Adsorption Effect and Adsorption Mechanism of High Content Zeolite Ceramsite on Asphalt VOCs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. 13X Zeolite and Attapulgite Clay
2.1.2. Asphalt Binder
2.2. Methods
2.2.1. Preparation of Zeolite Ceramsite
2.2.2. Preparation of Asphalt Mortar
2.2.3. Characterization of Zeolite Ceramsite
2.2.4. Adsorption Experiment of Asphalt VOCs
3. Results and Discussion
3.1. Basic Characterization of Adsorbent
3.1.1. Chemical Composition Analysis
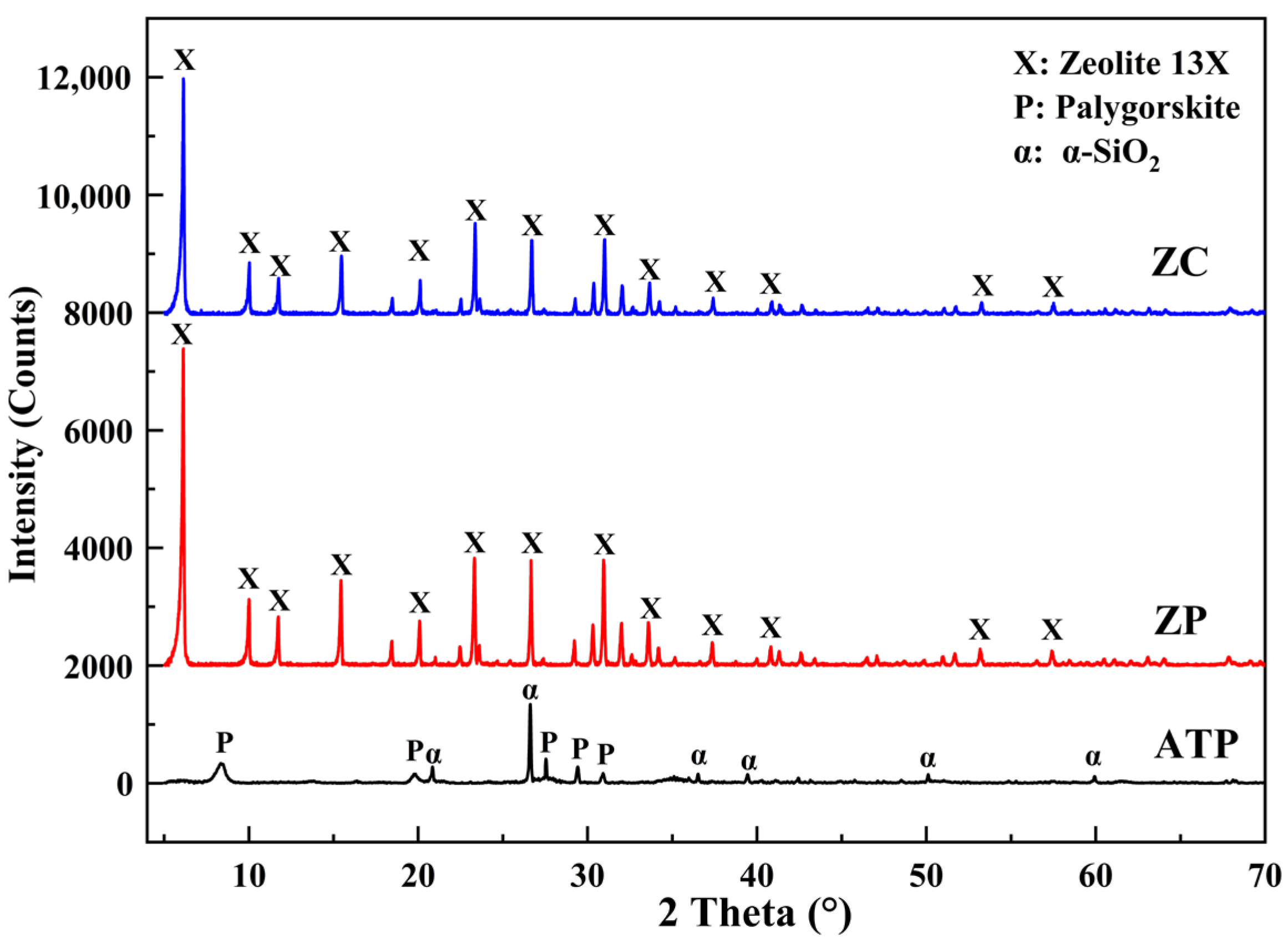
3.1.2. XRD Results
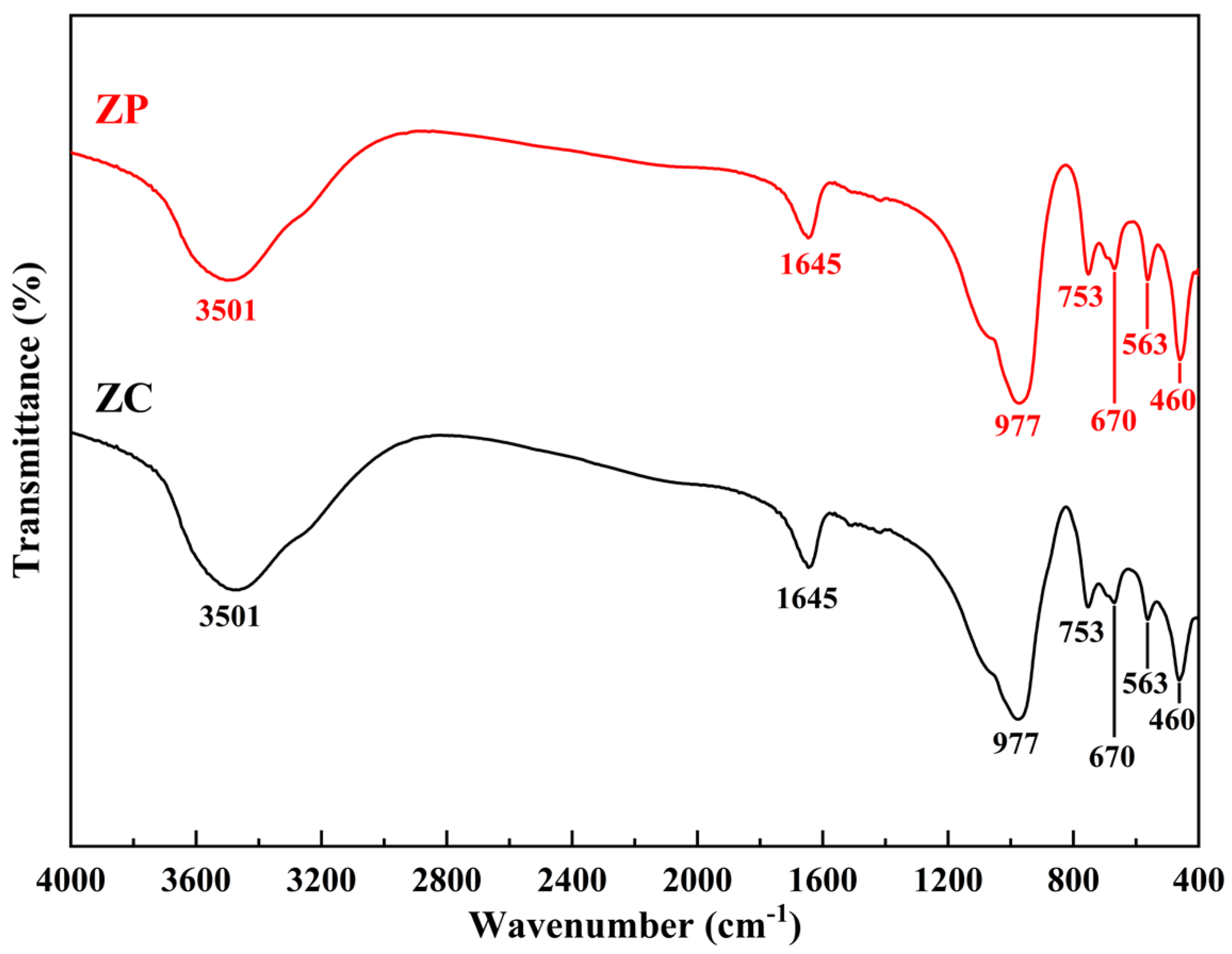
3.1.3. FTIR Analysis
3.1.4. Microscopic Study
3.2. Adsorption Effect of ZC on VOCs Emission
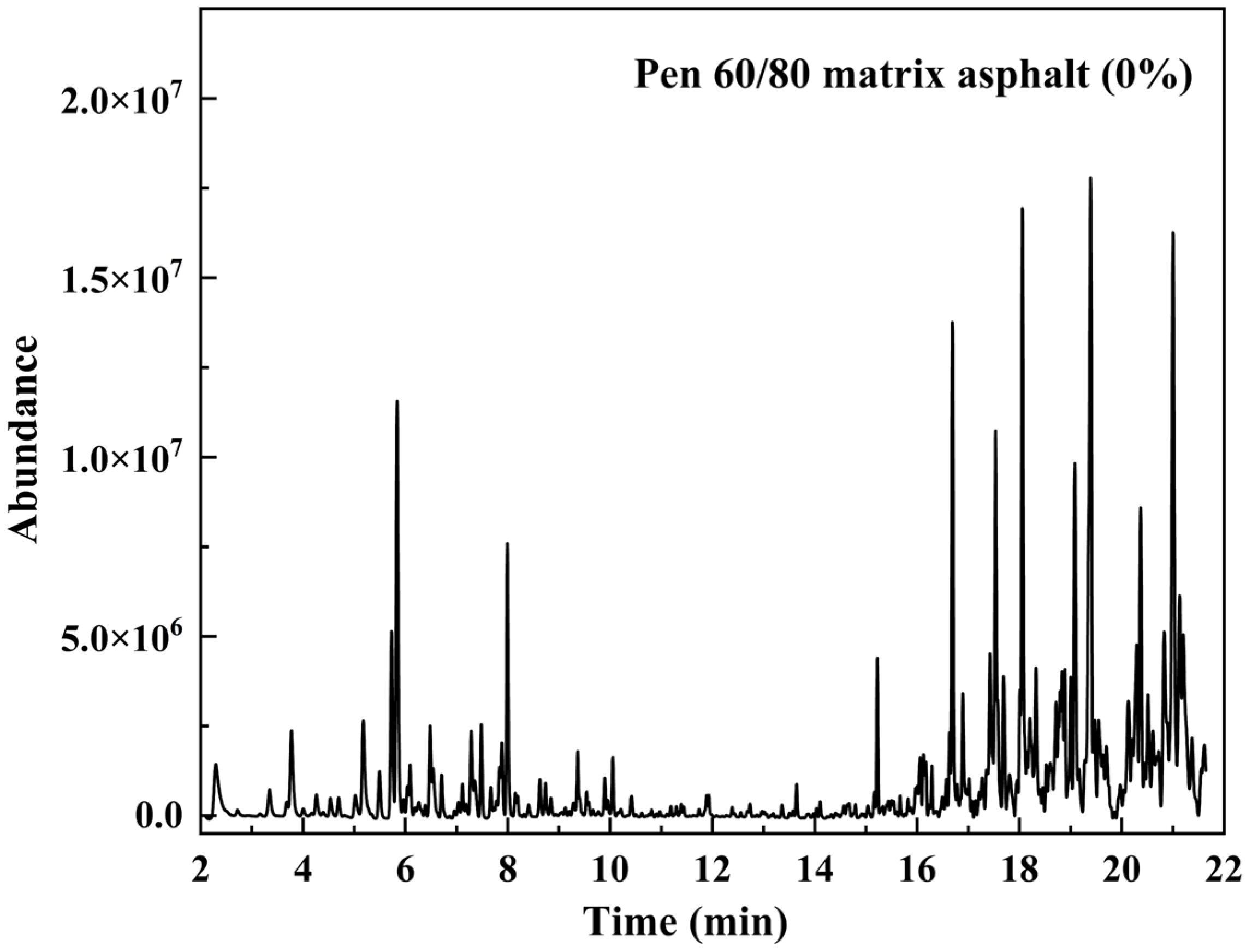
3.2.1. Analysis of Asphalt VOCs Composition
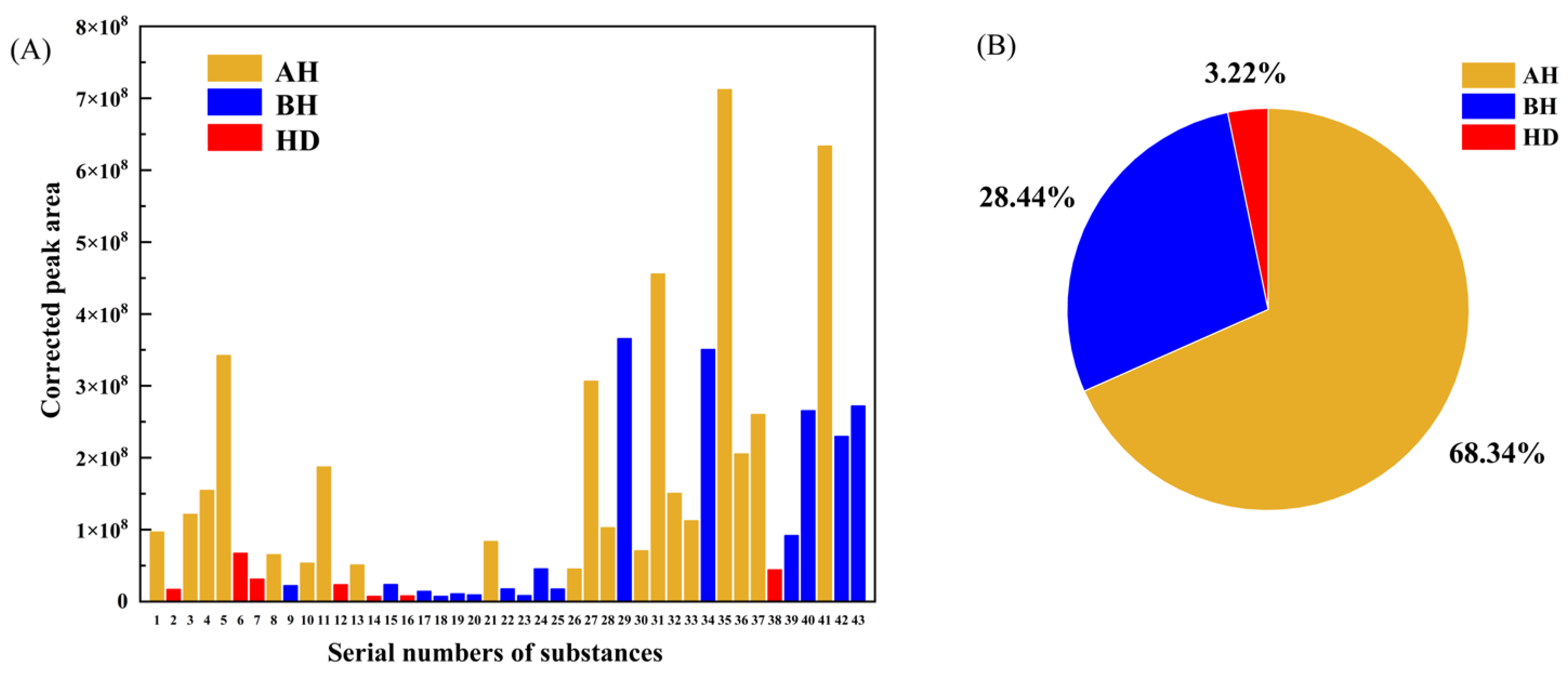
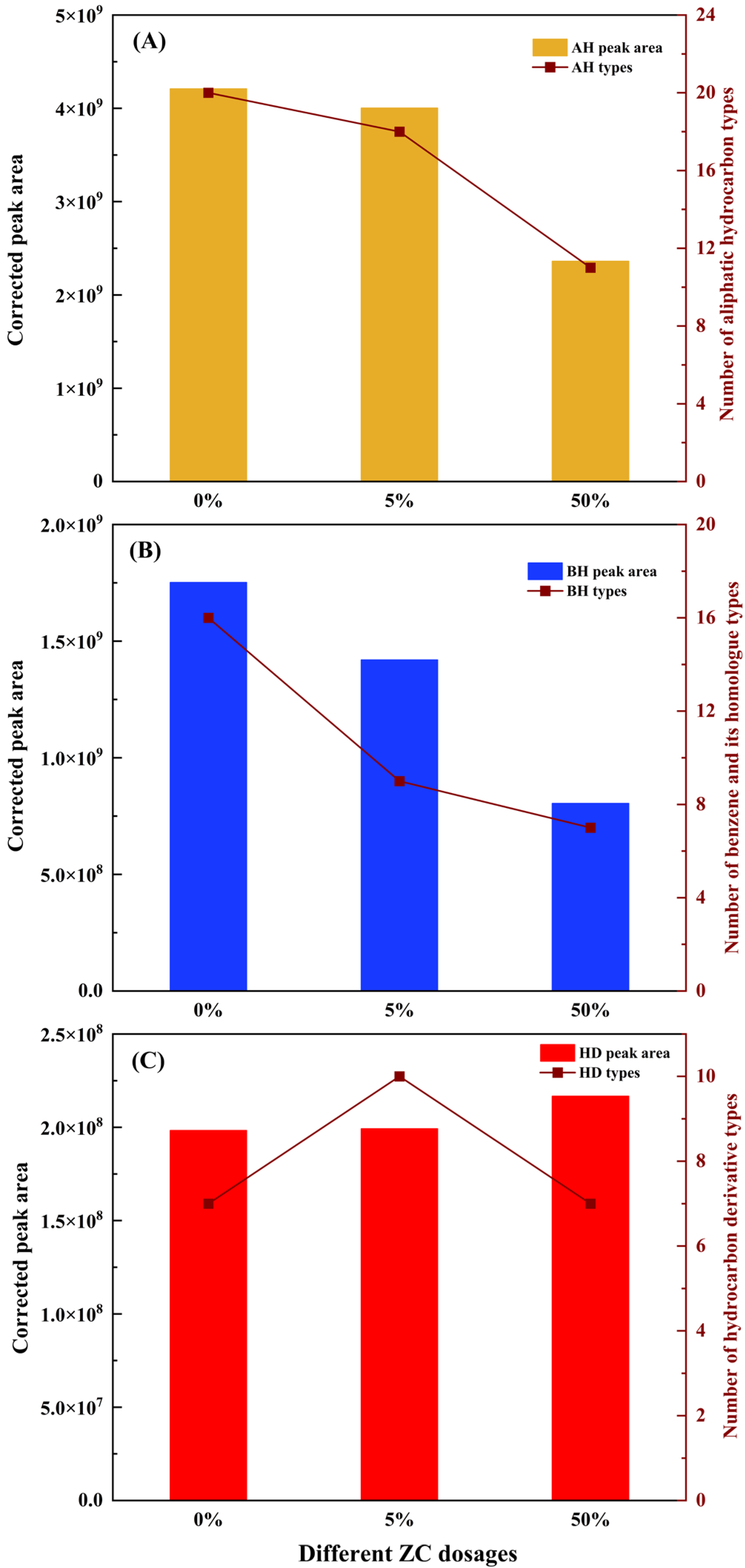
3.2.2. Adsorption Effect of Different ZC Dosage
3.2.3. Adsorption Processes of Asphalt VOCs
3.3. Adsorption Mechanism of Asphalt VOCs by ZC
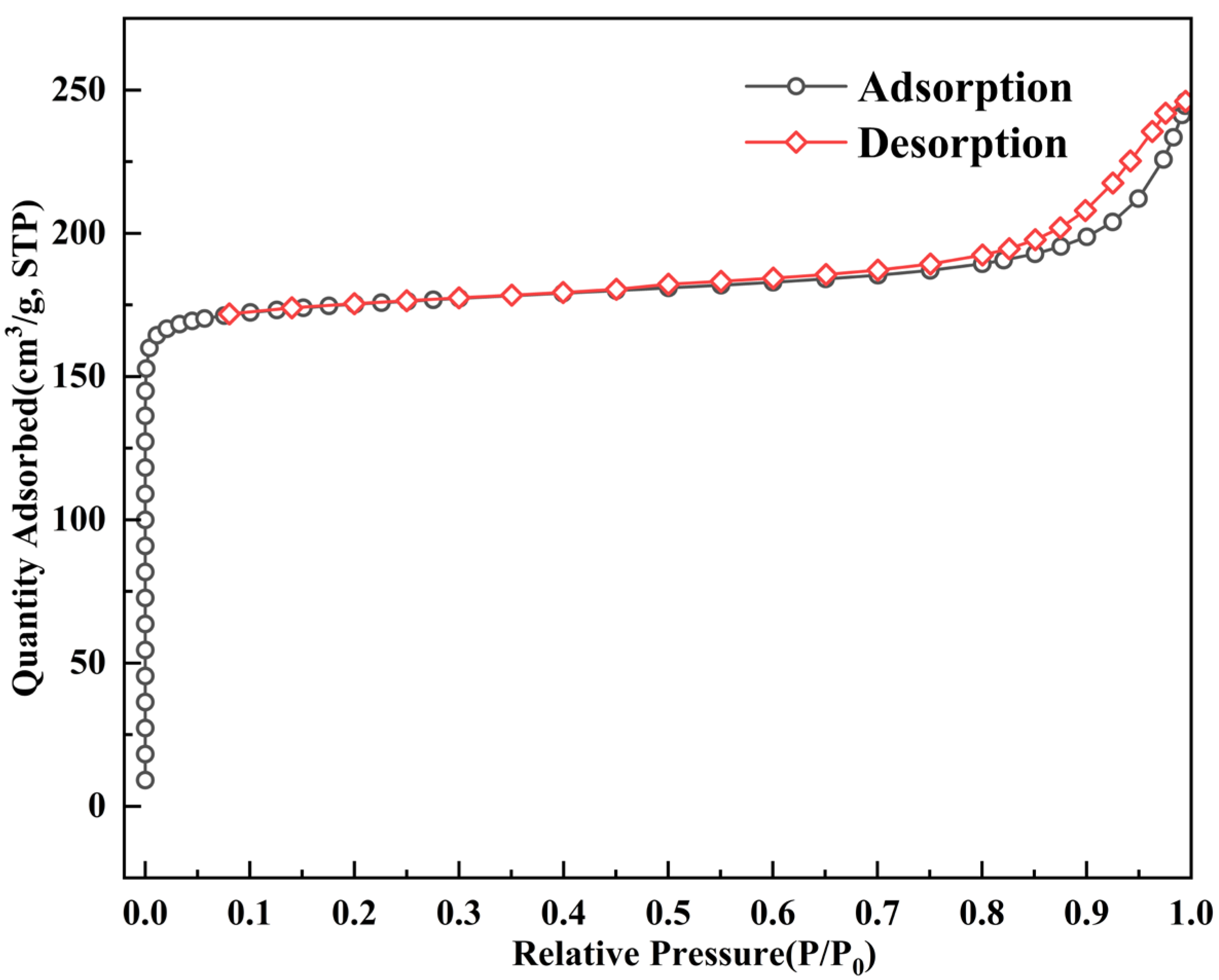
3.3.1. Isotherms of ZC
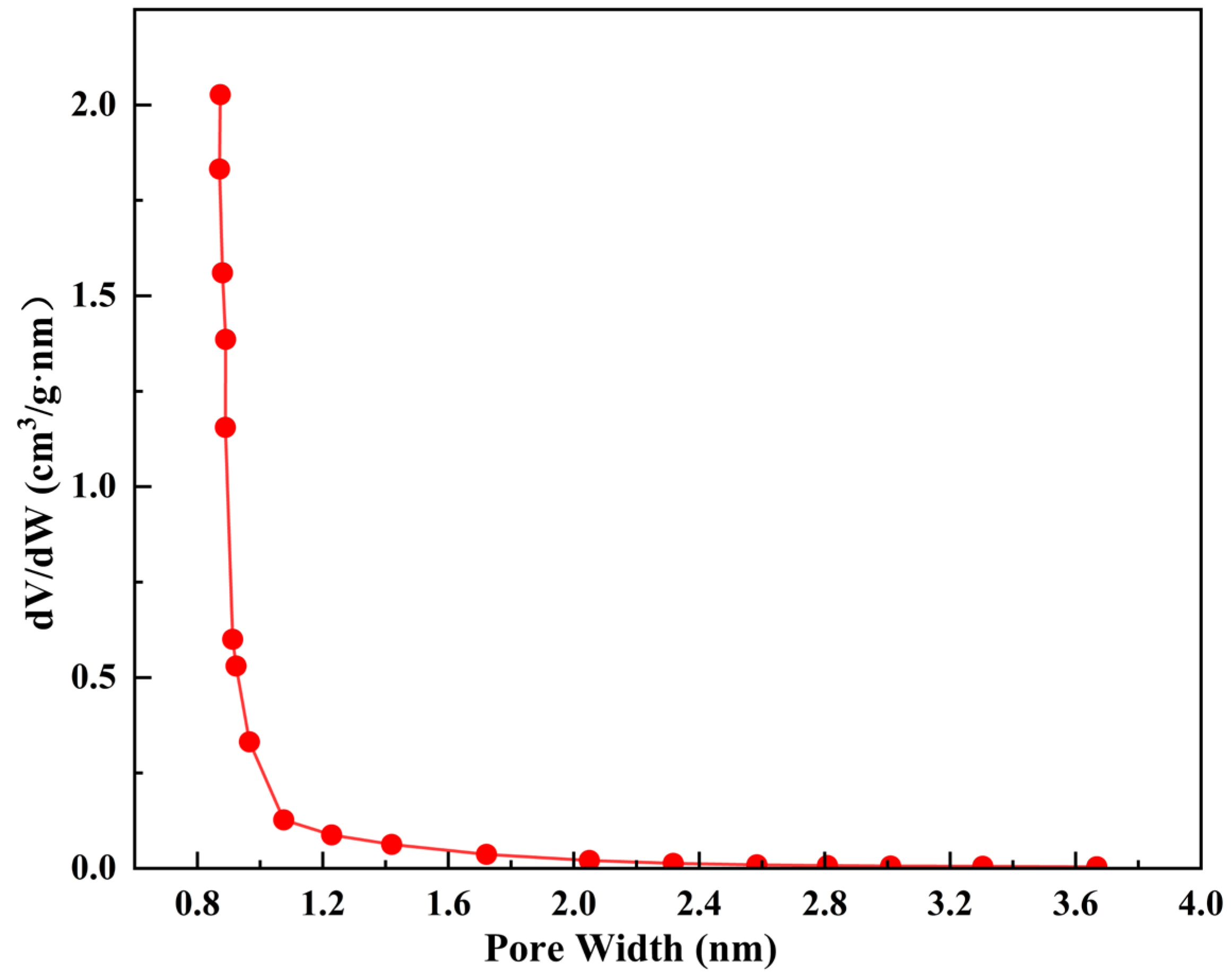
3.3.2. Pore Size Distribution of ZC
4. Conclusions
- (1)
- The prepared zeolite ceramsite contains a trace of chemical components from the attapulgite binder through XRF analysis. Meanwhile, XRD and FTIR results did not detect the presence of obvious impurity crystal phases and atoms in the ZC, which possesses a high degree of crystallinity, indicating that the major crystal phase of the prepared product is 13X zeolite.
- (2)
- The adsorption results of asphalt VOCs show that the volume reduction of total VOCs with high matching degrees exceeds 45% at a large dosage of 50%, indicating a significant VOCs inhibition effect. In comparison, with the addition at the dosage of 5%, the inhibitory effect of less than 10% is not ideal. Besides, the prepared zeolite ceramsite shows high selective adsorption of aromatic hydrocarbons in asphalt VOCs at both dosages, and their adsorption effect is about 10% higher than that of aliphatic hydrocarbons.
- (3)
- The nitrogen adsorption isotherm indicates the micropore domination within the zeolite ceramsite and contains some textural mesopores stemming from the attapulgite binder. The micropore size of zeolite ceramsite is 0.88 nm, of which a micropore volume of 0.27 cm3/g accounts for 71.05% of its total pore volume. The main pore structures in ZC can be classified as relatively narrower micropores. The BET surface area is 709.34 m2/g, whereas the external specific surface area of 58.86 m2/g only occupies 8.29% of the total specific surface area, indicating that intraparticle adsorption dominates the whole adsorption process.
- (4)
- The adsorption mechanism of asphalt VOCs by ZC is mainly physical adsorption. Under the narrow micropores within the material, the molecular kinetics diameter of most aromatic hydrocarbons is comparable to the pore size. The increase in the intraparticle diffusion resistance of aliphatic hydrocarbon molecules is found to be the important factor in obtaining high adsorption of aromatic hydrocarbons in asphalt VOCs.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bottin, M.C.; Gate, L.; Rihn, B.; Micillino, J.C.; Nathalie, M.; Martin, A.; Nunge, H.; Morel, G.; Wrobel, R.; Ayi-Fanou, L.; et al. Genotoxic Effects of Bitumen Fumes in Big Blue® Transgenic Rat Lung. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2006, 596, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, Q.; Wang, F.; Xie, J.; Li, Y.; Li, J.; Wu, S. Emission Behavior, Environmental Impact and Priority-Controlled Pollutants Assessment of Volatile Organic Compounds (VOCs) during Asphalt Pavement Construction Based on Laboratory Experiment. J. Hazard. Mater. 2020, 398, 122904. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Huang, S.; Yuan, H. Study on Asphalt Volatile Organic Compounds Emission Reduction: A State-of-the-Art Review. J. Clean. Prod. 2021, 318, 128596. [Google Scholar] [CrossRef]
- Chang, X.; Xiao, Y.; Long, Y.; Wang, F.; You, Z. Temperature Dependency of VOCs Release Characteristics of Asphalt Materials under Varying Test Conditions. J. Traffic Transp. Eng. 2022, 9, 280–292. [Google Scholar] [CrossRef]
- Cui, P.; Wu, S.; Xiao, Y.; Wan, M.; Cui, P. Inhibiting Effect of Layered Double Hydroxides on the Emissions of Volatile Organic Compounds from Bituminous Materials. J. Clean. Prod. 2015, 108, 987–991. [Google Scholar] [CrossRef]
- Tang, N.; Yang, K.; Alrefaei, Y.; Dai, J.-G.; Wu, L.-M.; Wang, Q. Reduce VOCs and PM Emissions of Warm-Mix Asphalt Using Geopolymer Additives. Constr. Build. Mater. 2020, 244, 118338. [Google Scholar] [CrossRef]
- Cheraghian, G.; Cannone Falchetto, A.; You, Z.; Chen, S.; Kim, Y.S.; Westerhoff, J.; Moon, K.H.; Wistuba, M.P. Warm Mix Asphalt Technology: An up to Date Review. J. Clean. Prod. 2020, 268, 122128. [Google Scholar] [CrossRef]
- Bolliet, C.; Kriech, A.J.; Juery, C.; Vaissiere, M.; Brinton, M.A.; Osborn, L.V. Effect of Temperature and Process on Quantity and Composition of Laboratory-Generated Bitumen Emissions. J. Occup. Environ. Hyg. 2015, 12, 438–449. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of Various Types of VOCs by Adsorption/Catalytic Oxidation: A Review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Batur, E.; Baytar, O.; Kutluay, S.; Horoz, S.; Şahin, Ö. A Comprehensive New Study on the Removal of Pb (II) from Aqueous Solution by Şırnak Coal-Derived Char. Environ. Technol. 2020, 42, 505–520. [Google Scholar] [CrossRef]
- Ece, M.Ş.; Kutluay, S.; Şahin, Ö. Silica-Coated Magnetic Fe3O4 Nanoparticles as Efficient Nano-Adsorbents for the Improvement of the Vapor-Phase Adsorption of Benzene. Int. J. Chem. Technol. 2021, 5, 33–41. [Google Scholar] [CrossRef]
- Ece, M.Ş.; Kutluay, S. Comparative and Competitive Adsorption of Gaseous Toluene, Ethylbenzene, and Xylene onto Natural Cellulose-Modified Fe3O4 Nanoparticles. J. Environ. Chem. Eng. 2022, 10, 107389. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and Kinetics of Toluene Adsorption-desorption on Activated Carbons with Varying Pore Structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Batur, E.; Kutluay, S. Dynamic Adsorption Behavior of Benzene, Toluene, and Xylene VOCs in Single- and Multi-Component Systems by Activated Carbon Derived from Defatted Black Cumin (Nigella Sativa L.) Biowaste. J. Environ. Chem. Eng. 2022, 10, 107565. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, P.; Tong, Z.; Zhao, Z.; Zhao, Z. Enhanced Hydrophobic MIL(Cr) Metal-Organic Framework with High Capacity and Selectivity for Benzene VOCs Capture from High Humid Air. Chem. Eng. J. 2017, 313, 1122–1131. [Google Scholar] [CrossRef]
- Long, Y.; Wu, S.; Xiao, Y.; Cui, P.; Zhou, H. VOCs Reduction and Inhibition Mechanisms of Using Active Carbon Filler in Bituminous Materials. J. Clean. Prod. 2018, 181, 784–793. [Google Scholar] [CrossRef]
- Zhou, X.; Moghaddam, T.B.; Chen, M.; Wu, S.; Adhikari, S. Biochar Removes Volatile Organic Compounds Generated from Asphalt. Sci. Total Environ. 2020, 745, 141096. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wei, X.; Zhao, H.; Wang, T.; Xiao, Y. Interaction of Spent FCC Catalyst and Asphalt Binder: Rheological Properties, Emission of VOCs and Immobilization of Metals. J. Clean. Prod. 2020, 259, 120830. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Lin, C.; Shang, H.; Yang, J.; Li, L.; Li, J. Effects of Different Alkali Metal Cations in FAU Zeolites on the Separation Performance of CO2/N2O. Chem. Eng. J. 2022, 431, 134257. [Google Scholar] [CrossRef]
- Jedli, H.; Almoneef, M.M.; Mbarek, M.; Jbara, A.; Slimi, K. Adsorption of CO2 onto Zeolite ZSM-5: Kinetic, Equilibrium and Thermodynamic Studies. Fuel 2022, 321, 124097. [Google Scholar] [CrossRef]
- David, E. Evaluation of Na-13X Zeolites Activity in the Catalytic Pyrolysis of Rapeseed Oil Cake to Produce Bio-Oil. Appl. Catal. A Gen. 2021, 617, 118126. [Google Scholar] [CrossRef]
- Wu, R.; Xiao, Y.; Zhang, P.; Lin, J.; Cheng, G.; Chen, Z.; Yu, R. Asphalt VOCs Reduction of Zeolite Synthesized from Solid Wastes of Red Mud and Steel Slag. J. Clean. Prod. 2022, 345, 131078. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Y.; Long, Y.; Chen, Z.; Cui, P.; Wu, R.; Chang, X. VOCs Reduction in Bitumen Binder with Optimally Designed Ca (OH)2-Incorporated Zeolite. Constr. Build. Mater. 2021, 279, 122485. [Google Scholar] [CrossRef]
- García, L.; Rodríguez, G.; Orjuela, A. Study of the Pilot-Scale Pan Granulation of Zeolite-Based Molecular Sieves. Braz. J. Chem. Eng. 2021, 38, 165–175. [Google Scholar] [CrossRef]
- Che, T.; Pan, B.; Ouyang, J. The Laboratory Evaluation of Incorporating Ceramsite into HMA as Fine Aggregates. Constr. Build. Mater. 2018, 186, 1239–1246. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, N.; An, S.; Cai, C.; Peng, J.; Xie, M.; Peng, J.; Song, X. Synthesis of Novel Hierarchical Porous Zeolitization Ceramsite from Industrial Waste as Efficient Adsorbent for Separation of Ammonia Nitrogen. Sep. Purif. Technol. 2022, 297, 121418. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, F.-S. Zeolite Loaded Ceramsite Developed from Construction and Demolition Waste. Mater. Lett. 2013, 93, 380–382. [Google Scholar] [CrossRef]
- Feng, N.-Q.; Xing, F.; Leng, F.-G. Zeolite Ceramsite Cellular Concrete. Mag. Concr. Res. 2000, 52, 117–122. [Google Scholar] [CrossRef]
- García, L.; Poveda, Y.A.; Khadivi, M.; Rodríguez, G.; Görke, O.; Esche, E.; Godini, H.R.; Wozny, G.; Orjuela, A. Synthesis and Granulation of a 5A Zeolite-Based Molecular Sieve and Adsorption Equilibrium of the Oxidative Coupling of Methane Gases. J. Chem. Eng. Data 2017, 62, 1550–1557. [Google Scholar] [CrossRef]
- Cao, J.-L.; Shao, G.-S.; Wang, Y.; Liu, Y.; Yuan, Z.-Y. CuO Catalysts Supported on Attapulgite Clay for Low-Temperature CO Oxidation. Catal. Commun. 2008, 9, 2555–2559. [Google Scholar] [CrossRef]
- Zhou, C.; Alshameri, A.; Yan, C.; Qiu, X.; Wang, H.; Ma, Y. Characteristics and Evaluation of Synthetic 13X Zeolite from Yunnan’s Natural Halloysite. J. Porous. Mater. 2013, 20, 587–594. [Google Scholar] [CrossRef]
- Shi, T.; Liu, Y.; Zhang, Y.; Lan, Y.; Zhao, Q.; Zhao, Y.; Wang, H. Calcined Attapulgite Clay as Supplementary Cementing Material: Thermal Treatment, Hydration Activity and Mechanical Properties. Int. J. Concr. Struct. Mater. 2022, 16, 10. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, X.; Ma, J.; Li, R. Adsorption of Carbon Dioxide on Alkali-Modified Zeolite 13X Adsorbents. Int. J. Greenh. Gas Control. 2007, 1, 355–359. [Google Scholar] [CrossRef]
- Chen, C.; Park, D.-W.; Ahn, W.-S. CO2 Capture Using Zeolite 13X Prepared from Bentonite. Appl. Surf. Sci. 2014, 292, 63–67. [Google Scholar] [CrossRef]
- Chen, D.; Hu, X.; Shi, L.; Cui, Q.; Wang, H.; Yao, H. Synthesis and Characterization of Zeolite X from Lithium Slag. Appl. Clay Sci. 2012, 59–60, 148–151. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Salim, C.; Hinode, H. Synthesis of Pure Na-X and Na-A Zeolite from Bagasse Fly Ash. Microporous Mesoporous Mater. 2012, 162, 6–13. [Google Scholar] [CrossRef]
- Buchwald, Z.; Sandomierski, M.; Voelkel, A. Calcium-Rich 13X Zeolite as a Filler with Remineralizing Potential for Dental Composites. ACS Biomater. Sci. Eng. 2020, 6, 3843–3854. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Wu, S.; Zhou, X.; Zhao, G.; Zhao, Y.; Cheng, M. Evaluation of VOCs Inhibited Effects and Rheological Properties of Asphalt with High-Content Waste Rubber Powder. Constr. Build. Mater. 2021, 300, 124320. [Google Scholar] [CrossRef]
- Aytas, S.O.; Akyil, S.; Eral, M. Adsorption and Thermodynamic Behavior of Uranium on Natural Zeolite. J. Radioanal. Nucl. Chem. 2004, 260, 119–125. [Google Scholar] [CrossRef]
- Zhu, C.; Luan, Z.; Wang, Y.; Shan, X. Removal of Cadmium from Aqueous Solutions by Adsorption on Granular Red Mud (GRM). Sep. Purif. Technol. 2007, 57, 161–169. [Google Scholar] [CrossRef]
- Yusof, A.M.; Keat, L.K.; Ibrahim, Z.; Majid, Z.A.; Nizam, N.A. Kinetic and Equilibrium Studies of the Removal of Ammonium Ions from Aqueous Solution by Rice Husk Ash-Synthesized Zeolite Y and Powdered and Granulated Forms of Mordenite. J. Hazard. Mater. 2010, 174, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Al. Haddabi, M.; Vuthaluru, H.; Znad, H.; Ahmed, M. Attapulgite as Potential Adsorbent for Dissolved Organic Carbon From Oily Water. Clean Soil Air Water 2015, 43, 1522–1530. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption Materials for Volatile Organic Compounds (VOCs) and the Key Factors for VOCs Adsorption Process: A Review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van Der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Chen, C.; Son, W.-J.; You, K.-S.; Ahn, J.-W.; Ahn, W.-S. Carbon Dioxide Capture Using Amine-Impregnated HMS Having Textural Mesoporosity. Chem. Eng. J. 2010, 161, 46–52. [Google Scholar] [CrossRef]
- Khoramzadeh, E.; Mofarahi, M.; Lee, C.-H. Equilibrium Adsorption Study of CO2 and N2 on Synthesized Zeolites 13X, 4A, 5A, and Beta. J. Chem. Eng. Data 2019, 64, 5648–5664. [Google Scholar] [CrossRef]
- Genli, N.; Kutluay, S.; Baytar, O.; Şahin, Ö. Preparation and Characterization of Activated Carbon from Hydrochar by Hydrothermal Carbonization of Chickpea Stem: An Application in Methylene Blue Removal by RSM Optimization. Int. J. Phytoremediation 2022, 24, 88–100. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Horvath, G. Energetic Interactions in Phase and Molecular Level Pore Characterisation in Nano-Range. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 295–304. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Horváth, G.; Kawazoe, K. Method for the Calculation of Effective Pore Size Distribution in Molecular Sieve Carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef]
- Saito, A.; Foley, H.C. Curvature and Parametric Sensitivity in Models for Adsorption in Micropores. AIChE J. 1991, 37, 429–436. [Google Scholar] [CrossRef]
- Kutluay, S. Excellent Adsorptive Performance of Novel Magnetic Nano-Adsorbent Functionalized with 8-Hydroxyquinoline-5-Sulfonic Acid for the Removal of Volatile Organic Compounds (BTX) Vapors. Fuel 2021, 287, 119691. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A Critical Review on VOCs Adsorption by Different Porous Materials: Species, Mechanisms and Modification Methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto Engineered Carbon Materials: A Review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Le-Minh, N.; Sivret, E.C.; Shammay, A.; Stuetz, R.M. Factors Affecting the Adsorption of Gaseous Environmental Odors by Activated Carbon: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 341–375. [Google Scholar] [CrossRef]
- Jiménez-Cruz, F.; Laredo, G.C. Molecular Size Evaluation of Linear and Branched Paraffins from the Gasoline Pool by DFT Quantum Chemical Calculations. Fuel 2004, 83, 2183–2188. [Google Scholar] [CrossRef]
- Funke, H.H.; Argo, A.M.; Baertsch, C.D.; Falconer, J.L.; Noble, R.D. Separation of Close-Boiling Hydrocarbons with Silicalite Zeolite Membranes. J. Chem. Soc. Faraday Trans. 1996, 92, 2499–2502. [Google Scholar] [CrossRef]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the Shape Selectivity of Zeolite Catalysts for Biomass Conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]


| Item | Results | Methods |
|---|---|---|
| Penetration (25 °C)/0.1 mm | 63 | ASTM D5 |
| Softening Point/°C | 51 | ASTM D36 |
| Ductility (15 °C)/cm | >100 | ASTM D113 |
| Density (15 °C)/g·cm−3 | 1.03 | ASTM D8188 |
| Solubility (Trichloroethylene)/% | 99.7 | ASTM D2042 |
| Oxides | Zeolite (%) | Attapulgite Clay (%) | Zeolite Ceramsite (%) |
|---|---|---|---|
| SiO2 | 41.02 | 52.06 | 42.38 |
| Al2O3 | 30.01 | 12.36 | 25.06 |
| Fe2O3 | 0.03 | 5.02 | 0.90 |
| CaO | 0.24 | 2.24 | 0.59 |
| MgO | 0.18 | 6.66 | 2.09 |
| K2O | 0.06 | 1.38 | 0.19 |
| Na2O | 15.84 | 0.56 | 12.48 |
| Items | 0ZC | 5ZC | 50ZC |
|---|---|---|---|
| AH types | 20 | 17 | 11 |
| AH volatilization | 4209044861 | 4003208713 | 2361028006 |
| AH adsorption effect | - | 4.89% | 43.91% |
| BH types | 16 | 9 | 7 |
| BH volatilization | 1751970027 | 1519753368 | 804337964 |
| BH adsorption effect | - | 13.25% | 54.09% |
| Total asphalt VOCs volatilization | 6159307657 | 5722215181 | 3637487695 |
| Total adsorption effect | - | 8.72% | 45.09% |
| Sample | SBET (m2/g) a | Sexternal (m2/g) b | Vtotal (cm3/g) c | Vmicro (cm3/g) d | Average Pore Size (nm) e | Micropore Size (nm) |
|---|---|---|---|---|---|---|
| ZC | 709.34 | 58.86 | 0.38 | 0.27 | 2.13 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhao, H.; Xue, Y.; Chang, X. Adsorption Effect and Adsorption Mechanism of High Content Zeolite Ceramsite on Asphalt VOCs. Materials 2022, 15, 6100. https://doi.org/10.3390/ma15176100
Chen W, Zhao H, Xue Y, Chang X. Adsorption Effect and Adsorption Mechanism of High Content Zeolite Ceramsite on Asphalt VOCs. Materials. 2022; 15(17):6100. https://doi.org/10.3390/ma15176100
Chicago/Turabian StyleChen, Wei, Hui Zhao, Yongjie Xue, and Xiwen Chang. 2022. "Adsorption Effect and Adsorption Mechanism of High Content Zeolite Ceramsite on Asphalt VOCs" Materials 15, no. 17: 6100. https://doi.org/10.3390/ma15176100




