A Two-Step Approach to Tune the Micro and Nanoscale Morphology of Porous Niobium Oxide to Promote Osteointegration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Anodic Oxidation
2.3. Etching
2.4. Surface Characterization Methods
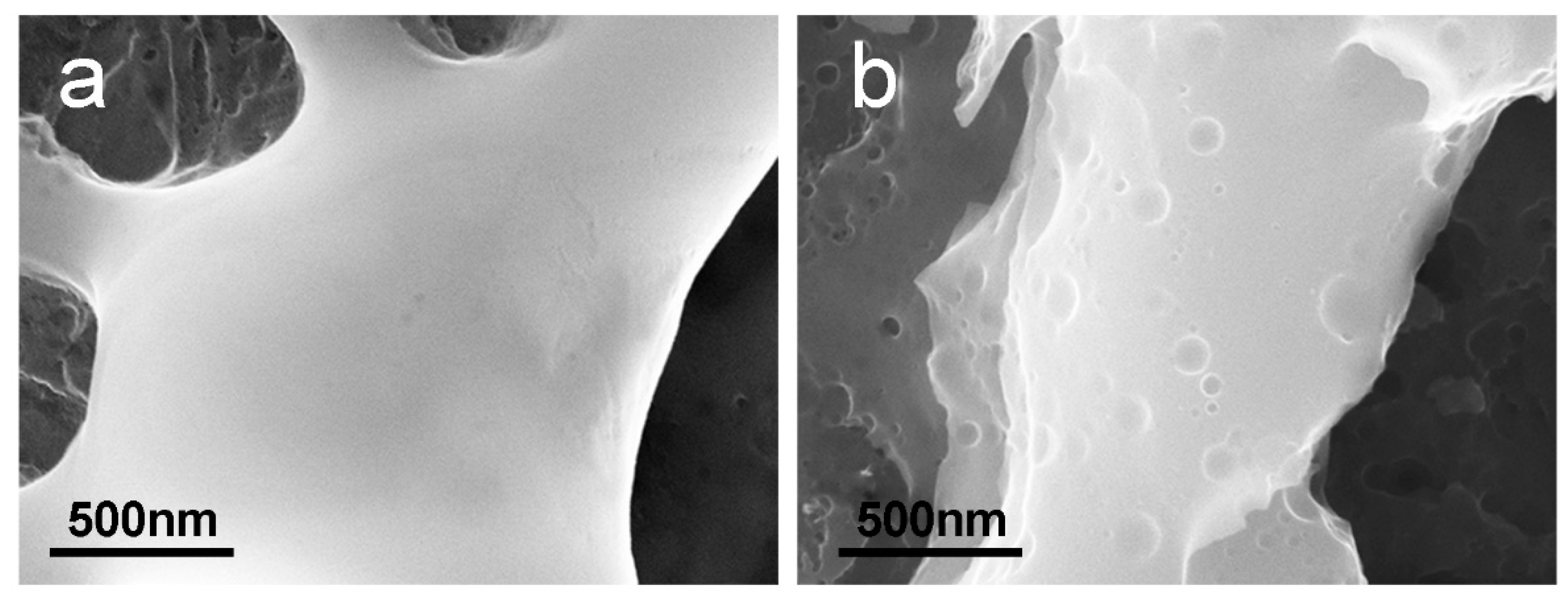
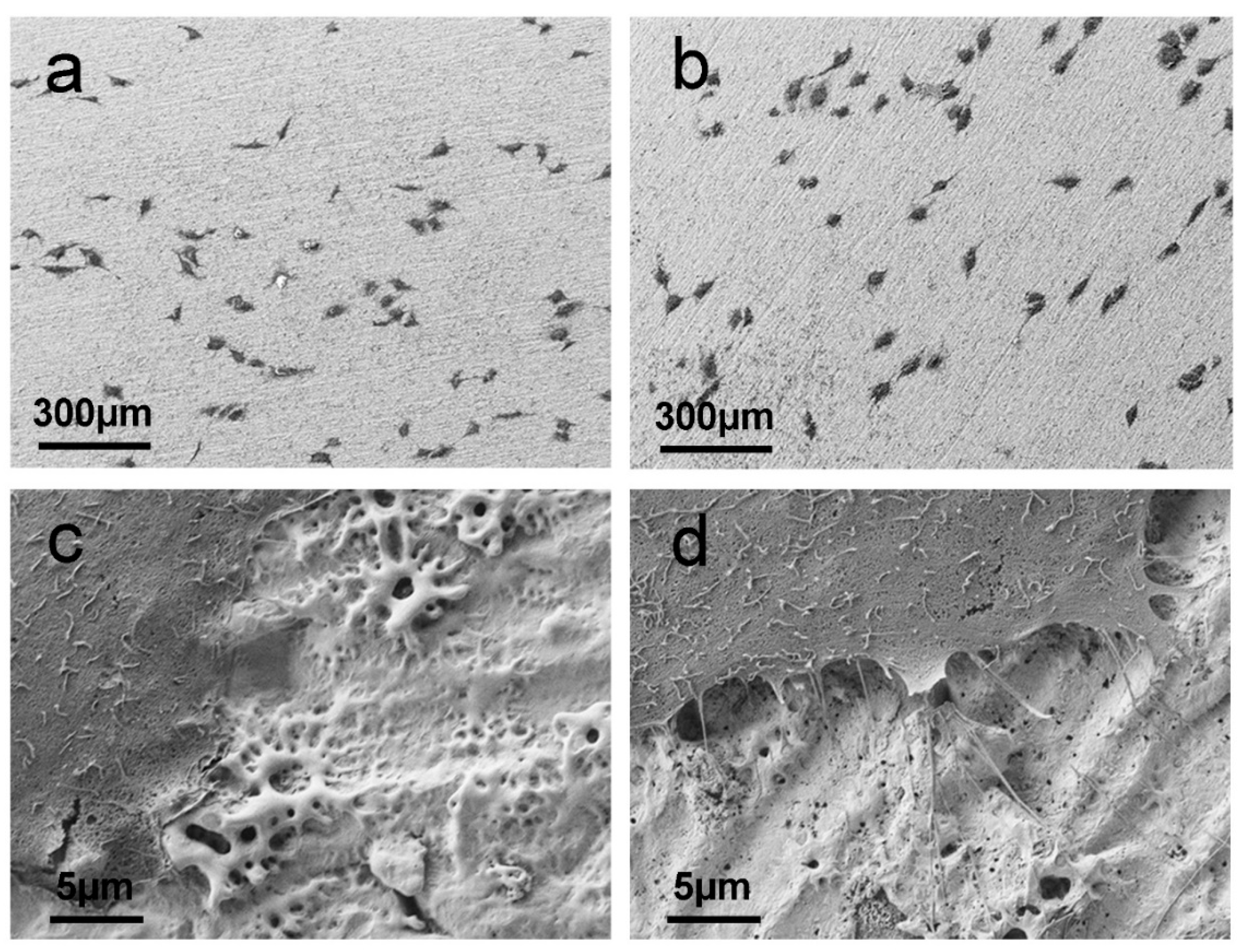
2.4.1. Surface Analysis (SEM)
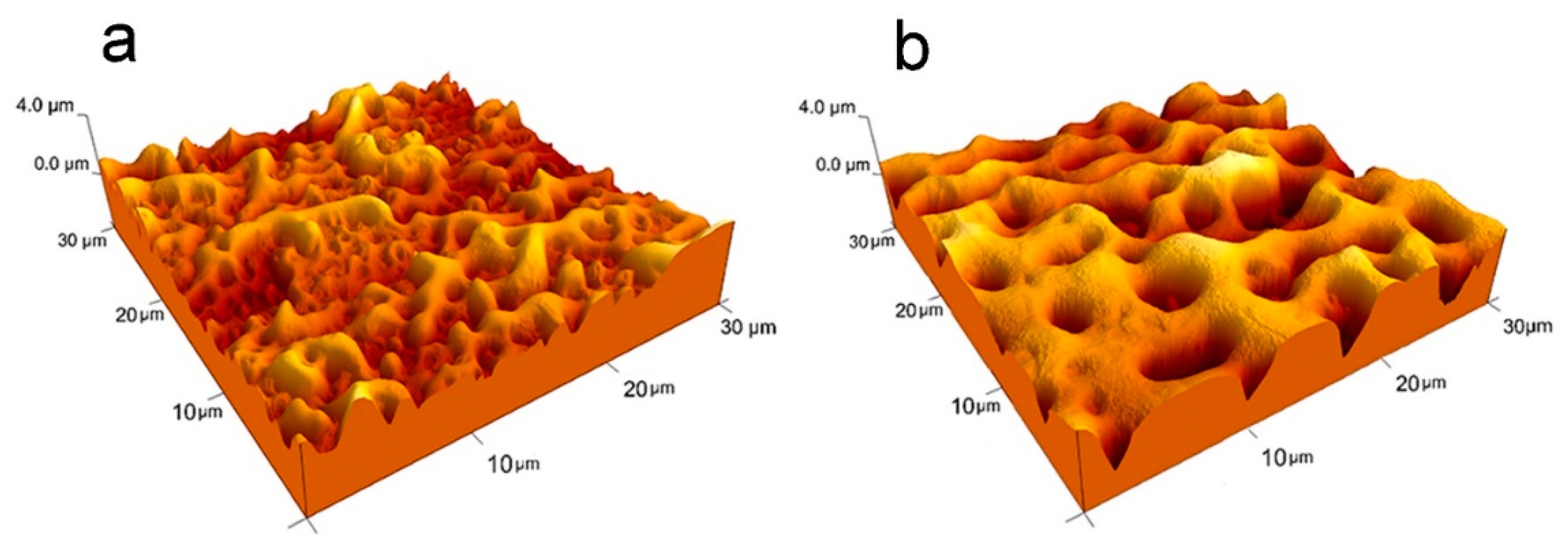
2.4.2. Roughness Analysis (AFM)
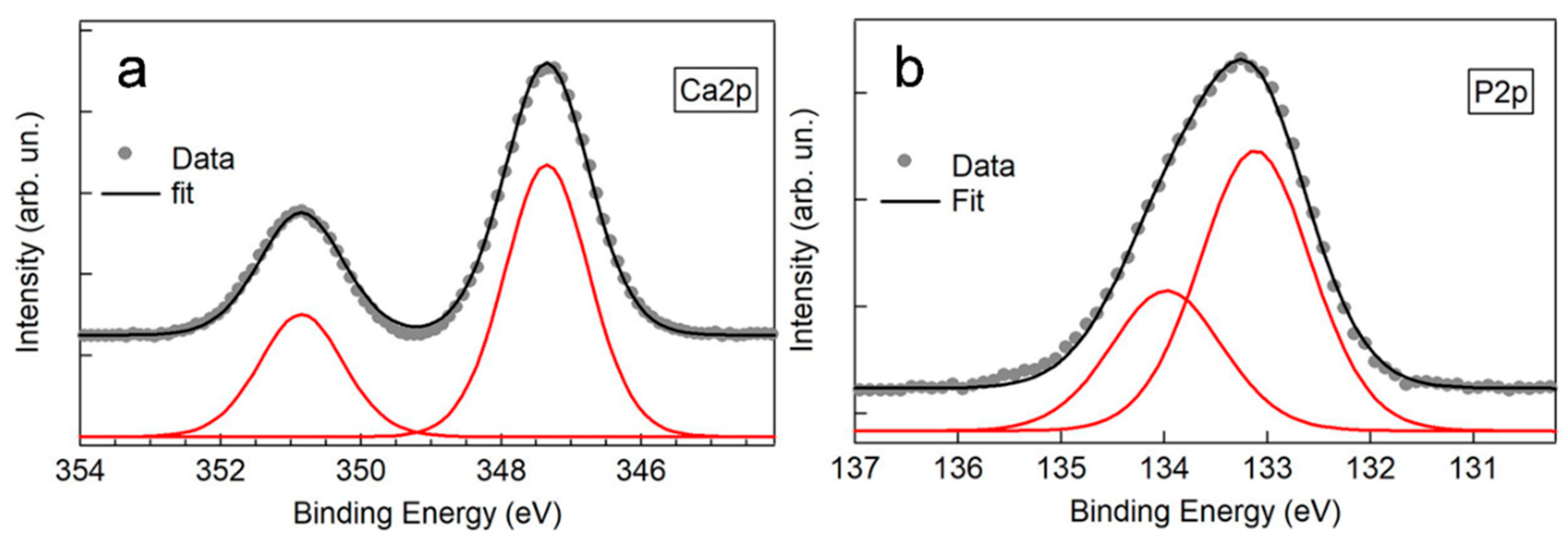
2.4.3. Chemical Analysis (XPS)
2.5. Cell Culture
3. Results
3.1. Anodizing
3.2. Post-Anodizing Treatments
3.3. In Vitro Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, X.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, J. Effects of Topography and Composition of Titanium Surface Oxides on Osteoblast Responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef]
- Gongadze, E.; Kabaso, D.; Sebastian, B.; Slivnik, T.; Schmuki, P.; Ursula, V.R.; Iglic, A. Adhesion of Osteoblasts to a Nanorough Titanium Implant Surface. Int. J. Nanomedicine. 2011, 6, 1801–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.A.F.; Balderrama, Í.d.F.; Karam, P.S.B.H.; de Oliveira, R.C.; de Oliveira, F.A.; Grandini, C.R.; Vicente, F.B.; Stavropoulos, A.; Zangrando, M.S.R.; Sant’Ana, A.C.P. Surface Roughness of Titanium Disks Influences the Adhesion, Proliferation and Differentiation of Osteogenic Properties Derived from Human. Int. J. Implant. Dent. 2020, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Rokosz, K.; Hryniewicz, T. Comparative SEM and EDX Analysis of Surface Coatings Created on Niobium and Titanium Alloys after Plasma Electrolytic Oxidation (PEO). Technical Gazette 2017, 24, 465–472. [Google Scholar] [CrossRef]
- Pereira, B.L.; Beilner, G.; Lepienski, C.M.; de Souza, G.B.; Kuromoto, N.K.; Szameitat, E.S.; Peng, A.N.S.; Lee, J.Y.; Claro, A.P.R.A.; Nugent, M.J.D. Scratch-Resistant and Well-Adhered Nanotube Arrays Produced via Anodizing Process on β-Titanium Alloy. Mater. Today Commun. 2021, 26, 101947. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Moneta, M.; Norek, M.; Michalska-Domańska, M.; Scarpellini, A.; Salerno, M. The Influence of Electrolyte Composition on the Growth of Nanoporous Anodic Alumina. Electrochim. Acta 2016, 211, 453–460. [Google Scholar] [CrossRef]
- Sowa, M.; Woszczak, M.; Kazek-Kęsik, A.; Dercz, G.; Korotin, D.M.; Zhidkov, I.S.; Kurmaev, E.Z.; Cholakh, S.O.; Basiaga, M.; Simka, W. Influence of Process Parameters on Plasma Electrolytic Surface Treatment of Tantalum for Biomedical Applications. Appl. Surf. Sci. 2017, 407, 52–63. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Han, Y. Structural Characteristics and Outward–Inward Growth Behavior of Tantalum Oxide Coatings on Tantalum by Micro-Arc Oxidation. Surf. Coat. Technol. 2013, 214, 110–116. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Yu, X. Microstructure and Biological Properties of Micro-Arc Oxidation Coatings on ZK60 Magnesium Alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1574–1586. [Google Scholar] [CrossRef]
- Sandhyarani, M.; Prasadrao, T.; Rameshbabu, N. Role of Electrolyte Composition on Structural, Morphological and in-Vitro Biological Properties of Plasma Electrolytic Oxidation Films Formed on Zirconium. Appl. Surf. Sci. 2014, 317, 198–209. [Google Scholar] [CrossRef]
- Sri Maha Vishnu, D.; Sure, J.; Liu, Y.; Vasant Kumar, R.; Schwandt, C. Electrochemical Synthesis of Porous Ti-Nb Alloys for Biomedical Applications. Mater. Sci. Eng. C 2019, 96, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Trincă, L.C.; Mareci, D.; Solcan, C.; Fântânariu, M.; Burtan, L.; Hriţcu, L.; Chiruţă, C.; Fernández-Mérida, L.; Rodríguez-Raposo, R.; Santana, J.J.; et al. New Ti-6Al-2Nb-2Ta-1Mo Alloy as Implant Biomaterial: In Vitro Corrosion and in Vivo Osseointegration Evaluations. Mater. Chem. Phys. 2020, 240, 122229. [Google Scholar] [CrossRef]
- Michalska, J.; Sowa, M.; Piotrowska, M.; Widziołek, M.; Tylko, G.; Dercz, G.; Socha, R.P.; Osyczka, A.M.; Simka, W. Incorporation of Ca Ions into Anodic Oxide Coatings on the Ti-13Nb-13Zr Alloy by Plasma Electrolytic Oxidation. Mater. Sci. Eng. C 2019, 104, 109957. [Google Scholar] [CrossRef]
- Caetano, G.L.; dos Santos Junior, J.; Pereira, B.L.; Benegra, M. One-Step Plasma Electrolytic Oxidation in Ti-30Nb-8Zr, Ti, and Nb Surfaces. Surf. Eng. 2021, 37, 1467–1475. [Google Scholar] [CrossRef]
- Quintero, D.; Gómez, M.A.; Araujo, W.S.; Echeverría, F.; Calderón, J.A. Influence of the Electrical Parameters of the Anodizing PEO Process on Wear and Corrosion Resistance of Niobium. Surf. Coat. Technol. 2019, 380, 125067. [Google Scholar] [CrossRef]
- Luz, A.R.; de Lima, G.G.; Santos, E.; Pereira, B.L.; Sato, H.H.; Lepienski, C.M.; Lima, D.B.; Laurindo, C.; Grandini, C.R.; Kuromoto, N.K. Tribo-Mechanical Properties and Cellular Viability of Electrochemically Treated Ti-10Nb and Ti-20Nb Alloys. J. Alloy. Compd. 2019, 779, 129–139. [Google Scholar] [CrossRef]
- Xu, Z.; Yate, L.; Qiu, Y.; Aperador, W.; Coy, E.; Jiang, B.; Moya, S.; Wang, G.; Pan, H. Potential of Niobium-Based Thin Films as a Protective and Osteogenic Coating for Dental Implants: The Role of the Nonmetal Elements. Mater. Sci. Eng. C 2019, 96, 166–175. [Google Scholar] [CrossRef]
- Pereira, B.L.; Lepienski, C.M.; Mazzaro, I.; Kuromoto, N.K. Apatite Grown in Niobium by Two-Step Plasma Electrolytic Oxidation. Mater. Sci. Eng. C 2017, 77, 1235–1241. [Google Scholar] [CrossRef]
- Pereira, B.L.; da Luz, A.R.; Lepienski, C.M.; Mazzaro, I.; Kuromoto, N.K. Niobium Treated by Plasma Electrolytic Oxidation with Calcium and Phosphorus Electrolytes. J. Mech. Behav. Biomed. Mater. 2018, 77, 347–352. [Google Scholar] [CrossRef]
- Sowa, M.; Worek, J.; Dercz, G.; Korotin, D.M.; Kukharenko, A.I.; Kurmaev, E.Z.; Cholakh, S.O.; Basiaga, M.; Simka, W. Surface Characterisation and Corrosion Behaviour of Niobium Treated in a Ca- and P-Containing Solution under Sparking Conditions. Electrochim. Acta 2016, 198, 91–103. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Electrochemical Behavior of Plasma Electrolytically Oxidized Niobium in Simulated Physiological Environment. Surf. Coat. Technol. 2018, 344, 121–131. [Google Scholar] [CrossRef]
- Canepa, P.; Ghiara, G.; Spotorno, R.; Canepa, M.; Cavalleri, O. Structural vs. Electrochemical Investigation of Niobium Oxide Layers Anodically Grown in a Ca and P Containing Electrolyte. J. Alloy. Compd. 2021, 851, 156937. [Google Scholar] [CrossRef]
- Canepa, P.; Firpo, G.; Mattera, L.; Canepa, M.; Cavalleri, O. Calcium and Phosphorous Enrichment of Porous Niobium and Titanium Oxides for Biomaterial Applications. Surf. Coat. Technol. 2020, 389, 125634. [Google Scholar] [CrossRef]
- Yu, M.; Wan, Y.; Ren, B.; Wang, H.; Zhang, X.; Qiu, C.; Liu, A.; Liu, Z. 3D Printed Ti–6Al–4V Implant with a Micro/Nanostructured Surface and Its Cellular Responses. ACS Omega 2020, 5, 31738–31743. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Nanostructured Niobium Oxide Coatings Influence Osteoblast Adhesion. J. Biomed. Mater. Res. 2006, 79A, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xu, S.; Shen, M.; Cheng, B.; Li, Y.; Liu, X.; Qin, D.; Bellare, A.; Kong, L. Osteogenic Activity of Titanium Surfaces with Hierarchical Micro/Nano-Structures Obtained by Hydrofluoric Acid Treatment. Int. J. Nanomedicine. 2017, 12, 1317–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qadir, M.; Lin, J.; Biesiekierski, A.; Li, Y.; Wen, C. Effect of Anodized TiO2–Nb2O5–ZrO2 Nanotubes with Different Nanoscale Dimensions on the Biocompatibility of a Ti35Zr28Nb Alloy. ACS Appl. Mater. Interfaces 2020, 12, 6776–6787. [Google Scholar] [CrossRef] [PubMed]
- Lauria, I.; Kutz, T.N.; Böke, F.; Rütten, S.; Zander, D.; Fischer, H. Influence of Nanoporous Titanium Niobium Alloy Surfaces Produced via Hydrogen Peroxide Oxidative Etching on the Osteogenic Differentiation of Human Mesenchymal Stromal Cells. Mater. Sci. Eng. C 2019, 98, 635–648. [Google Scholar] [CrossRef]
- Variola, F.; Yi, J.-H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the Surface Properties of Ti6Al4V by Controlled Chemical Oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef]
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants. Materials 2017, 10, 1302. [Google Scholar] [CrossRef] [Green Version]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The Relationship between the Nanostructure of Titanium Surfaces and Bacterial Attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A Review of the Biomaterials Technologies for Infection-Resistant Surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Encinas, N.; Yang, C.-Y.; Geyer, F.; Kaltbeitzel, A.; Baumli, P.; Reinholz, J.; Mailänder, V.; Butt, H.-J.; Vollmer, D. Submicrometer-Sized Roughness Suppresses Bacteria Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 21192–21200. [Google Scholar] [CrossRef]
- Cao, Y.; Su, B.; Chinnaraj, S.; Jana, S.; Bowen, L.; Charlton, S.; Duan, P.; Jakubovics, N.S.; Chen, J. Nanostructured Titanium Surfaces Exhibit Recalcitrance towards Staphylococcus Epidermidis Biofilm Formation. Sci. Rep. 2018, 8, 1071. [Google Scholar] [CrossRef]
- Demirci, S.; Dikici, T.; Güllüoğlu, A.N. Micro/Nanoscale Surface Modification of Ti6Al4V Alloy for Implant Applications. J. Mater. Eng. Perform. 2021. [Google Scholar] [CrossRef]
- Kohler, R.; Sowards, K.; Medina, H. Numerical Model for Acid-Etching of Titanium: Engineering Surface Roughness for Dental Implants. J. Manuf. Process. 2020, 59, 113–121. [Google Scholar] [CrossRef]
- Doe, Y.; Ida, H.; Seiryu, M.; Deguchi, T.; Takeshita, N.; Sasaki, S.; Sasaki, S.; Irie, D.; Tsuru, K.; Ishikawa, K.; et al. Titanium Surface Treatment by Calcium Modification with Acid-Etching Promotes Osteogenic Activity and Stability of Dental Implants. Materialia 2020, 12, 100801. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.-F.; Yin, M.; Ren, L.-F.; Lin, H.; Shi, G. Effect of Heat Treatment on H2O2/HCl Etched Pure Titanium Dental Implant: An in Vitro Study. Med. Sci Monit. 2012, 18, BR265–BR272. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Ren, L.; Wang, L.; Lin, H.; Wang, S.; Tong, Y. H2O2/HCl and Heat-Treated Ti-6Al-4V Stimulates Pre-Osteoblast Proliferation and Differentiation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 368–375. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, C.; Deng, J.; Xu, R.; Yang, Y.; Deng, F. Micro/Nano Topography of Selective Laser Melting Titanium Inhibits Osteoclastogenesis via Mediation of Macrophage Polarization. Biochem. Biophys. Res. Commun. 2021, 581, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Takai, M.; Kim, H.-M.; Ishihara, K. Preparation of Nano-Structured Titanium Oxide Film for Biosensor Substrate by Wet Corrosion Process. Curr. Appl. Phys. 2009, 9, e266–e269. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Matsuno, R.; Ishihara, K.; Takai, M. Electrical Transport Ability of Nanostructured Potassium-Doped Titanium Oxide Film. Appl. Phys. Express 2011, 4, 025803. [Google Scholar] [CrossRef]
- Kim, C.; Kendall, M.R.; Miller, M.A.; Long, C.L.; Larson, P.R.; Humphrey, M.B.; Madden, A.S.; Tas, A.C. Comparison of Titanium Soaked in 5 M NaOH or 5 M KOH Solutions. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Tobimatsu, H.; Maruyama, Y.; Tanaki, T.; Jerkiewicz, G. Preparation and Characterization of Microporous Layers on Titanium. ACS Appl. Mater. Interfaces 2009, 1, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.B.J.G.; Eiras, C.; Bulhões, L.O.S. Breakdown of the Niobium Oxide Film under Galvanostatic Polarisation and in Acid Solutions. Corros. Sci. 2004, 46, 1051–1060. [Google Scholar] [CrossRef]
- Simka, W.; Krząkała, A.; Korotin, D.M.; Zhidkov, I.S.; Kurmaev, E.Z.; Cholakh, S.O.; Kuna, K.; Dercz, G.; Michalska, J.; Suchanek, K.; et al. Modification of a Ti–Mo Alloy Surface via Plasma Electrolytic Oxidation in a Solution Containing Calcium and Phosphorus. Electrochim. Acta 2013, 96, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Frauchiger, V.M.; Schlottig, F.; Gasser, B.; Textor, M. Anodic Plasma-Chemical Treatment of CP Titanium Surfaces for Biomedical Applications. Biomaterials 2004, 25, 593–606. [Google Scholar] [CrossRef]
- Lokeshkumar, E.; Manojkumar, P.; Saikiran, A.; Premchand, C.; Hariprasad, S.; Rama Krishna, L.; Rameshbabu, N. Fabrication of Ca and P Containing Niobium Oxide Ceramic Coatings on Niobium by PEO Coupled EPD Process. Surf. Coat. Technol. 2021, 416, 127161. [Google Scholar] [CrossRef]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Mei, S.; Chu, P.K.; Zhang, Y.; Wu, Z. The Influence of Hierarchical Hybrid Micro/Nano-Textured Titanium Surface with Titania Nanotubes on Osteoblast Functions. Biomaterials 2010, 31, 5072–5082. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Hayakawa, S.; Tsuru, K.; Osaka, A. Bioactive Titania Gel Layers Formed by Chemical Treatment of Ti Substrate with a H2O2/HCl Solution. Biomaterials 2002, 23, 1353–1357. [Google Scholar] [CrossRef]
- Ossowska, A.; Olive, J.-M.; Zieliński, A.; Wojtowicz, A. Effect of Double Thermal and Electrochemical Oxidation on Titanium Alloys for Medical Applications. Appl. Surf. Sci. 2021, 563, 150340. [Google Scholar] [CrossRef]

| Anodizing Conditions | Galvanostatic | 60 s | 300 s | 30 min |
|---|---|---|---|---|
| 200 V | 480 | 570 | 550 | 660 |
| 250 V | 710 | 820 | 620 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canepa, P.; Firpo, G.; Gatta, E.; Spotorno, R.; Giannoni, P.; Quarto, R.; Canepa, M.; Cavalleri, O. A Two-Step Approach to Tune the Micro and Nanoscale Morphology of Porous Niobium Oxide to Promote Osteointegration. Materials 2022, 15, 473. https://doi.org/10.3390/ma15020473
Canepa P, Firpo G, Gatta E, Spotorno R, Giannoni P, Quarto R, Canepa M, Cavalleri O. A Two-Step Approach to Tune the Micro and Nanoscale Morphology of Porous Niobium Oxide to Promote Osteointegration. Materials. 2022; 15(2):473. https://doi.org/10.3390/ma15020473
Chicago/Turabian StyleCanepa, Paolo, Giuseppe Firpo, Elena Gatta, Roberto Spotorno, Paolo Giannoni, Rodolfo Quarto, Maurizio Canepa, and Ornella Cavalleri. 2022. "A Two-Step Approach to Tune the Micro and Nanoscale Morphology of Porous Niobium Oxide to Promote Osteointegration" Materials 15, no. 2: 473. https://doi.org/10.3390/ma15020473







