Investigation on Application Prospect of Refractories for Hydrogen Metallurgy: The Enlightenment from the Reaction between Commercial Brown Corundum and Hydrogen
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermodynamic Calculations
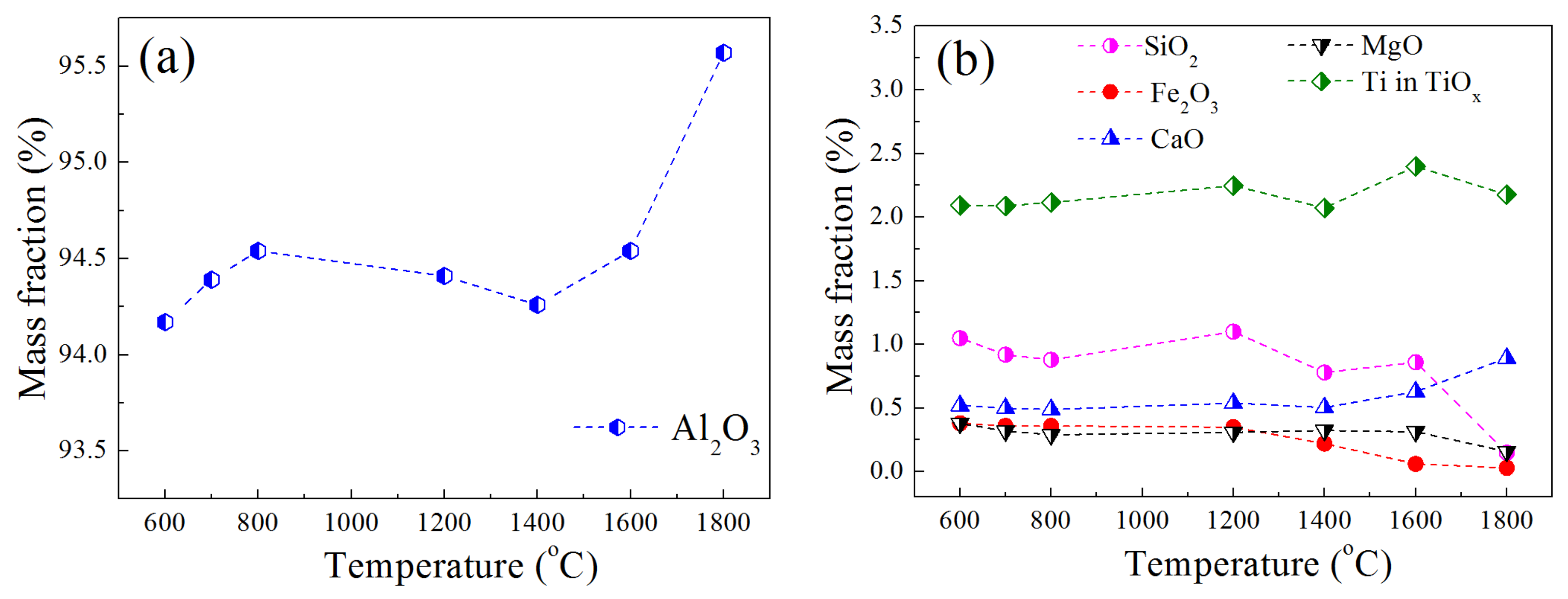
3.2. Weight Loss of Brown Corundum
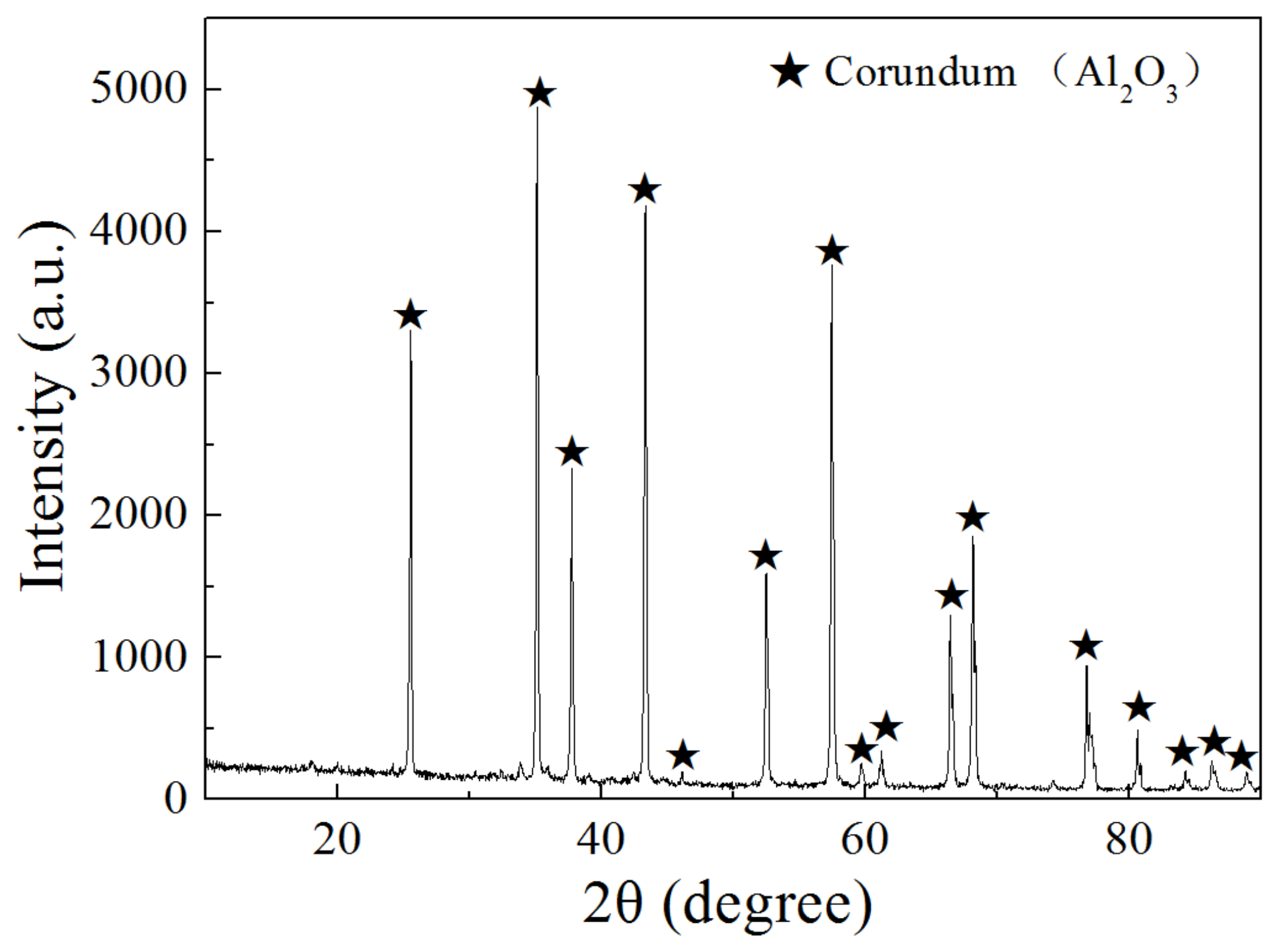
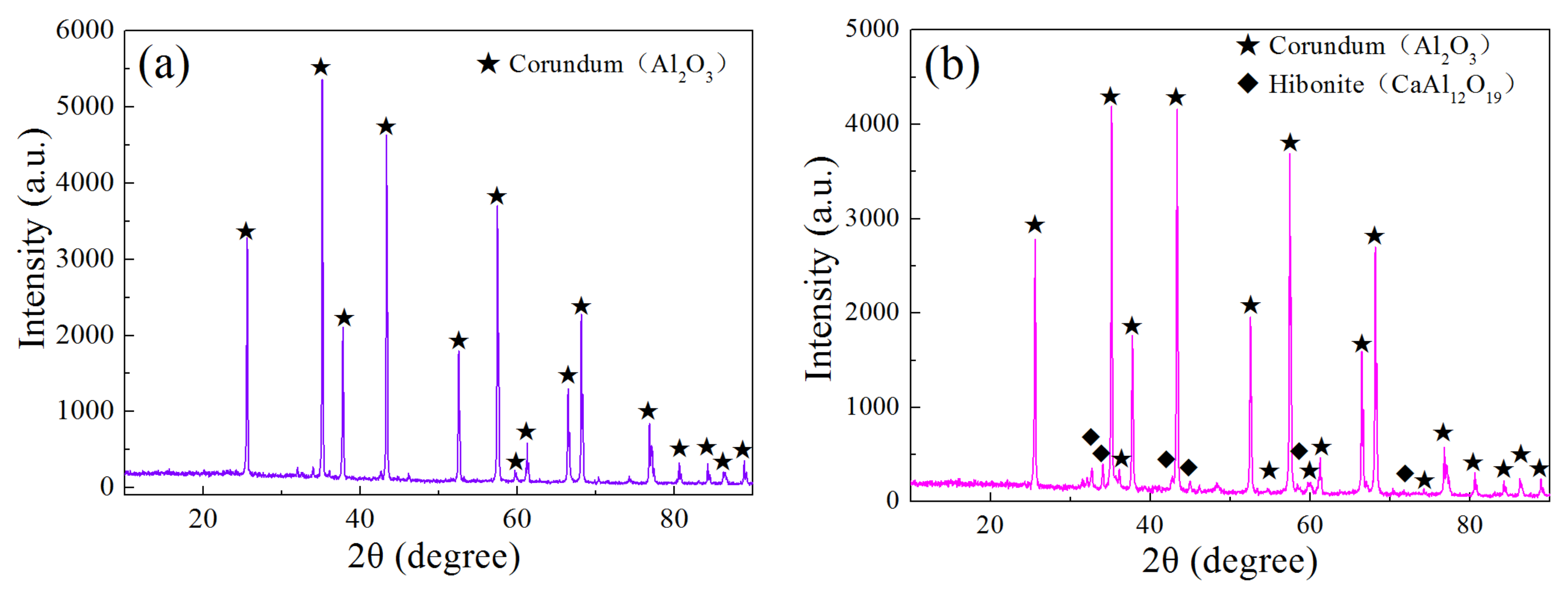
3.3. Phase Compositions of Brown Corundum after Reduction by Hydrogen
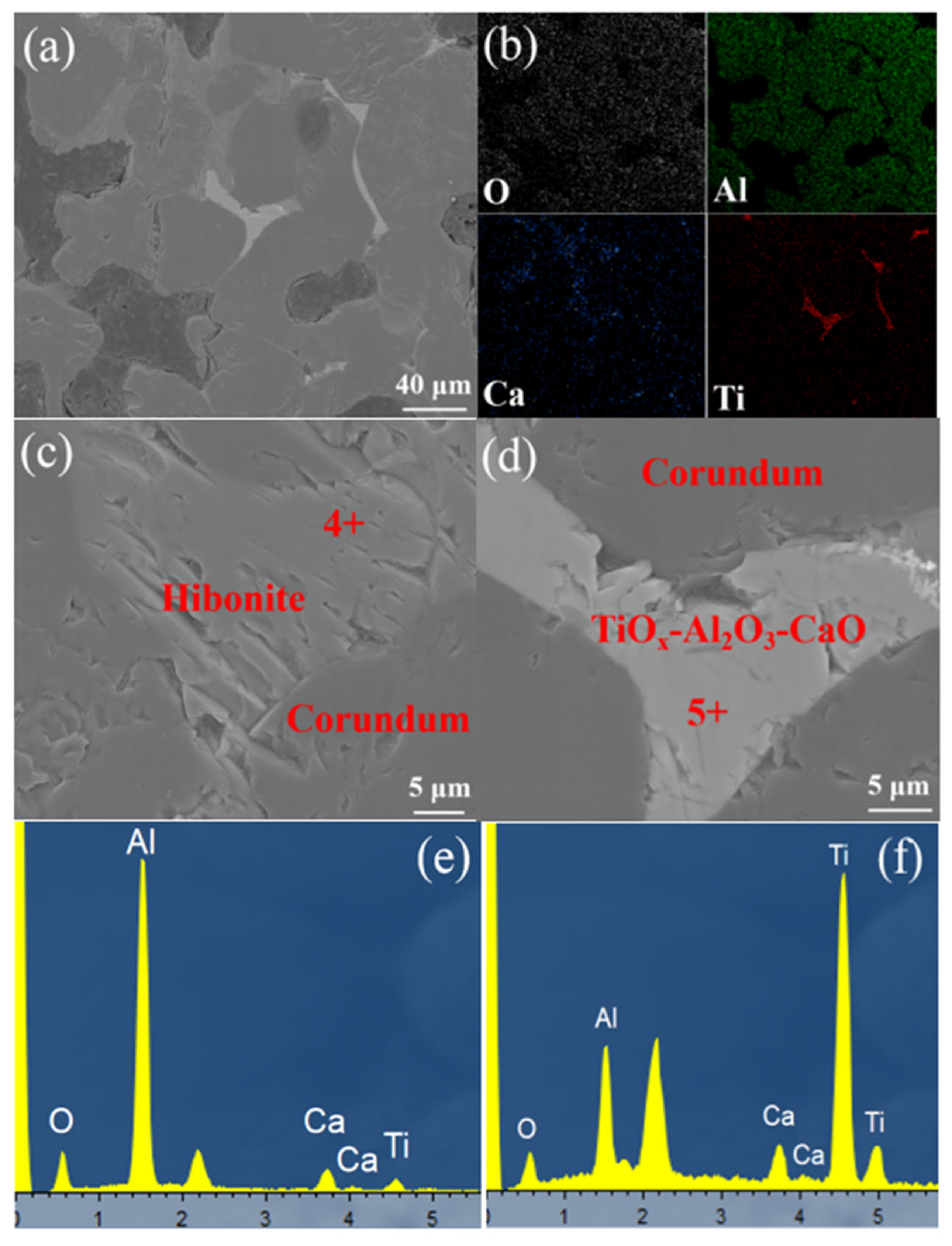
3.4. Microstructure Evaluation of Brown Corundum after Reduction by Hydrogen
4. Conclusions
- (1)
- The thermodynamic stability of the oxides under high-purity hydrogen was in the order of Al2O3 > CaO > MgO > SiO2 > TiO2 > Fe2O3 at the temperature lower than 1400 °C, when continuously increasing the temperature, the SiO2 performed worse in stability compared with TiO2 and MgO. The Al2O3 could remain stable even at 1800 °C. Obvious weight loss appeared when raising the temperature to 1400 °C. The reduction in Fe2O3, SiO2 and MgO contributed a lot to the weight loss.
- (2)
- The pressures of Ca (g) and H2O (g) caused by the reaction between CaAl12O19 and H2 (g) was just 1.62 Pa at 1800 °C. The CaO contents in the brown corundum remained stable owing to the formation of hibonite (CaAl12O19).
- (3)
- The corundum (Al2O3) and hibonite (CaAl12O19) performed excellently in stability under the high-purity hydrogen atmosphere, even at 1800 °C, which indicated that high-purity Al2O3 and CaAl12O19 -based refractories were suitable for lining materials in the hydrogen metallurgy field.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, P.; Dong, P.L. Carbon emission cannot be ignored in future of Chinese steel industry. Iron Steel 2018, 53, 1–7. [Google Scholar]
- Benjamin, N.I.; Lin, B. Quantile analysis of carbon emissions in China metallurgy industry. J. Clean. Prod. 2020, 243, 118534. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Li, F.; Feng, C.; Liu, Z.G.; Zhou, Y.S. Development and progress on hydrogen metallurgy. Int. J. Min. Met. Mater. 2020, 27, 713–723. [Google Scholar] [CrossRef]
- Tso, S.T.; Pask, J.A. Reaction of fused silica with hydrogen gas. J. Am. Ceram. Soc. 1982, 65, 457–460. [Google Scholar] [CrossRef]
- Herbell, T.P.; Eckel, A.J.; Hull, D.R.; Misra, A.K. Effect of Hydrogen on the Strength and Microstructure of Selected Ceramics. Available online: https://ntrs.nasa.gov/api/citations/19910005169/downloads/19910005169.pdf (accessed on 1 October 2022).
- Wang, R.R.; Zhang, J.L.; Liu, Y.R.; Zheng, A.Y.; Liu, Z.J.; Liu, X.L.; Li, Z.G. Thermal performance and reduction kinetic analysis of cold-bonded pellets with CO and H2 mixtures. Int. J. Min. Met. Mater. 2018, 25, 752–761. [Google Scholar] [CrossRef]
- Klimova, V.; Pakhaluev, V.; Shcheklein, S. On the problem of efficient production of hydrogen reducing gases for metallurgy utilizing nuclear energy. Int. J. Hydrogen Energy 2016, 41, 3320–3325. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, Q.; Chai, X.; Wang, Q.; Lu, Y.; Ji, W. Analysis of process parameters on energy utilization and environmental impact of hydrogen metallurgy. J. Clean. Prod. 2022, 361, 132289. [Google Scholar] [CrossRef]
- Cavaliere, P. Direct reduced iron: Most efficient technologies for greenhouse emissions abatement. In Clean Ironmaking and Steelmaking Processes; Springer: Cham, Switzerland, 2019; pp. 419–484. [Google Scholar]
- Li, L.; Niu, L.; Guo, H.J.; Duan, S.C.; Li, Y.Q.; Meng, X.L. Experimental research and kinetics analysis on hydrogen reduction of limonite. Chin. J. Eng. 2015, 37, 13–19. [Google Scholar]
- Bai, M.H.; Li, L.Y.; Xu, K.; Han, S.F.; Lu, J.C.; Dong, J.B. Numerical analysis and optimization on hydrogen utilization in DR shaft furnace. J. Yanshan Univ. 2016, 40, 481–486. [Google Scholar]
- Yao, X.; Guo, H.J.; Li, Y.Q.; Sun, G.Y.; Li, L. Direct reduction of magnetite under different reducing conditions by hydrogen. Nonferrous Met. Sci. Eng. 2015, 6, 12–16. [Google Scholar]
- Hamadeh, H.; Mirgaux, O.; Patisson, F. Detailed modeling of the direct reduction of iron ore in a shaft furnace. Materials 2018, 11, 1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.Y.; Li, B.; Yang, W.S.; Guo, J.; Guo, H.J. Analysis of energy consumption of the reduction of Fe2O3 by hydrogen and carbon monoxide mixtures. Energies 2020, 13, 1986. [Google Scholar] [CrossRef]
- Xu, C.Y.; Zheng, A.Y.; Zhang, J.L.; Wang, R.R.; Li, Y.; Wang, Y.Z.; Liu, Z.J. Effect of H2/CO ratio on gas consumption and energy utilization rate of gas-based direct reduction process. In Proceedings of the 10th International Symposium on High-Temperature Metallurgical Processing; Springer: Cham, Switzerland, 2019; pp. 631–647. [Google Scholar]
- Guo, X.; Wu, S.; Xu, J.P.; Du, K. Reducing gas composition optimization for COREX® pre-reduction shaft furnace based on CO-H2 mixture. Procedia Eng. 2011, 15, 4702–4706. [Google Scholar] [CrossRef] [Green Version]
- Li, J.L.; Li, F.; Zhu, X.H.; Lin, D.B.; Li, Q.F.; Liu, W.H.; Xu, Z. Colossal dielectric permittivity in hydrogen-reduced rutile TiO2 crystals. J. Alloys Compd. 2017, 692, 375–380. [Google Scholar] [CrossRef]
- Chen, W.P.; Wang, Y.; Dai, J.Y.; Lu, S.G.; Wang, X.X.; Lee, P.F.; Chan, H.L.W.; Choy, C.L. Spontaneous recovery of hydrogen-degraded TiO2 ceramic capacitors. Appl. Phys. Lett. 2004, 84, 103–105. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|
| 1.12 | 92.97 | 0.2 | 0.26 | 0.17 | 0.094 | 0.038 | 2.13 |
| Reactions | 1200 °C | 1400 °C | 1600 °C | 1800 °C | |
|---|---|---|---|---|---|
| SiO2 (s) + H2 (g) = SiO (g) + H2O (g) | PSiO | 2.7 | 41.3 | 345.2 | 1884.3 |
| PH2O | 2.7 | 41.3 | 345.2 | 1884.3 | |
| MgO (s) + H2 (g) = Mg (g) + H2O (g) | PMg | 2.2 | 23.2 | 146.2 | 639.4 |
| PH2O | 2.2 | 23.2 | 146.2 | 639.4 | |
| Al2O3 (s) + 3H2 (g) = 2Al (g) + 3H2O (g) | PAl | 9.3 × 10−22 | 7.0 × 10−18 | 7.5 × 10−15 | 2.0 × 10−12 |
| PH2O | 1.4 × 10−21 | 1.0 × 10−17 | 1.1 × 10−14 | 3.0 × 10−12 | |
| CaO (s) + H2 (g) = Ca (g) + H2O (g) | PCa | 0.3 | 2.0 | 11.6 | 60.9 |
| PH2O | 0.3 | 2.0 | 11.6 | 60.9 | |
| 2TiO2 (s) + H2 (g) = Ti2O3 (s) + H2O (g) | PH2O | 77.4 | 201.7 | 394.1 | 625.5 |
| Fe2O3 (s) + 3H2 (g) = 2Fe + 3H2O (g) | PH2O | 1.5 × 105 | 1.3 × 105 | 9.8 × 104 | 7.3 × 104 |
| Reactions | 1200 °C | 1400 °C | 1600 °C | 1800 °C | |
|---|---|---|---|---|---|
| CaAl12O19 (s) + H2 (g) = Ca (g) + H2O (g) + 6Al2O3 | PCa | 3.6 × 10−3 | 4.7 × 10−2 | 0.35 | 1.62 |
| PH2O | 3.6 × 10−3 | 4.7 × 10−2 | 0.35 | 1.62 | |
| CaO (s) + H2 (g) = Ca (g) + H2O (g) | PCa | 0.3 | 2.0 | 11.6 | 60.9 |
| PH2O | 0.3 | 2.0 | 11.6 | 60.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, D.; Gu, H.; Huang, A.; Fu, L. Investigation on Application Prospect of Refractories for Hydrogen Metallurgy: The Enlightenment from the Reaction between Commercial Brown Corundum and Hydrogen. Materials 2022, 15, 7022. https://doi.org/10.3390/ma15197022
Li S, Chen D, Gu H, Huang A, Fu L. Investigation on Application Prospect of Refractories for Hydrogen Metallurgy: The Enlightenment from the Reaction between Commercial Brown Corundum and Hydrogen. Materials. 2022; 15(19):7022. https://doi.org/10.3390/ma15197022
Chicago/Turabian StyleLi, Shaofei, Ding Chen, Huazhi Gu, Ao Huang, and Lvping Fu. 2022. "Investigation on Application Prospect of Refractories for Hydrogen Metallurgy: The Enlightenment from the Reaction between Commercial Brown Corundum and Hydrogen" Materials 15, no. 19: 7022. https://doi.org/10.3390/ma15197022






