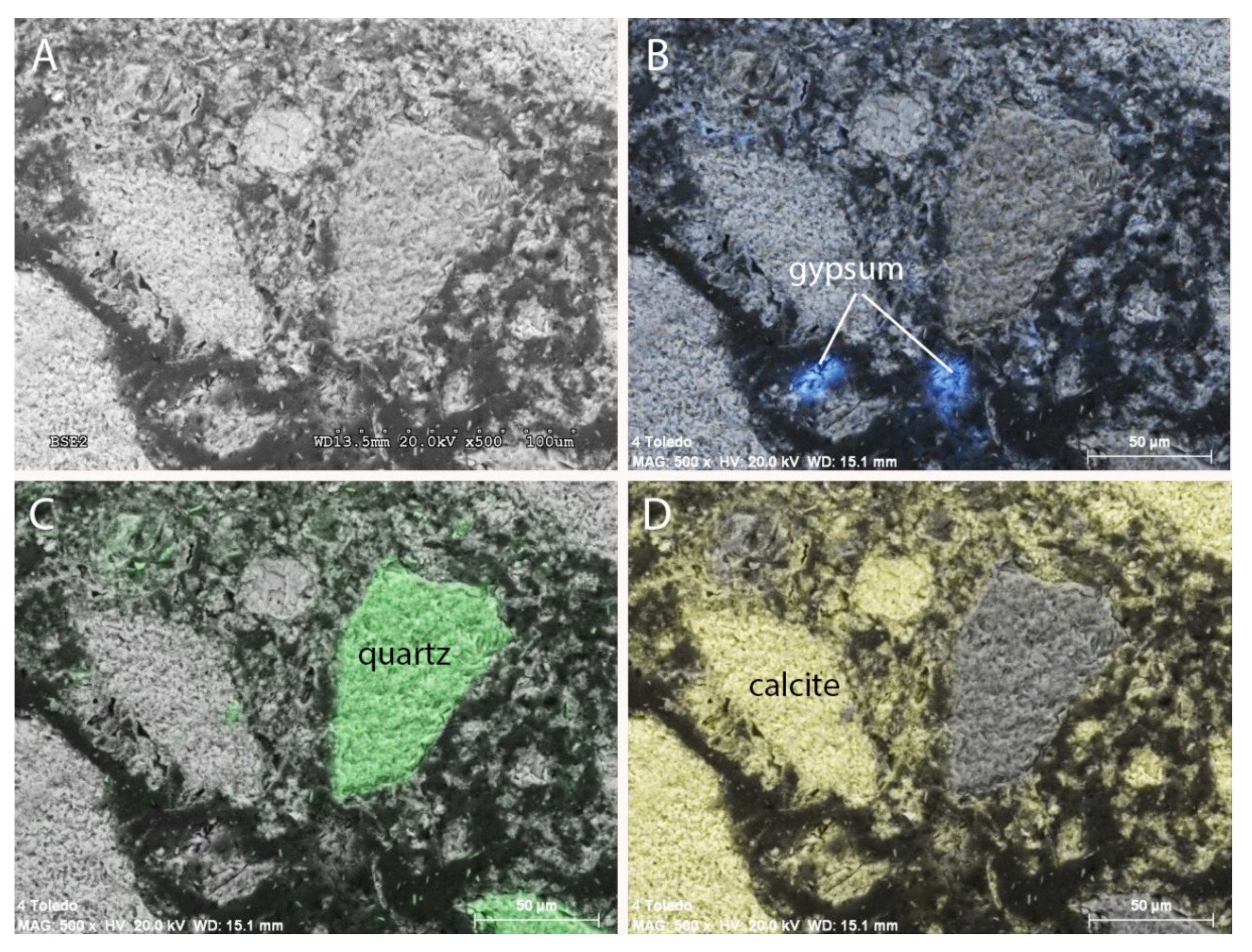
- SEM-EDX analyses were carried out with a scanning electron microscope Hitachi S3000N model. This microscope has an X-ray detector Bruker brand XFlash 3001 model for microanalysis and mapping. The use of energy-dispersive X-rays (EDX) allowed us to obtain the images on which mappings of elements were subsequently superimposed. Both secondary electron analysis (SEM) and backscattered electron analysis (BSE) have been used.
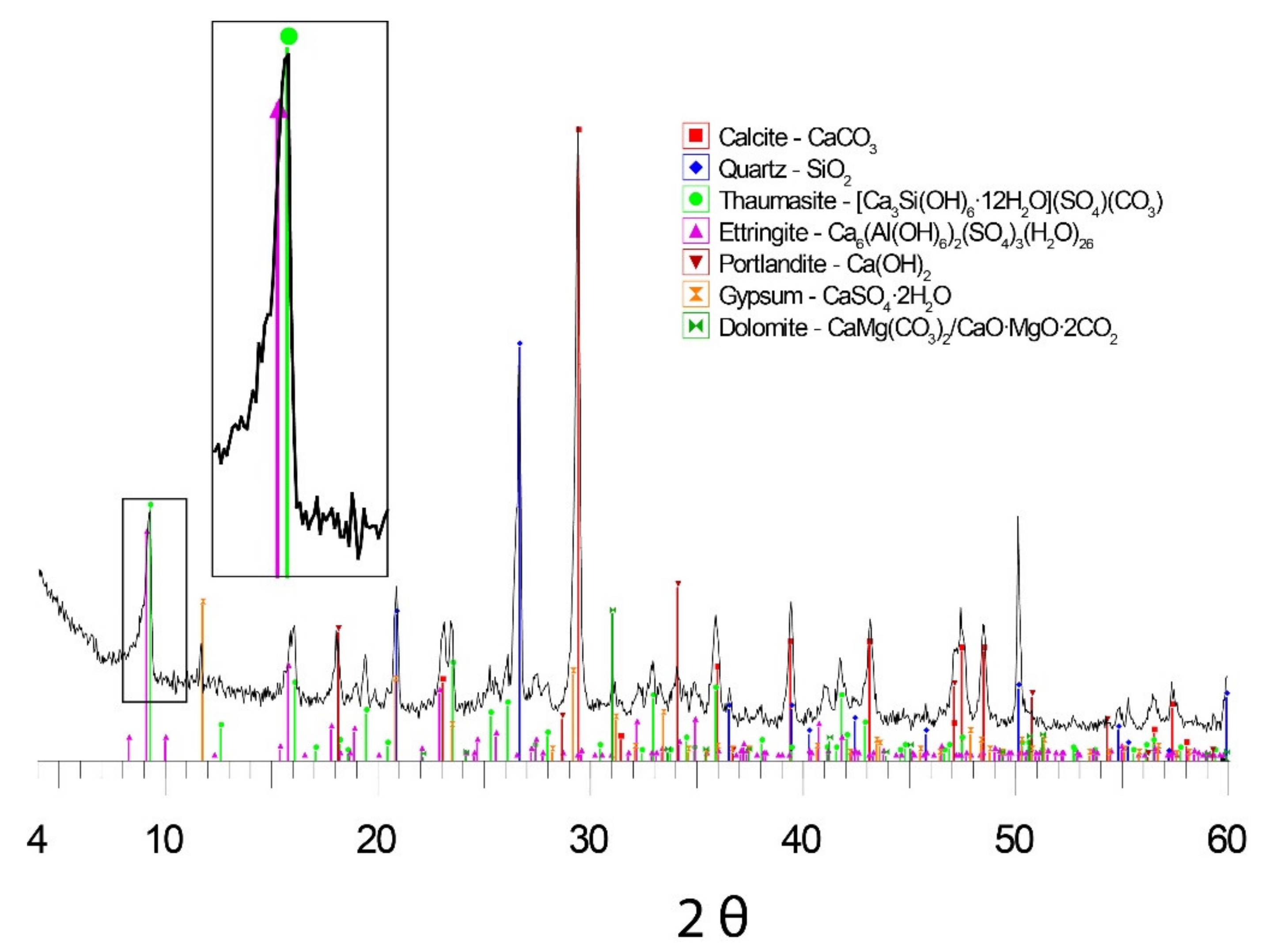
- XRD analyses were performed on a Bruker D8-Advance with an X-ray generator KRISTALLOFLEX K 760-80F and a Cu Kα tube. The samples were crushed in an agate mortar until they reached a size of ca. 40 μm, after which a sweep was performed from 4 to 60 degrees of 2θ at a rate of 1 °/min.
3. Results
3.1. Optical Microscopy (Stereomicroscope)
3.2. Scanning Electron Microscopy (SEM and BSE)
3.3. X-ray Diffraction (XRD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- The UK Government Thaumasite Expert Group. The Thaumasite Form of Sulfate Attack: Risks, Diagnosis, Remedial Works and Guidance on New Construction; Department of the Environment, Transport and the Regions: Rotherham, UK, 1999. [Google Scholar]
- Aguilera, J.; Varela, M.T.B.; Vazquez, T. Procedure of synthesis of thaumasite. Cem. Concr. Res. 2001, 31, 1163–1168. [Google Scholar] [CrossRef]
- Masárová, A.; Fridrichová, M.; Dvořák, K. Synthetic Preparation of Thaumasite—Several Possible Routes for Thaumasite Formation. Procedia Eng. 2016, 151, 313–320. [Google Scholar] [CrossRef]
- Chinchón-Payá, S.; Aguado, A.; Chinchón, S. Ettringite dependace in thaumasite formation. IOP Conf. Ser. Mater. Sci. Eng. 2020, 897, 12002. [Google Scholar] [CrossRef]
- Crammond, N. The occurrence of thaumasite in modern construction—A review. Cem. Concr. Compos. 2002, 24, 393–402. [Google Scholar] [CrossRef]
- Crammond, N. The thaumasite form of sulfate attack in the UK. Cem. Concr. Compos. 2003, 25, 809–818. [Google Scholar] [CrossRef]
- Freyburg, E.; Berninger, A.M. Field experiences in concrete deterioration by thaumasite formation: Possibilities and problems in thaumasite analysis. Cem. Concr. Compos. 2003, 25, 1105–1110. [Google Scholar] [CrossRef]
- Hagelia, P. Thaumasite and secondary calcite in some Norwegian concretes. Cem. Concr. Compos. 2003, 25, 1131–1140. [Google Scholar] [CrossRef]
- Hobbs, D.W.; Taylor, M.G. Nature of the thaumasite sulfate attack mechanism in field concrete. Cem. Concr. Res. 2000, 30, 529–533. [Google Scholar] [CrossRef]
- Wimpenny, D.; Slater, D. Evidence from the highways agency thaumasite investigation in Gloucestershire to support or contradict postulated mechanisms of thaumasite formation (TF) and thaumasite sulfate attack (TSA). Cem. Concr. Compos. 2003, 25, 879–888. [Google Scholar] [CrossRef]
- Van Hees, R.P.J.; Wijffels, T.J.; van der Klugt, L.J.A.R. Thaumasite swelling in historic mortars: Field observations and laboratory research. Cem. Concr. Compos. 2003, 25, 1165–1171. [Google Scholar] [CrossRef]
- Collepardi, M. Thaumasite formation and deterioration in historic buildings. Cem. Concr. Compos. 1999, 21, 147–154. [Google Scholar] [CrossRef]
- Corinaldesi, V.; Moriconi, G.; Tittarelli, F. Thaumasite: Evidence for incorrect intervention in masonry restoration. Cem. Concr. Compos. 2003, 25, 1157–1160. [Google Scholar] [CrossRef]
- Crammond, N.J.; Halliwell, M.A. The thaumasite form of sulfate attack in concretes containing a source of carbonate ions—A microstructural overview. Adv. Concr. Technol. 1995, 154, 357–380. [Google Scholar]
- Irassar, E.F. Sulfate attack on cementitious materials containing limestone filler—A review. Cem. Concr. Res. 2009, 39, 241–254. [Google Scholar] [CrossRef]
- Collett, G.; Crammond, N.J.; Swamy, R.N.; Sharp, J.H. The role of carbon dioxide in the formation of thaumasite. Cem. Concr. Res. 2004, 34, 1599–1612. [Google Scholar] [CrossRef]
- Sahu, S.; Exline, D.L.; Nelson, M.P. Identification of thaumasite in concrete by Raman chemical imaging. Cem. Concr. Compos. 2002, 24, 347–350. [Google Scholar] [CrossRef]
- Chinchón-Payá, S.; Aguado, A.; Nugteren, H.W.; Chinchón, S. External sulfate attack in dam concretes with thaumasite formation. Mater. Constr. 2015, 65, e042. [Google Scholar] [CrossRef]
- Ramezanianpour, A.M.; Hooton, R.D. Thaumasite sulfate attack in Portland and Portland-limestone cement mortars exposed to sulfate solution. Constr. Build. Mater. 2013, 40, 162–173. [Google Scholar] [CrossRef]
- Gaze, M.E.; Crammond, N.J. The formation of thaumasite in a cement : Lime : Sand mortar exposed to cold magnesium and potassium sulfate solutions. Cem. Concr. Compos. 2000, 22, 209–222. [Google Scholar] [CrossRef]
- Colman, C.; Bulteel, D.; Thiery, V.; Rémond, S.; Michel, F.; Courard, L. Internal sulfate attack in mortars containing contaminated fine recycled concrete aggregates. Constr. Build. Mater. 2021, 272, 121851. [Google Scholar] [CrossRef]
- Pipilikaki, P.; Papageorgiou, D.; Teas, C.; Chaniotakis, E.; Katsioti, M. The effect of temperature on thaumasite formation. Cem. Concr. Compos. 2008, 30, 964–969. [Google Scholar] [CrossRef]
- Sahu, S.; Badger, S.; Thaulow, N. Evidence of thaumasite formation in Southern California concrete. Cem. Concr. Compos. 2002, 24, 379–384. [Google Scholar] [CrossRef]
- Skaropoulou, A.; Kakali, G.; Tsivilis, S. Thaumasite form of sulfate attack in limestone cement concrete: The effect of cement composition, sand type and exposure temperature. Constr. Build. Mater. 2012, 36, 527–533. [Google Scholar] [CrossRef]
- Martinez-Ramirez, S.; Blanco-Varela, M.T.; Rapazote, J. Thaumasite formation in sugary solutions: Effect of temperature and sucrose concentration. Constr. Build. Mater. 2011, 25, 21–29. [Google Scholar] [CrossRef]
- Diamond, S. Thaumasite in Orange County, Southern California: An inquiry into the effect of low temperature. Cem. Concr. Compos. 2003, 25, 1161–1164. [Google Scholar] [CrossRef]
- Araújo, G.S.; Chinchón, S.; Aguado, A. Evaluation of the behavior of concrete gravity dams suffering from internal sulfate attack. IBRACON Struct. Mater. J. 2008, 1, 84–112. [Google Scholar]
- Espinós, J.; Aguado, A.; López, C.; Campos, A.; Chinchón-Payá, S. Expansion studies for the Paso Nuevo dam. In Proceedings of the 2nd International Congress on Dam Maintenance and Rehabilitation, Zaragoza, Spain, 23–25 November 2010; pp. 143–151. [Google Scholar]
- Chinchón-Payá, S.; Oliveira, I.; Aguado, A.; Chinchón, S. The sulfate attack in concrete by degradation of iron sulfides and the effect of the host rock. In Proceedings of the XII DBMC International Conference on Durability of Building Materials and Components, Porto, Portugal, 12–15 April 2011. [Google Scholar]
- Chinchón, J.S.; Ayora, C.; Aguado, A.; Guirado, F. Influence of weathering of iron sulfides contained in aggregates on concrete durability. Cem. Concr. Res. 1995, 25, 1264–1272. [Google Scholar] [CrossRef]
- Chinchón-Payá, S.; Aguado, A.; Chinchón, S. A comparative investigation of the degradation of pyrite and pyrrhotite under simulated laboratory conditions. Eng. Geol. 2012, 127, 75–80. [Google Scholar] [CrossRef]
- Ayora, C.; Chinchón, S.; Aguado, A.; Guirado, F. Weathering of iron sulfides and concrete alteration: Thermodynamic model and observation in dams from Central Pyrenees, Spain. Cem. Concr. Res. 1998, 28, 1223–1235. [Google Scholar] [CrossRef]
- Chinchón Payá, S.; Chinchón Payá, E.; Chinchón Yepes, J.; Andrade_Perdrix, M. Indicador del Frente de Avance de la Carbonatación del Hormigón Sustituto de la Fenolftaleína. Patent Spain P201431556, 2014. [Google Scholar]
- Chinchón-Payá, S.; Aguado, A.; Coloma, F.; Chinchón, S. Study of aggregate samples with iron sulfides through micro X-ray fluorescence (μXRF) and X-ray photoelectron spectroscopy (XPS). Mater. Struct. 2013, 48, 1285–1290. [Google Scholar] [CrossRef]
- Chinchón, J.S.; López-Soler, A.; Travería, A.; Vaquer, R. XRF analysis of sulphur in aggregates used in concrete by the addition of Li2SO4. Mater. Struct. 1991, 24, 13–14. [Google Scholar] [CrossRef]
- Martin, J.D. XPowder 12; Qualitative, Quantitative and Microtextural Powder X-ray Diffaction Analysis; 2012. Available online: https://www.xpowder.com/ (accessed on 12 July 2022).
- Martucci, A.; Cruciani, G. In Situ time resolved synchrotron powder diffraction study of thaumasite. Phys. Chem. Miner. 2006, 33, 723–731. [Google Scholar] [CrossRef]
- Barnett, S.J.; Adam, C.D.; Jackson, A.R.W. Solid solutions between ettringite, Ca6Al2(SO4)3(OH)12·26H2O, and thaumasite, Ca3SiSO4CO3(OH)6·12H2O. J. Mater. Sci. 2000, 35, 4109–4114. [Google Scholar] [CrossRef]
- Barnett, S.J.; Macphee, D.E.; Lachowski, E.E.; Crammond, N.J. XRD, EDX and IR analysis of solid solutions between thaumasite and ettringite. Cem. Concr. Res. 2002, 32, 719–730. [Google Scholar] [CrossRef]





| Thaumasite | a (nm) | b (nm) | c (nm) | α | β | γ | Vol (nm)3 |
|---|---|---|---|---|---|---|---|
| Table Data | 11.0575 | 11.0575 | 10.4163 | 90 | 90 | 120 | 1.10296 |
| PDF 240038 | |||||||
| refinement | 1.10430 ± 0.00210 | 1.10430 ± 0.00210 | 1.04138 ± 0.00204 | 90 | 90 | 120 | 1.09980 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinchón-Payá, S.; Aguado de Cea, A.; Saval Pérez, J.M.; Chinchón Yepes, J.S. Internal Sulphate Attack in Masonry Mortars with Thaumasite Formation. Materials 2022, 15, 5708. https://doi.org/10.3390/ma15165708
Chinchón-Payá S, Aguado de Cea A, Saval Pérez JM, Chinchón Yepes JS. Internal Sulphate Attack in Masonry Mortars with Thaumasite Formation. Materials. 2022; 15(16):5708. https://doi.org/10.3390/ma15165708
Chicago/Turabian StyleChinchón-Payá, Servando, Antonio Aguado de Cea, José Miguel Saval Pérez, and José Servando Chinchón Yepes. 2022. "Internal Sulphate Attack in Masonry Mortars with Thaumasite Formation" Materials 15, no. 16: 5708. https://doi.org/10.3390/ma15165708






