Preparation of Cemented Carbide and Study of Copper-Accelerated Salt Spray Corrosion and Erosion Behavior
Abstract
:1. Introduction
2. Experimental Design
2.1. WC-Co Cemented Carbide Sample Preparation
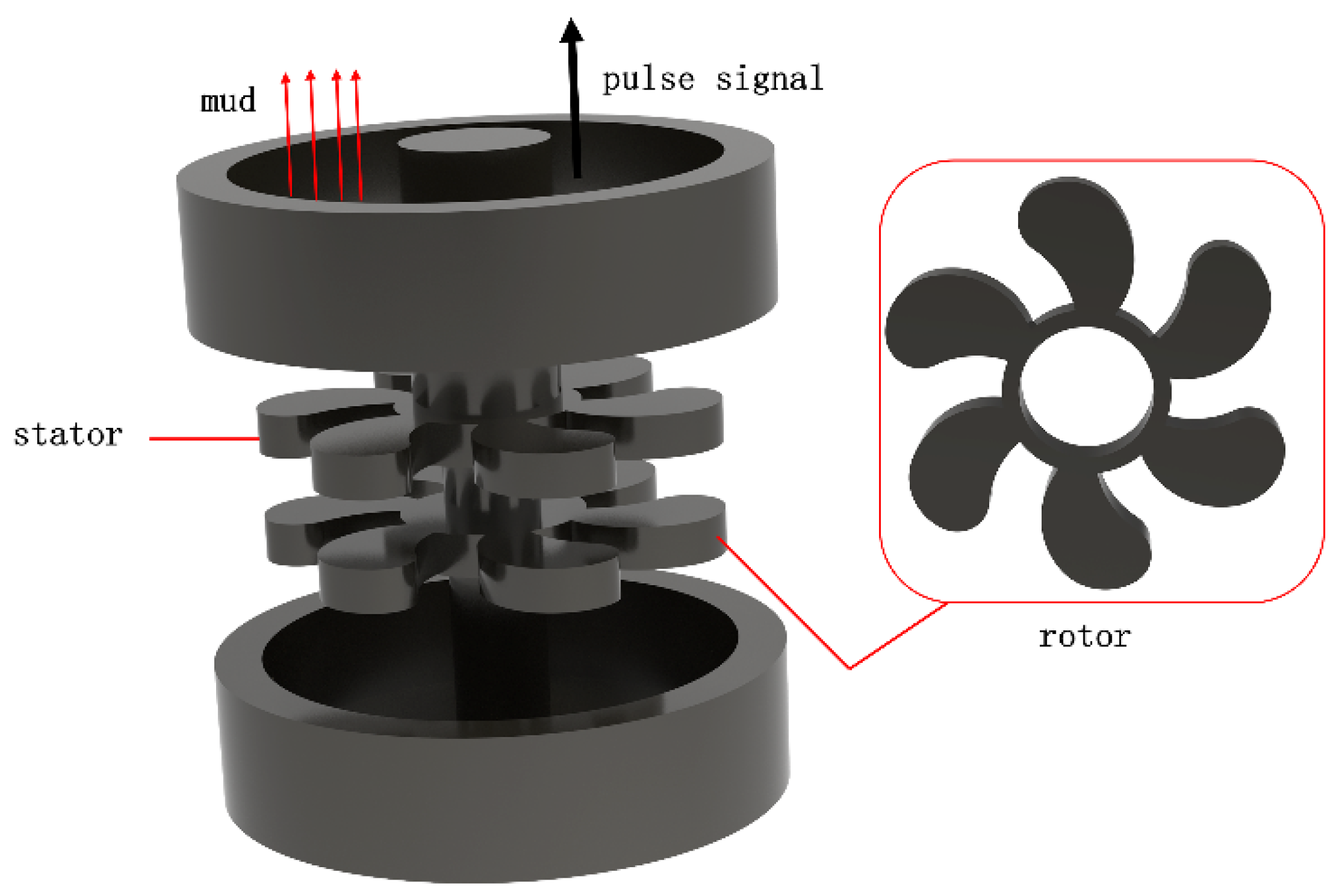
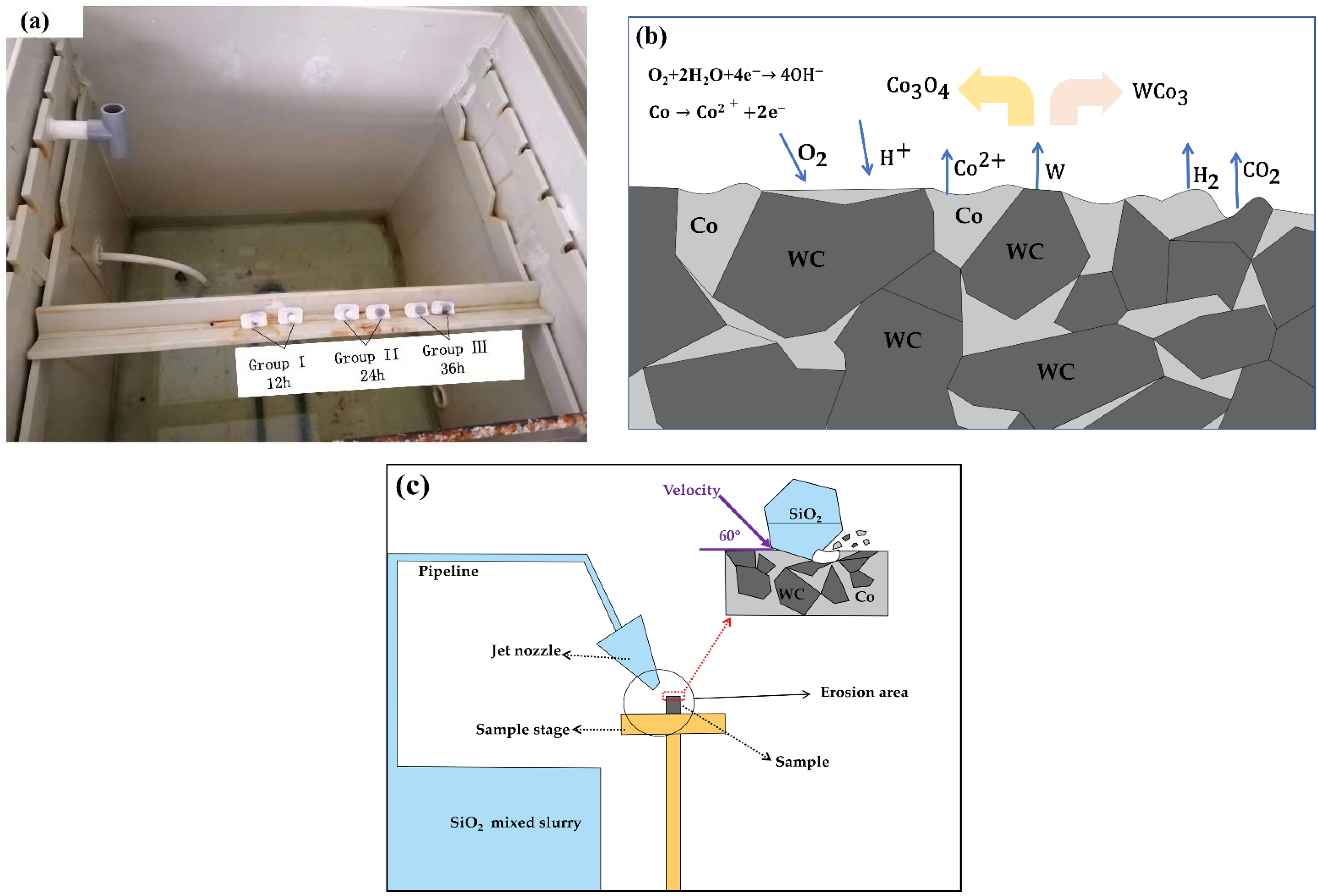
2.2. Corrosion and Erosion Experiments
3. Results
3.1. Cemented Carbide Samples
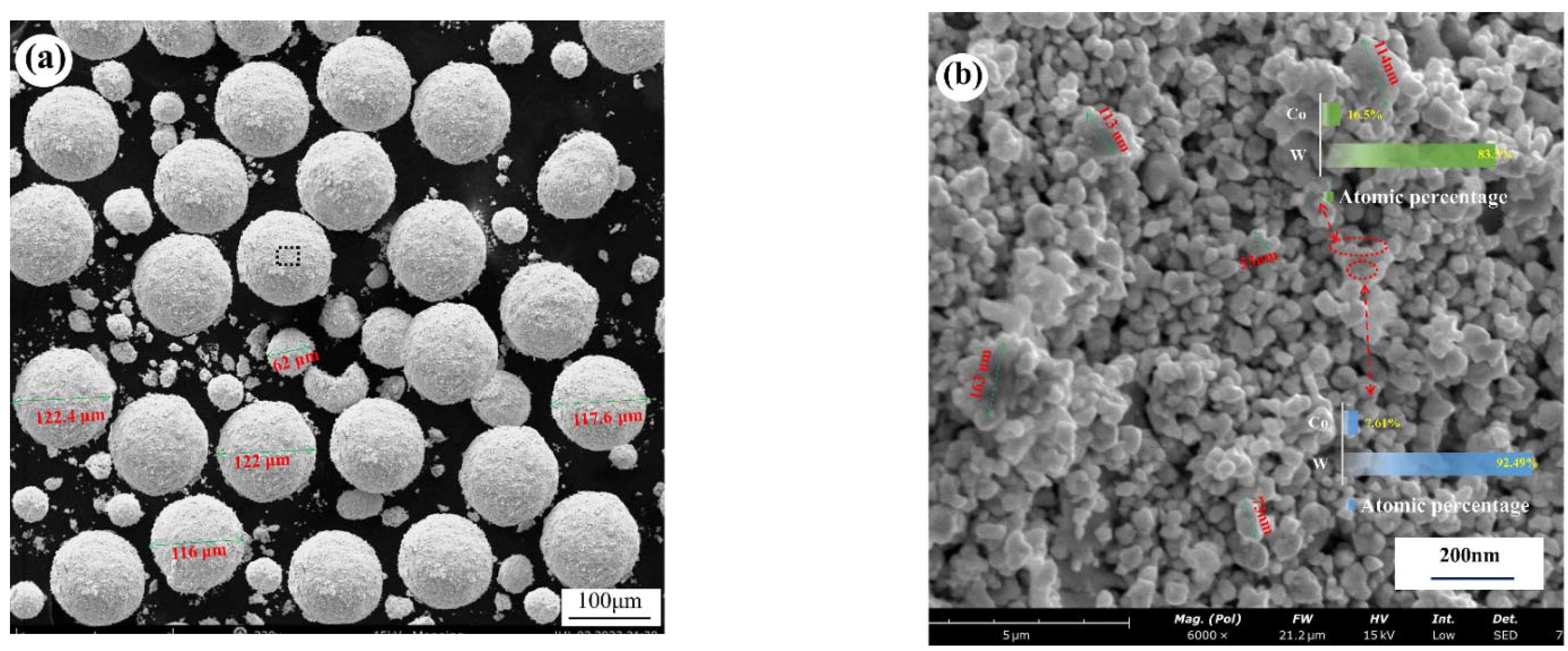
3.1.1. Microstructure of Cemented Carbide Powder Raw Materials
3.1.2. Structure of Cemented Carbide Specimens

3.2. Sample Corrosion Performance Analysis
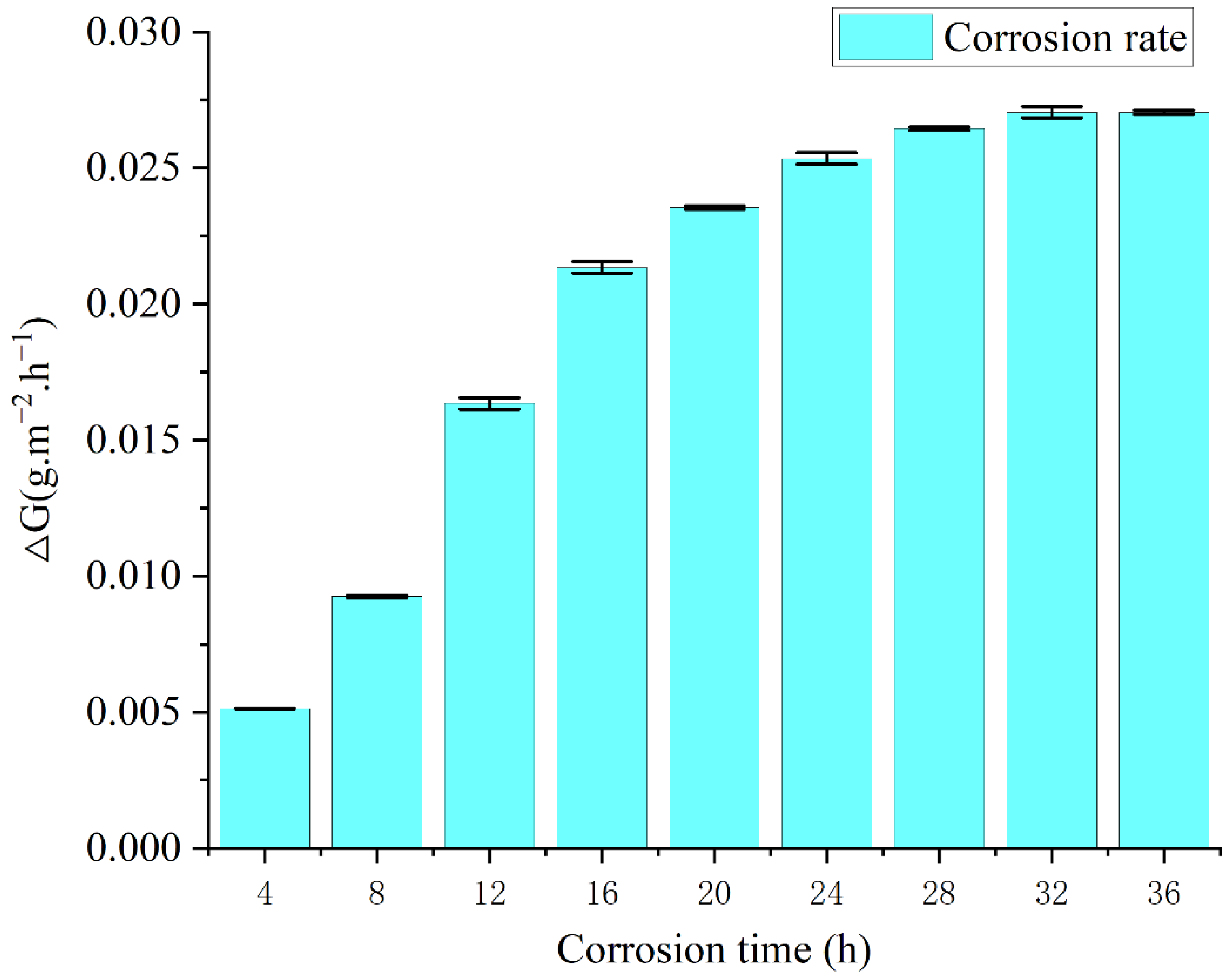
3.2.1. Corrosion Morphology and Rate

| Sample | m0 (g) | m1 (g) | Corrosion Time (h) |
|---|---|---|---|
| Y33 | 8.795 | 8.7949 | 4 |
| Y33 | 8.795 | 8.7946 | 8 |
| Y33 | 8.795 | 8.7940 | 12 |
| Y33 | 8.795 | 8.7933 | 16 |
| Y33 | 8.795 | 8.7926 | 20 |
| Y33 | 8.795 | 8.7919 | 24 |
| Y33 | 8.795 | 8.7913 | 28 |
| Y33 | 8.795 | 8.7907 | 32 |
| Y33 | 8.795 | 8.7901 | 36 |
3.2.2. SEM and EDS Analysis of Corroded WC-Co Surface
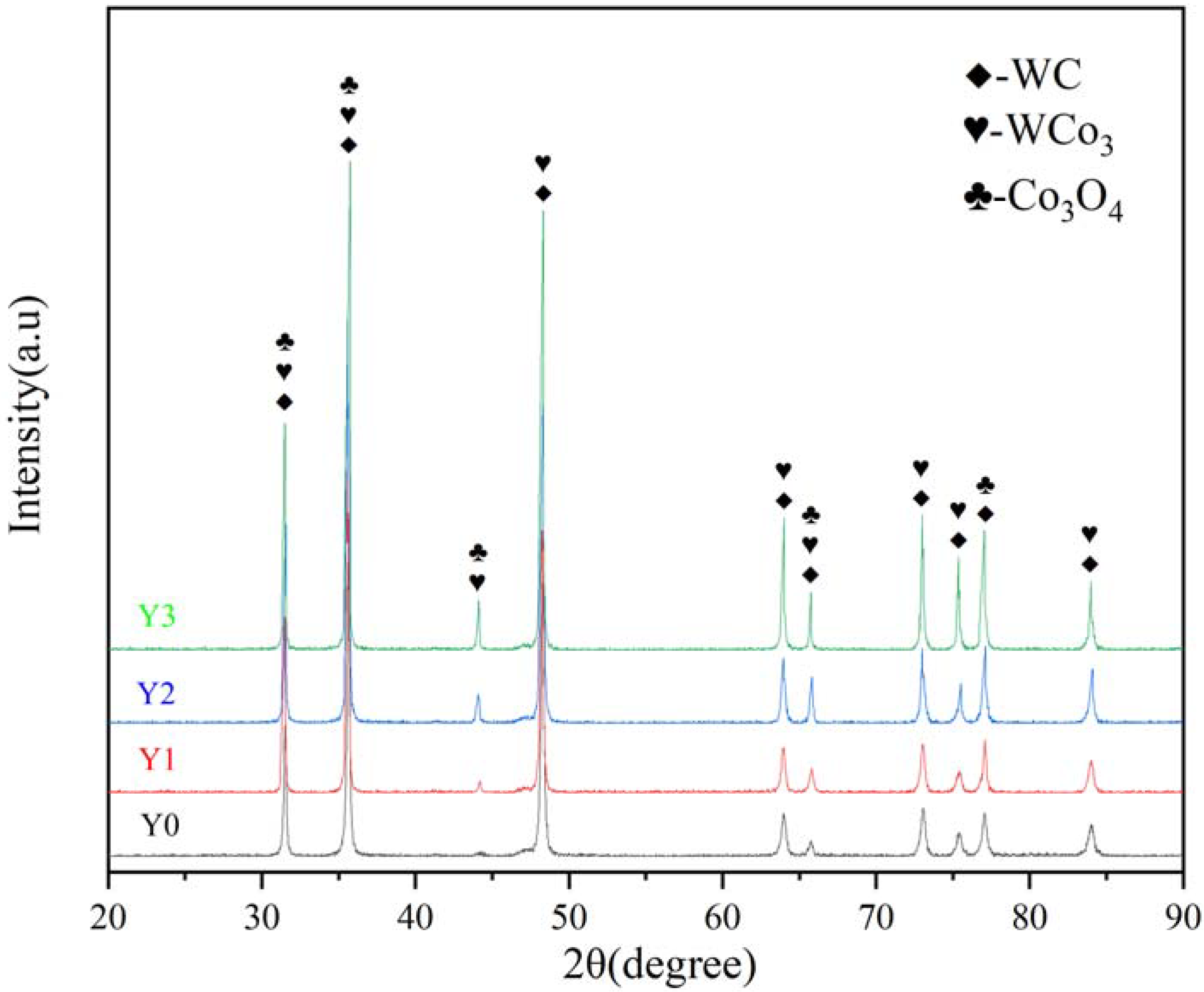
3.2.3. XRD Analysis
3.3. Erosion Corrosion Behavior of WC-Co Materials
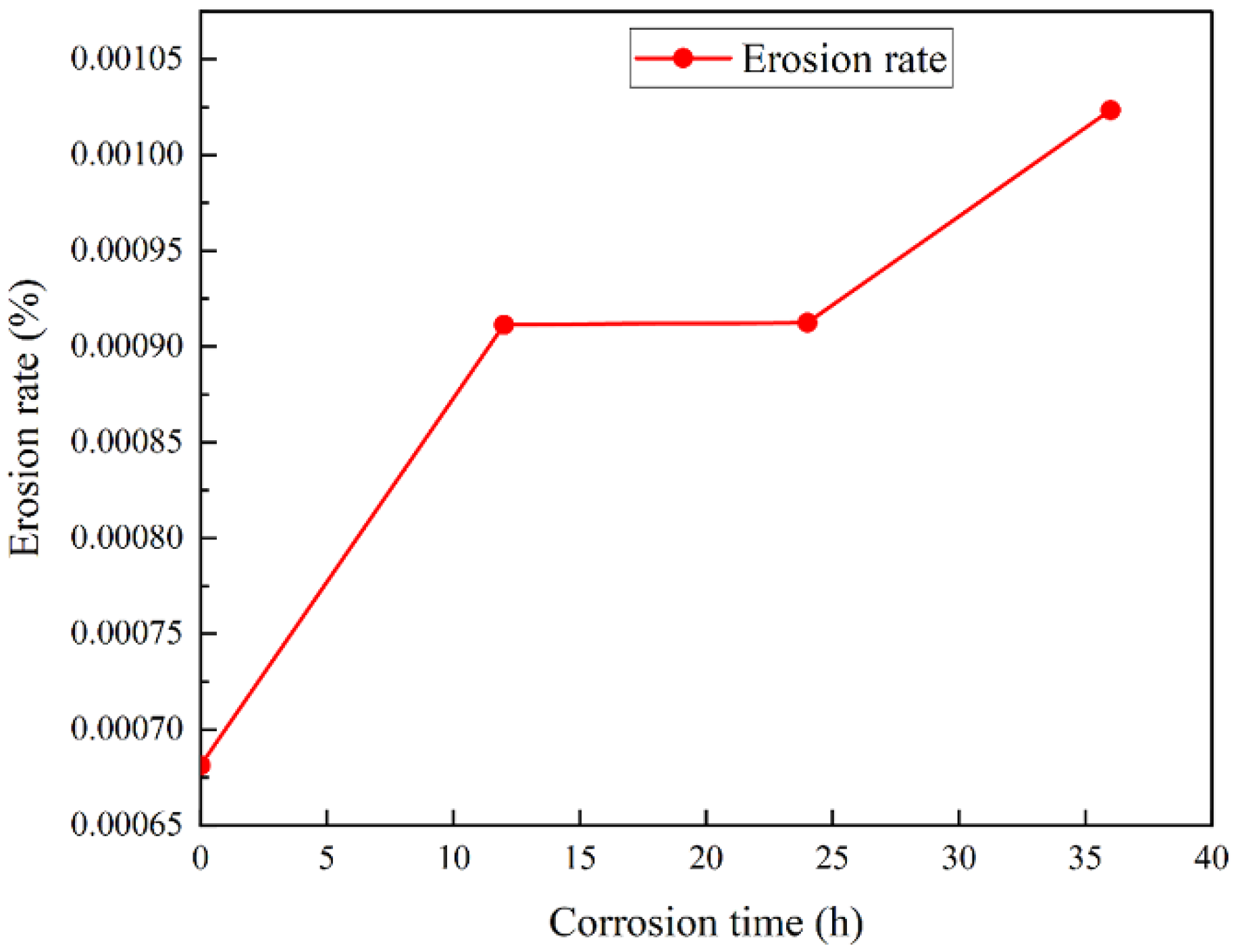
3.3.1. Erosion Morphology and Rate

3.3.2. SEM Analysis of the Erosion Morphology
4. Conclusions
- (1)
- The research and quality control of the mixed powder used for the sintering enabled the sintering of the carbide samples with a carbon content and grain size that met the requirements, and the measured coercive magnetic force and magnetic saturation strength were 17.89 KA/m and 14.42 G·cm3/g, respectively, meeting the carbide production standards.
- (2)
- The acidic copper-accelerated salt spray corrosion experiments on cemented carbide showed that the corrosion rate was different with different corrosion time periods. The corrosion rate was fast at the 4–16 h stage, and the generation of corrosion in the corrosion process slowed down the corrosion rate. A large number of rust spots and corrosion holes appeared on the surface during the corrosion process and generated corrosion products of WCo3 and Co3O4, which caused a large amount of cobalt and a small amount of tungsten oxidation loss.
- (3)
- After corrosion, the erosion experiments show that the corrosion of the carbide had a great impact on the anti-corrosion performance. The longer the corrosion time was, the greater the erosion rate was, reaching 0.00104. The long corrosion time led to surface loosening, which reduced the erosion resistance. Erosion occurred after a large number of erosion pits formed, and surface flaking seriously affected the durability and accuracy of the material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corrosion Inhibitors in the Oil and Gas Industry; John Wiley & Sons: Hoboken, NJ, USA, 2020.
- Zhang, W.; Zhang, S.; Dai, W.; Wang, X.; Liu, B. Erosion wear characteristics of cemented carbide for mud pulser rotor. Int. J. Refract. Met. Hard Mater. 2021, 101, 105666. [Google Scholar] [CrossRef]
- Berro, M.J.; Reich, M. Laboratory investigations of a hybrid mud pulse telemetry (HMPT)—A new approach for speeding up the transmitting of MWD/LWD data in deep boreholes. J. Pet. Sci. Eng. 2019, 183, 106374. [Google Scholar] [CrossRef]
- Yan, Z.; Geng, Y.; Wei, C.; Wang, T.; Gao, T.; Shao, J.; Hu, X.; Yuan, M. Design of a continuous wave mud pulse generator for data transmission by fluid pressure fluctuation. Flow Meas. Instrum. 2018, 59, 28–36. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Maurya, R.; Singh, P.K. Failure Behavior of Cemented Tungsten Carbide Materials: A Case Study of Mining Drill Bits. J. Mater. Eng. Perform. 2021, 30, 6090–6106. [Google Scholar] [CrossRef]
- Katiyar, P.K. A comprehensive review on synergy effect between corrosion and wear of cemented tungsten carbide tool bits: A mechanistic approach. Int. J. Refract. Met. Hard Mater. 2020, 92, 105315. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Randhawa, N.S. Corrosion behavior of WC-Co tool bits in simulated (concrete, soil, and mine) solutions with and without chloride additions. Int. J. Refract. Met. Hard Mater. 2019, 85, 105062. [Google Scholar] [CrossRef]
- Mohammad, E.; Yuan, P.; Muhammad, S.; Zhang, H. Erosion and Structural Integrity of Mud Pulse Telemetry Tools: Numerical Simulation and Field Studies. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2019. [Google Scholar] [CrossRef]
- Prakash, L.J. Application of fine grained tungsten carbide based cemented carbides. Int. J. Refract. Met. Hard Mater. 1995, 13, 257–264. [Google Scholar] [CrossRef]
- Guo, S.; Bao, R.; Yang, J.; Chen, H.; Yi, J. Effect of Mo and Y2O3 additions on the microstructure and properties of fine WC-Co cemented carbides fabricated by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2017, 69, 1–10. [Google Scholar] [CrossRef]
- Zhu, E.; Zhang, J.; Guo, S.; Yang, X.; Zhang, X.; Yang, J. Effect of Co on morphology and preparation of in situ synthesis of WC-Co composite powders. Mater. Res. Express 2019, 6, 86522. [Google Scholar] [CrossRef]
- Shengda, G.; Tao, S.; Rui, B.; Jiangao, Y.; Jianhong, Y. Synthesis and Characterization of WC-6Co Nanocrystalline Composite Powder. Rare Met. Mater. Eng. 2018, 47, 1986–1992. [Google Scholar] [CrossRef]
- Zhang, F.L.; Wang, C.Y.; Zhu, M. Nanostructured WC/Co composite powder prepared by high energy ball milling. Scr. Mater. 2003, 49, 1123–1128. [Google Scholar] [CrossRef]
- Bonache, V.; Salvador, M.D.; Busquets, D.; Burguete, P.; Martínez, E.; Sapiña, F.; Sánchez, E. Synthesis and processing of nanocrystalline tungsten carbide: Towards cemented carbides with optimal mechanical properties. Int. J. Refract. Met. Hard Mater. 2011, 29, 78–84. [Google Scholar] [CrossRef]
- Trends in Oil and Gas Corrosion Research and Technologies: Production and Transmission; Woodhead Publishing: Cambridge, UK, 2017.
- Wu, Z.; Long, J.; Jin, P. Effect of nickel, chromium on the acid salt spray corrosion performance of cemented carbide. Cem. Carbide 2019, 36, 277–282. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, Y.; Yi, D. Corrosion properties of WC-9Ni-0.57Cr cemented carbide in simulated seawater. J. Mater. Sci. Eng. 2008, 5, 750–753. (In Chinese) [Google Scholar]
- Human, A.M.; Exner, H.E. The relationship between electrochemical behaviour and in-service corrosion of WC based cemented carbides. Int. J. Refract. Met. Hard Mater. 1997, 15, 65–71. [Google Scholar] [CrossRef]
- Guo, S.; Bao, R.; Li, S.; Ye, Y.; Zhu, E.; Wang, W.; Ye, Y. The role of Y2O3, Cu, Mo and Mo2C additives on optimizing the corrosion resistance of WC-6Co cemented carbide in HCl and NaOH solutions. J. Alloy. Compd. 2020, 827, 154269. [Google Scholar] [CrossRef]
- Beste, U.; Hammerström, L.; Engqvist, H.; Rimlinger, S.; Jacobson, S. Particle erosion of cemented carbides with low Co content. Wear 2001, 250, 809–817. [Google Scholar] [CrossRef]
- Wang, G.; Lu, X.; Ding, M.; Liu, Y.; Tang, W.; Zhang, B. Diamond coatings deposited on cemented carbide substrates with SiC as interlayers: Preparation and erosion resistance tests. Diam. Relat. Mater. 2017, 73, 105–113. [Google Scholar] [CrossRef]
- McAuliffe, C.D. Crude-Oil-Water Emulsions to Improve Fluid Flow in an Oil Reservoir. J. Pet. Technol. 1973, 25, 721–726. [Google Scholar] [CrossRef]
- Camargo, R.; Palermo, T. Rheological properties of hydrate suspensions in an asphaltenic crude oil. In Proceedings of the 4th International Conference on Gas Hydrates, Yokohama, Japan, 19–23 May 2002; Volume 1, pp. 880–885. [Google Scholar]
- Ansell, T.Y.; Hanneman, T.; Gonzalez-Perez, A.; Park, C.; Nieto, A. Effect of high energy ball milling on spherical metallic powder particulates for additive manufacturing. Part. Sci. Technol. 2021, 39, 981–989. [Google Scholar] [CrossRef]
- Dai, Y. Fabricating Theory and Super High Temperature Atomizing Mechanism for Spherical Tungsten Carbide; Central South University: Changsha, China, 2008; pp. 24–89. [Google Scholar]
- Zhao, W. Study on the Gas Flow Field in Spray Deposition Deposition and the Breakup Mechanism; Harbin Institute of Technology: Harbin, China, 2012; pp. 71–90. [Google Scholar]
- Bor, A.; Jargalsaikhan, B.; Uranchimeg, K.; Lee, J.; Choi, H. Particle morphology control of metal powder with various experimental conditions using ball milling. Powder Technol. 2021, 394, 181–190. [Google Scholar] [CrossRef]
- Rios, J.; Restrepo, A.; Zuleta, A.; Bolívar, F.; Castaño, J.; Correa, E.; Echeverria, F. Effect of Ball Size on the Microstructure and Morphology of Mg Powders Processed by High-Energy Ball Milling. Metals 2021, 11, 1621. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Zhang, Y. Study on the flowability of titanium alloy powder. Powder Metall. Technol. 2016, 34, 330–335. [Google Scholar] [CrossRef]
- Leturia, M.; Benali, M.; Lagarde, S.; Ronga, I.; Saleh, K. Characterization of flow properties of cohesive powders: A comparative study of traditional and new testing methods. Powder Technol. 2014, 253, 406–423. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Chen, Y.; Feng, P.; Lai, H.; Li, J.; Luo, X. Novel Co3O4 Nanoparticles/Nitrogen-Doped Carbon Composites with Extraordinary Catalytic Activity for Oxygen Evolution Reaction (OER). Nano-Micro Lett. 2017, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Frykholm, R.; Ekroth, M.; Jansson, B. Effect of cubic phase composition on gradient zone formation in cemented carbides. Int. J. Refract. Met. Hard Mater. 2001, 19, 527–538. [Google Scholar] [CrossRef]
- Zhang, W.; Du, Y.; Peng, Y. Effect of TaC and NbC addition on the microstructure and hardness in graded cemented carbides: Simulations and experiments. Ceram. Int. 2016, 42, 428–435. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Q.; Zhu, Q.; Xiao, S.; Xu, C.; Yang, L.; Zheng, B.; Mao, S.; Song, Z. Formation of anti-shell/core structure of heavy rare earth elements (Tb, Dy) in sintered Nd-Fe-B magnet after grain boundary diffusion process. Scr. Mater. 2019, 163, 40–43. [Google Scholar] [CrossRef]




| Chemical Composition | Physical Properties | |||||
|---|---|---|---|---|---|---|
| WC (wt%) | Co (wt%) | Ta (wt%) | Tb (wt%) | Bulk Density (g/cm−3) | Particle Size (μm) | Hall Flow Rate (s/50 g) |
| 90.10 ± 0.05 | 9.85± 0.30 | 0.42 | 0.15 | 3.03 | 0.7 | 40.7 |
| Element | WC | Co | Ta | Tb |
|---|---|---|---|---|
| Wt% | 90.1 | 9.56 | 0.55 | 0.32 |
| Temperature (°C) | Relative Humidity (%) | Salt Mist Pressure (kPa) | Salt Mist Deposition Rate (mL·h−1) | pH |
|---|---|---|---|---|
| 25 ± 2 | 93 ± 3 | 98~112 | 1.0~2.0 | 3.5~4.2 |
| Grain Size (μm) | Density (g·cm−3) | Hardness (GPa) | Erosion Angle | Erosion Times (min) | Erosion Pressure (Mpa) |
|---|---|---|---|---|---|
| 200 | 2.6 | 8.5~9.8 | 60° | 10 | 3.0 ± 0.2 |
| Sample | mb (g) | ma (g) |
|---|---|---|
| Y0 | 8.805 | 8.799 |
| Y12 | 8.777 | 8.769 |
| Y22 | 8.767 | 8.759 |
| Y32 | 8.795 | 8.786 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Li, Y.; Wang, R.; Yang, H.; Guo, Z.; Lin, R.; Huang, Q.; Zhou, Y. Preparation of Cemented Carbide and Study of Copper-Accelerated Salt Spray Corrosion and Erosion Behavior. Materials 2022, 15, 7023. https://doi.org/10.3390/ma15197023
Wei S, Li Y, Wang R, Yang H, Guo Z, Lin R, Huang Q, Zhou Y. Preparation of Cemented Carbide and Study of Copper-Accelerated Salt Spray Corrosion and Erosion Behavior. Materials. 2022; 15(19):7023. https://doi.org/10.3390/ma15197023
Chicago/Turabian StyleWei, Shasha, Yuanyou Li, Renxin Wang, Hu Yang, Ziming Guo, Rongchuan Lin, Qingmin Huang, and Yuhui Zhou. 2022. "Preparation of Cemented Carbide and Study of Copper-Accelerated Salt Spray Corrosion and Erosion Behavior" Materials 15, no. 19: 7023. https://doi.org/10.3390/ma15197023





