Laser-Based Synthesis of TiO2-Pt Photocatalysts for Hydrogen Generation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Material Characterization
2.3. Studies of Photocatalytic HER Activity
3. Results and Discussion
3.1. Structure and Optical Properties of Samples
3.2. Photocatalytic Activity of Titania Prepared by PLAL
3.3. Effect of Pt Addition on the Photocatalytic Activity of Titania Obtained by PLAL
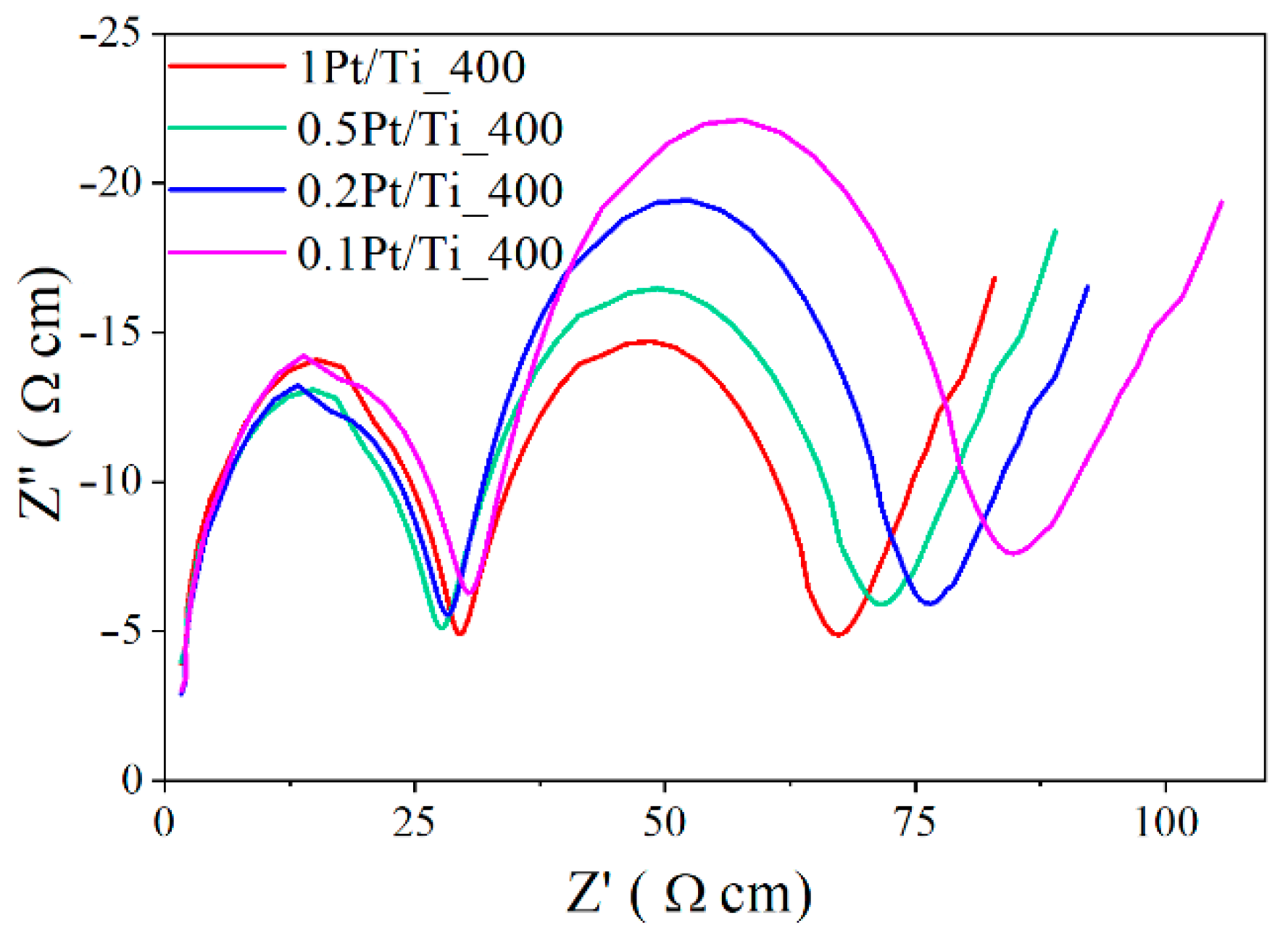
3.4. Electrochemical Analysis of the Photocatalytic Activity of Dark Pt/TiO2 Obtained by PLAL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, F.; Du, M.; Dai, L.; Qian, Y.; Pang, H. Review on synthesis of porous TiO2-based catalysts for energy conversion systems. Ceramics 2021, 47, 25177–25200. [Google Scholar] [CrossRef]
- Dominguez-Espindola, R.B.; Arias, D.M.; Rodriguez-Gonzalez, C.; Sebastian, P.J. A critical review on advances in TiO2-based photocatalytic systems for CO2 reduction. Appl. Therm. Eng. 2022, 216, 119009. [Google Scholar] [CrossRef]
- Ismael, M. Latest progress on the key operating parameters affecting the photocatalytic activity of TiO2-based photocatalysts for hydrogen fuel production: A comprehensive review. Fuel 2021, 303, 121207. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Diaz, L.; Rodriguez, V.D.; Gonzalez-Rodriguez, M.; Rodriguez-Castellon, E.; Algarra, M.; Nunez, P.; Moretti, E. M/TiO2 (M = Fe, Co, Ni, Cu, Zn) catalysts for photocatalytic hydrogen production under UV and visible light irradiation. Inorg. Chem. Front. 2021, 8, 3491–3500. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Esrafili, A.; Salimi, M.; Jafari, A.J.; Sobhi, H.R.; Gholami, M.; Kalantary, R.R. Pt-based TiO2 photocatalytic systems: A systematic review. J. Mol. Liq. 2022, 352, 118685. [Google Scholar] [CrossRef]
- Nur, A.S.M.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V.; Kuznetsov, V.N.; Ryabchuk, V.K. Second Generation Visible-Light-Active Photocatalysts: Preparation, Optical Properties, and Consequences of Dopants on the Band Gap Energy of TiO2. In Environmentally Benign Photocatalysts. Nanostructure Science and Technology; Anpo, M., Kamat, P., Eds.; Springer: New York, NY, USA, 2010; pp. 35–111. [Google Scholar] [CrossRef]
- Shabalina, A.; Fakhrutdinova, E.; Chen, Y.-W.; Lapin, I. Preparation of Gold-Modified F,N-TiO2 Visible Light Photocatalysts and Their Structural Features Comparative Analysis. J. Sol. Gel. Sci. Technol. 2015, 75, 617–624. [Google Scholar] [CrossRef]
- Dai, L.; Sun, F.; Fan, Q.; Li, H.; Yang, K.; Guo, T.; Zheng, L.; Fu, P. Carbon-based titanium dioxide materials for hydrogen production in water-methanol reforming: A review. J. Environ. Chem. Eng. 2022, 10, 107326. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Hezam, A.; Hossain, M.K.; Qamar, M.; Yamani, Z.H.; Byrappa, K. A novel Cs2O–Bi2O3–TiO2–ZnO heterostructure with direct Z-Scheme for efficient photocatalytic water splitting. Ceramics 2019, 45, 23756–23764. [Google Scholar] [CrossRef]
- Al-Ahmed, A. Photocatalytic properties of graphitic carbon nitrides (g-C3N4) for sustainable green hydrogen production: Recent advancement. Fuel 2022, 316, 123381. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, F.; Li, Y. A review on the effects of TiO2 surface point defects on CO2 photoreduction with H2O. J. Mater. 2017, 3, 17–32. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, X.; Zhang, Y.; Gao, J.; Chen, Z.; Lu, Z. Synergism of oxygen vacancies, Ti3+ and N dopants on the visible-light photocatalytic activity of N-doped TiO2. J. Photochem. Photobiol. A 2019, 382, 111928. [Google Scholar] [CrossRef]
- Lira, E.; Wendt, S.; Huo, P.P.; Hansen, J.; Streber, R.; Porsgaard, S.; Wei, Y.Y.; Bechstein, R.; Laegsgaard, E.; Besenbacher, F. The importance of bulk Ti3+ defects in the oxygen chemistry on titania surfaces. J. Am. Chem. Soc. 2011, 133, 6529–6532. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Gao, S.; Dai, G.; Ren, J.; Wang, D. Structure and photocatalytic activity of Ti3+ self-doped TiO2 flower shaped nanospheres. Surf. Interfaces 2020, 18, 100426. [Google Scholar] [CrossRef]
- Xiong, L.-B.; Li, J.-L.; Yang, B.; Yu, Y. Ti3+ in the Surface of Titanium Dioxide: Generation, Properties and Photocatalytic Application. J. Nanomater. 2011, 2012, 831524. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tian, H.; Wang, X.; Xue, G.; Tian, Z.; Zhang, J.; Yuan, S.; Yu, T.; Zou, Z. The role of oxygen vacancy-Ti3+ states on TiO2 nanotubes’ surface in dye-sensitized solar cells. Mater. Lett. 2013, 100, 51–53. [Google Scholar] [CrossRef]
- Xing, M.; Fang, W.; Nasir, M.; Ma, Y.; Zhang, J.; Anpo, M. Self-doped Ti3+-enhanced TiO2 nanoparticles with a high-performance photocatalysis. J. Catal. 2013, 297, 236–243. [Google Scholar] [CrossRef]
- Wanga, J.; Yang, P.; Huang, B. Self-doped TiO2−x nanowires with enhanced photocatalytic activity: Facile synthesis and effects of the Ti3+. Appl. Surf. Sci. 2015, 356, 391–398. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Jiao, S.; Fang, Z.; Liu, X.; Xu, Y.; Pang, G.; Feng, S. Synthesis, microstructure, and properties of black anatase and B phase TiO2 nanoparticles. Mater. Des. 2016, 100, 235–240. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Kim, S.-J. Blue TiO2 nanosheets as a high-performance electrode material for Supercapacitors. J. Colloid Interface Sci. 2019, 536, 62–70. [Google Scholar] [CrossRef]
- Su, J.; Zou, X.X.; Zou, Y.C.; Li, G.D.; Wang, P.P.; Chen, J.S. Porous titania with heavily self-doped Ti3+ for specific sensing of CO at room temperature. Inorg. Chem. 2013, 52, 5924–5930. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Han, M.; Konkin, A.; Koppe, T.; Wang, D.; Andreu, T.; Chen, G.; Vetter, U.; Morantee, J.R.; Schaaf, P. Slightly hydrogenated TiO2 with enhanced photocatalytic performance. J. Mater. Chem. A 2014, 2, 12708–12716. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.B.; Pillaid, S.C.; Periyat, P. Black TiO2 Nanomaterials: A Review of Recent Advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Negishi, N.; Kutsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A Chem. 2000, 161, 205–212. [Google Scholar] [CrossRef]
- Gurbatov, S.O.; Modin, E.; Puzikov, V.; Tonkaev, P.; Storozhenko, D.; Sergeev, A.; Mintcheva, N.; Yamaguchi, S.; Tarasenka, N.N.; Chuvilin, A.; et al. Black Au-Decorated TiO2 Produced via Laser Ablation in Liquid. ACS Appl. Mater. 2021, 13, 6522–6531. [Google Scholar] [CrossRef] [PubMed]
- Kibis, L.S.; Stadnichenko, A.I.; Svintsitskiy, D.A.; Slavinskaya, E.M.; Romanenko, A.V.; Fedorova, E.A.; Stonkus, O.A.; Svetlichnyi, V.A.; Fakhrutdinova, E.D.; Vorokhta, M.; et al. In situ probing of Pt/TiO2 activity in low-temperature ammonia oxidation. Catal. Sci. Technol. 2021, 11, 250–263. [Google Scholar] [CrossRef]
- Shukla, G.; Khare, A. Optical emission spectroscopic studies on laser ablated TiO2 plasma. Appl. Surf. Sci. 2009, 255, 8730–8737. [Google Scholar] [CrossRef]
- Qiao, M.; Yan, J.; Gao, B. Ablation of TiO2 surface with a double-pulse femtosecond laser. Opt. Commun. 2019, 441, 49–54. [Google Scholar] [CrossRef]
- Giorgetti, E.; Miranda, M.M.; Caporali, S.; Canton, P.; Marsili, P.; Vergari, C.; Giammanco, F. TiO2 nanoparticles obtained by laser ablation in water: Influence of pulse energy and duration on the crystalline phase. J. Alloys Compd. 2015, 643, S75–S79. [Google Scholar] [CrossRef]
- Nath, A.; Laha, S.S.; Khare, A. Synthesis of TiO2 nanoparticles via laser ablation at titanium-water interface. Integr. Ferroelectr. 2010, 121, 58–64. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Joshi, M.P.; Mondal, P.; Sinha, A.K.; Srivastava, A.K. Growth of anatase and rutile phase TiO2 nanoparticles using pulsed laser ablation in liquid: Influence of surfactant addition and ablation time variation. Appl. Surf. Sci. 2017, 396, 303–309. [Google Scholar] [CrossRef]
- Sasaki, T.; Liang, C.; Nichols, W.T.; Shimizu, Y.; Koshizaki, N. Fabrication of oxide base nanostructures using pulsed laser ablation in aqueous solutions. Appl. Phys. A 2004, 79, 1489–1492. [Google Scholar] [CrossRef]
- Liang, C.H.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. Preparation of ultrafine TiO2 nanocrystals via pulsed-laser ablation of titanium metal in surfactant solution. Appl. Phys. A 2005, 80, 819–822. [Google Scholar] [CrossRef]
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Muller, A.M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Sugioka, K. Laser ablation in liquids for nanomaterial synthesis: Diversities of targets and liquids. J. Phys. Photonics 2021, 3, 042002. [Google Scholar] [CrossRef]
- Tarasenka, N.; Shustava, E.; Butsen, A.; Kuchmizhak, A.A.; Pashayan, S.; Kulinich, S.A.; Tarasenko, N. Laser-assisted fabrication and modification of copper and zinc oxide nanostructures in liquids for photovoltaic applications. Appl. Surf. Sci. 2021, 554, 149570. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Kulinich, S.A.; Svetlichnyi, V.A. Green laser ablation-based synthesis of functional nanomaterials for generation, storage and detection of hydrogen. Curr. Opin. Green Sustain. Chem. 2022, 33, 100566. [Google Scholar] [CrossRef]
- Fakhrutdinova, E.D.; Shabalina, A.V.; Gerasimova, M.A.; Nemoykina, A.L.; Vodyankina, O.V.; Svetlichnyi, V.A. Highly defective dark nano titanium dioxide: Preparation via pulsed laser ablation and application. Materials 2020, 13, 2054. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, Z.P.; Gerasimova, M.A.; Fakhrutdinova, E.D.; Svetlichnyi, V.A. Effect of laser and temperature treatment on the optical properties of titanium dioxide nanoparticles prepared via pulse laser ablation. Rus. Phys. J. 2022, 64, 2115–2122. [Google Scholar] [CrossRef]
- Guayaquil-Sosa, J.F.; Serrano-Rosales, B.; Valades-Pelayo, P.J.; de Lasa, H. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. B 2017, 211, 337–348. [Google Scholar] [CrossRef]
- Semaltianos, N.G. Nanoparticles by Laser Ablation of Bulk Target Materials in Liquids. In Book Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer: New York, NY, USA, 2016; pp. 67–92. [Google Scholar]
- Liang, S.X.; Zhang, L.S.; Reichenberger, S.; Barcikowski, S. Design and perspective of amorphous metal nanoparticles from laser synthesis and processing. Phys. Chem. Chem. Phys. 2021, 23, 11121–11154. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Buccheri, M.A.; Cacciato, G.; Sanz, R.; Rappazzo, G.; Boninelli, S.; Reitano, R.; Romano, L.; Privitera, V.; Grimaldi, M.G. Photocatalytical and antibacterial activity of TiO2 nanoparticles obtained by laser ablation in water. Appl. Catal. B 2015, 165, 487–494. [Google Scholar] [CrossRef]
- Zhang, J.; Claverie, J.; Chaker, M.; Ma, D. Colloidal metal nanoparticles preparedby laser ablationand their applications. ChemPhysChem 2017, 18, 986–1006. [Google Scholar] [CrossRef]
- Nichols, W.T.; Sasaki, T.; Koshizaki, N. Laser ablation of a platinum target in water. III. Laser induced reactions. J. Appl. Phys. 2006, 100, 114913. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic hydrogen production: Role of sacrificial reagents on the activity of oxide, carbon, and sulfide catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Pedrono, F. New insights into the mechanism of photocatalytic reforming on Pd/TiO2. Appl. Catal. B 2011, 107, 205–209. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Sauer, M.C.; Gosztola, D. Efficient, rapid photooxidation of chemisorbed polyhydroxyl alcohols and carbohydrates by TiO2 nanoparticles in an aqueous solution. J. Phys. Chem. B 2004, 108, 12512–12517. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Sauer, M.C. Hole scavenging and photo-stimulated recombination of electron—hole pairs in aqueous TiO2 nanoparticles. J. Phys. Chem. B 2004, 108, 12497–12511. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Wang, X.; Leung, D.Y.; Gu, Q.; Chen, S.; Huang, H. Photocatalytic reforming of C3-polyols for H2 production: Part (I). Role of their OH groups. Appl. Catal. B 2011, 106, 681–688. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, C.; Dona-Rodriguez, J.M.; Gonzalez-Diaz, O.; Seck, I.; Zerbani, D.; Portillo, D.; Perez-Pena, J. Synthesis of highly photoactive TiO2 and Pt/TiO2 nanocatalysts for substrate-specific photocatalytic applications. Appl. Catal. B 2012, 125, 383–389. [Google Scholar] [CrossRef]
- Wei, P.; Liu, J.; Li, Z. Effect of Pt loading and calcination temperature on the photocatalytic hydrogen production activity of TiO2 microspheres. Ceramics 2013, 39, 5387–5391. [Google Scholar] [CrossRef]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Sun, S.; Wu, X.; Huang, Z.; Shen, H.; Zhao, H.; Jing, G. Engineering stable Pt nanoclusters on defective two-dimensional TiO2 nanosheets by introducing SMSI for efficient ambient formaldehyde oxidation. Chem. Eng. J. 2022, 435, 135035. [Google Scholar] [CrossRef]
- Oh, S.; Ha, H.; Choi, H.; Jo, C.; Cho, J.; Choi, H.; Ryoo, R.; Kim, H.Y.; Park, J.Y. Oxygen activation on the interface between Pt nanoparticles and mesoporous defective TiO2 during CO oxidation. J. Chem. Phys. 2019, 151, 234716. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Shen, S.; Mao, S.S. Black TiO2 for solar hydrogen conversion. J. Mater. 2017, 3, 96–111. [Google Scholar]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Maslova, V.; Fasolini, A.; Offidani, M.; Albonetti, S.; Basile, F. Solar-driven valorization of glycerol towards production of chemicals and hydrogen. Catal. Today 2021, 380, 147–155. [Google Scholar] [CrossRef]

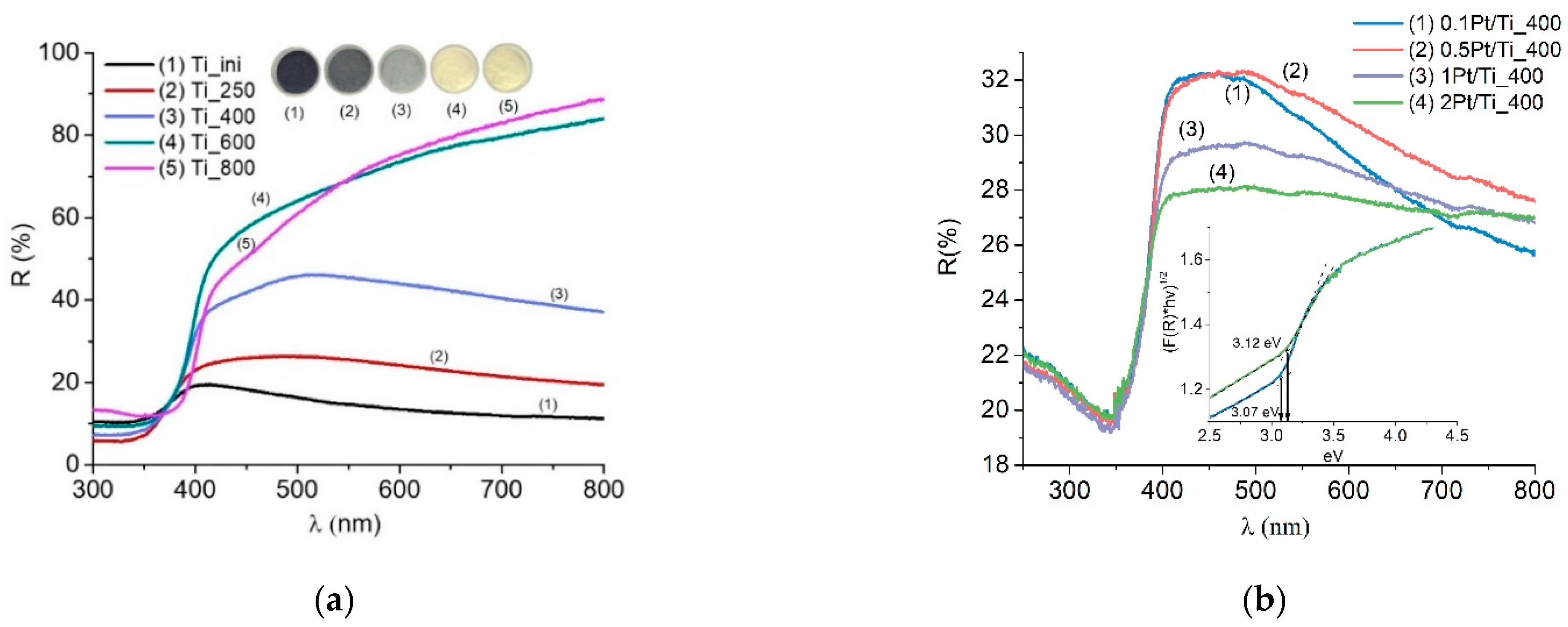
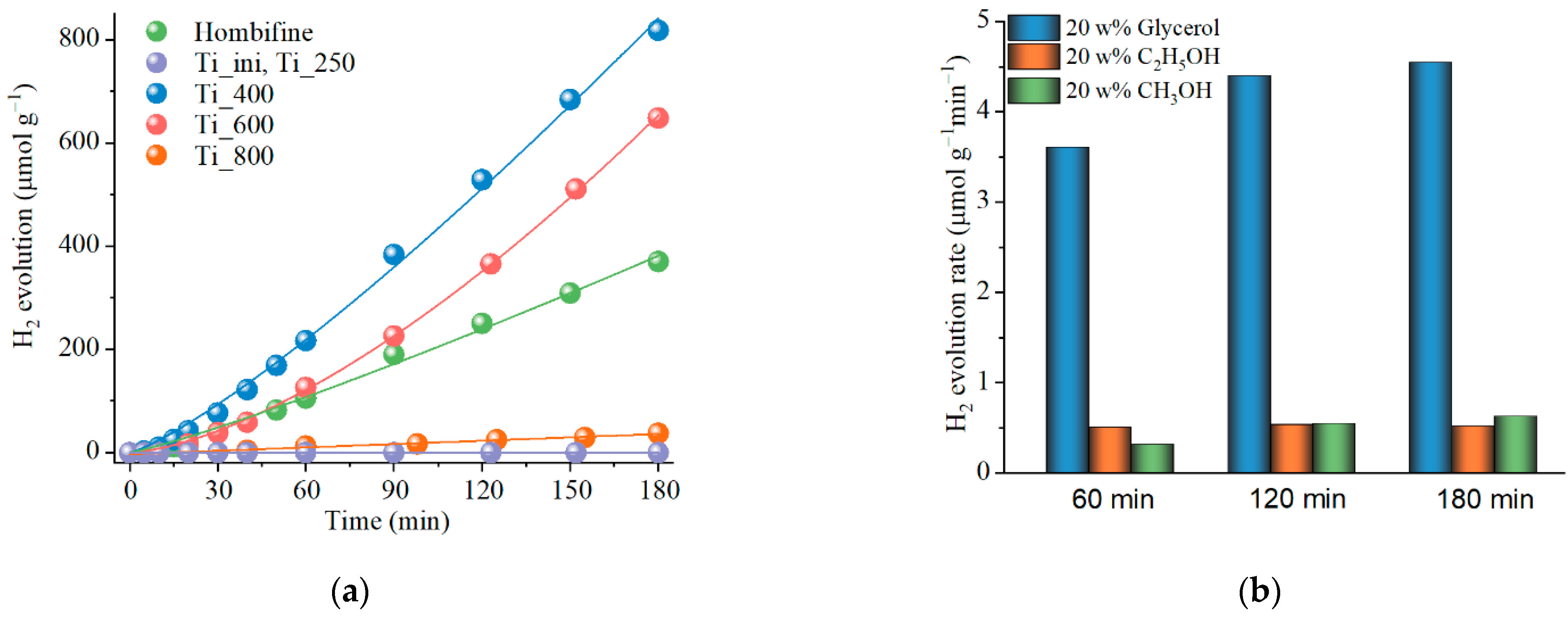
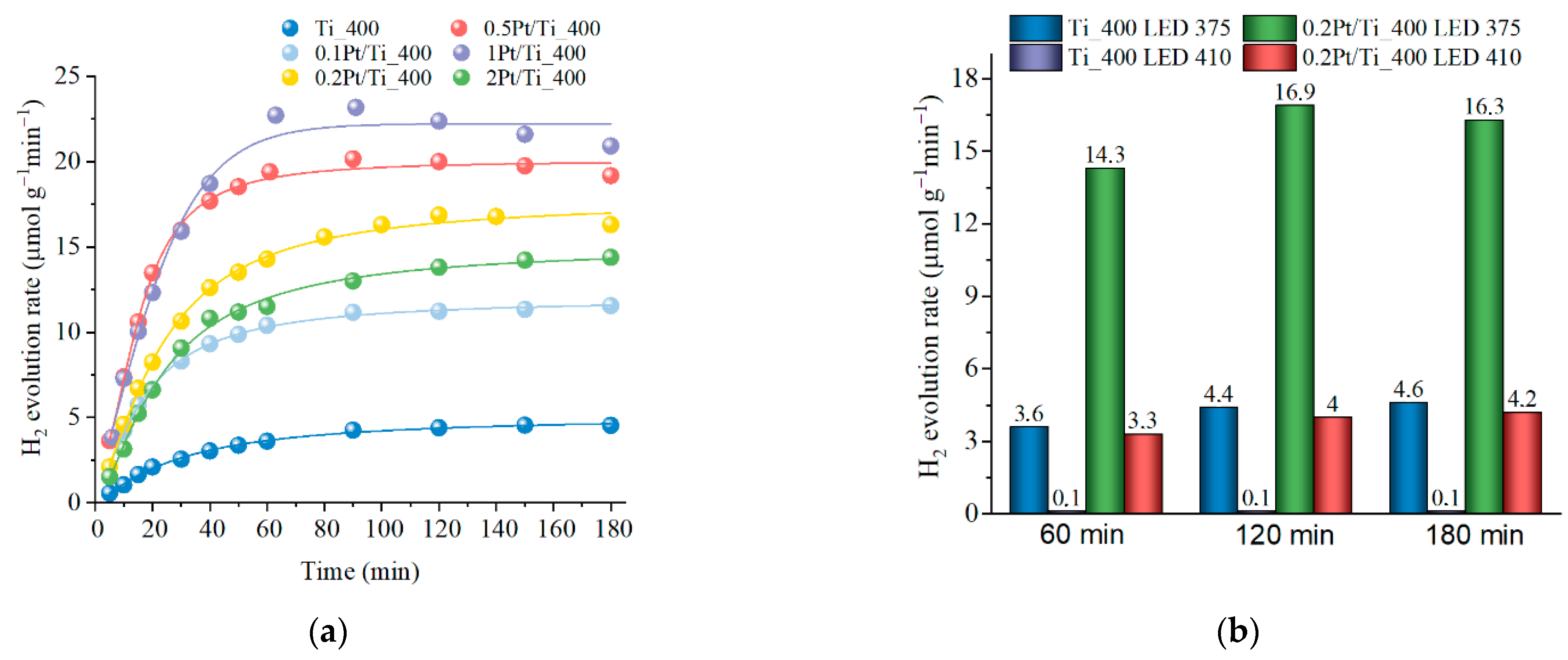
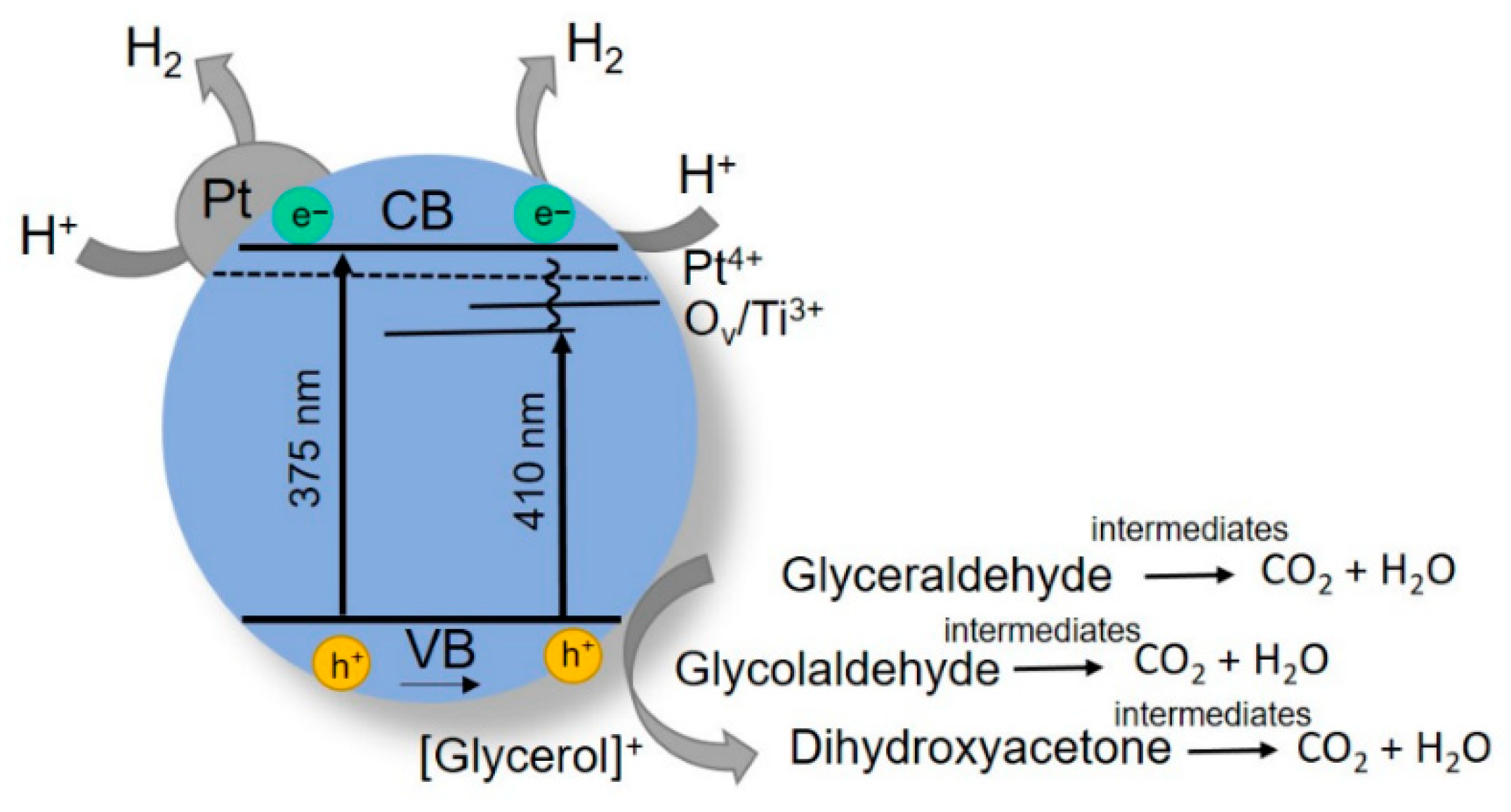

| Sample | Phase Content Titania (%) | CSR Size (nm) | Band Gap (eV) | SBET (m2/g) | ||
|---|---|---|---|---|---|---|
| Anatase | Rutile | Brookite | ||||
| Hombifine | 95 | 5 | – | 15 | 3.03 | 296 |
| Ti_ini | – | – | – | – | 3.06 | 227 |
| Ti_250 | – | – | – | – | 3.06 | 170 |
| Ti_400 | 90 | 10 | – | 24 | 3.03 | 86 |
| Ti_600 | 66 | 34 | – | 38 | 3.01 | 50 |
| Ti_800 | 42 | 58 | – | 105 | 3.00 | 7 |
| 0.1Pt/Ti_400 | 80 | 12 | 8 | 24 | 3.07 | 108 |
| 0.2Pt/Ti_400 | 80 | 12 | 8 | 23 | 3.07 | 94 |
| 0.5Pt/Ti_400 | 80 | 12 | 8 | 24 | 3.07 | 95 |
| 1Pt/Ti_400 | 75 | 12 | 13 | 23 | 3.09 | 95 |
| 2Pt/Ti_400 | 71 | 12 | 17 | 25 | 3.12 | 90 |
| Sample | pHo | ζo, mV | pHIEP |
|---|---|---|---|
| Ti_400 | 7.2 | –13 | 4.7 |
| 0.1Pt/Ti_400 | 5.3 | –15 | 3.6 |
| 0.2Pt/Ti_400 | 5.7 | –20 | 3.4 |
| 0.5Pt/Ti_400 | 5.3 | –19 | 2.6 |
| 1Pt/Ti_400 | 5.2 | –12 | 4.1 |
| 2Pt/Ti_400 | 7.2 | –13 | 4.7 |
| Pt | 4.7 | –38 | 2.1 |
| Sample | LED 375 | LED 410 | ||
|---|---|---|---|---|
| HER (mmol/g) | AQY | HER (mmol/g) | AQY | |
| Hombifine | 0.37 | 0.04 | – | – |
| Ti_400 | 0.82 | 0.08 | 0.02 | ~10−8 |
| 0.1Pt/Ti_400 | 2.08 | 0.21 | – | – |
| 0.2Pt/Ti_400 | 2.94 | 0.29 | 0.76 | 0.01 |
| 0.5Pt/Ti_400 | 3.45 | 0.34 | – | – |
| 1Pt/Ti_400 | 3.77 | 0.38 | – | – |
| 2Pt/Ti_400 | 2.59 | 0.26 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhrutdinova, E.; Reutova, O.; Maliy, L.; Kharlamova, T.; Vodyankina, O.; Svetlichnyi, V. Laser-Based Synthesis of TiO2-Pt Photocatalysts for Hydrogen Generation. Materials 2022, 15, 7413. https://doi.org/10.3390/ma15217413
Fakhrutdinova E, Reutova O, Maliy L, Kharlamova T, Vodyankina O, Svetlichnyi V. Laser-Based Synthesis of TiO2-Pt Photocatalysts for Hydrogen Generation. Materials. 2022; 15(21):7413. https://doi.org/10.3390/ma15217413
Chicago/Turabian StyleFakhrutdinova, Elena, Olesia Reutova, Liubov Maliy, Tamara Kharlamova, Olga Vodyankina, and Valery Svetlichnyi. 2022. "Laser-Based Synthesis of TiO2-Pt Photocatalysts for Hydrogen Generation" Materials 15, no. 21: 7413. https://doi.org/10.3390/ma15217413





