Bacteriostatic Poly Ethylene Glycol Plasma Coatings for Orthodontic Titanium Mini-Implants
Abstract
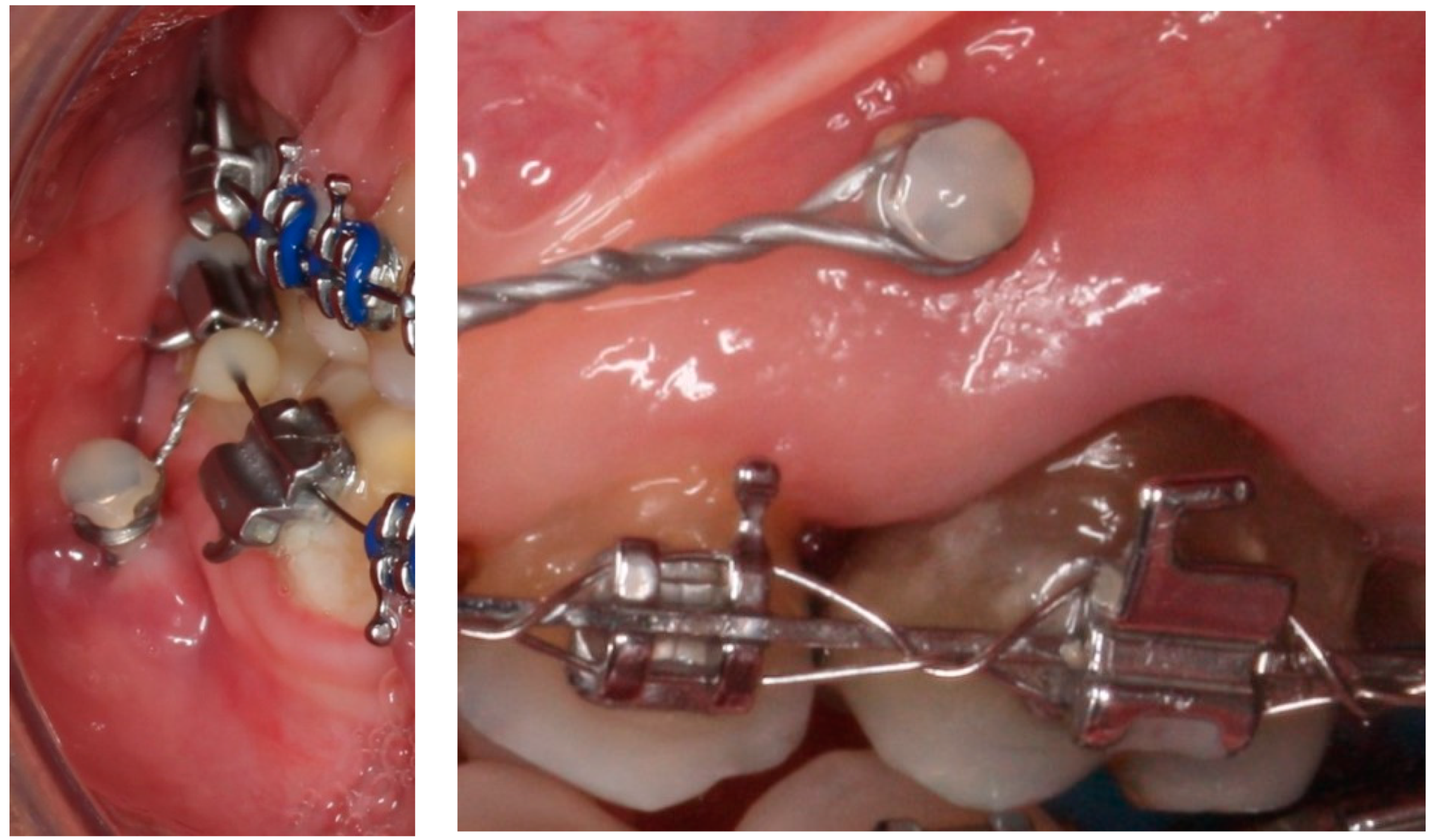
:1. Introduction
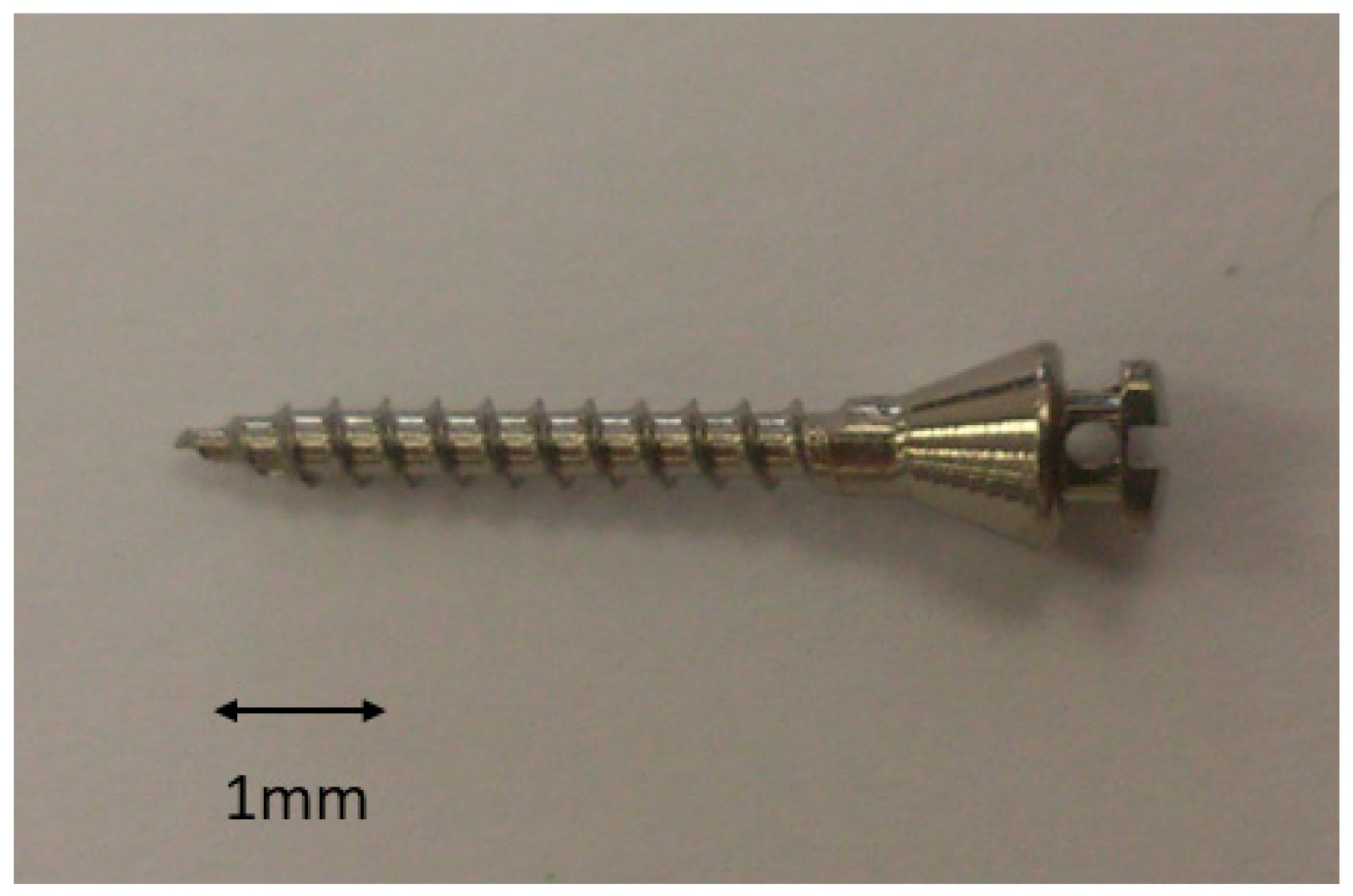
2. Materials and Methods
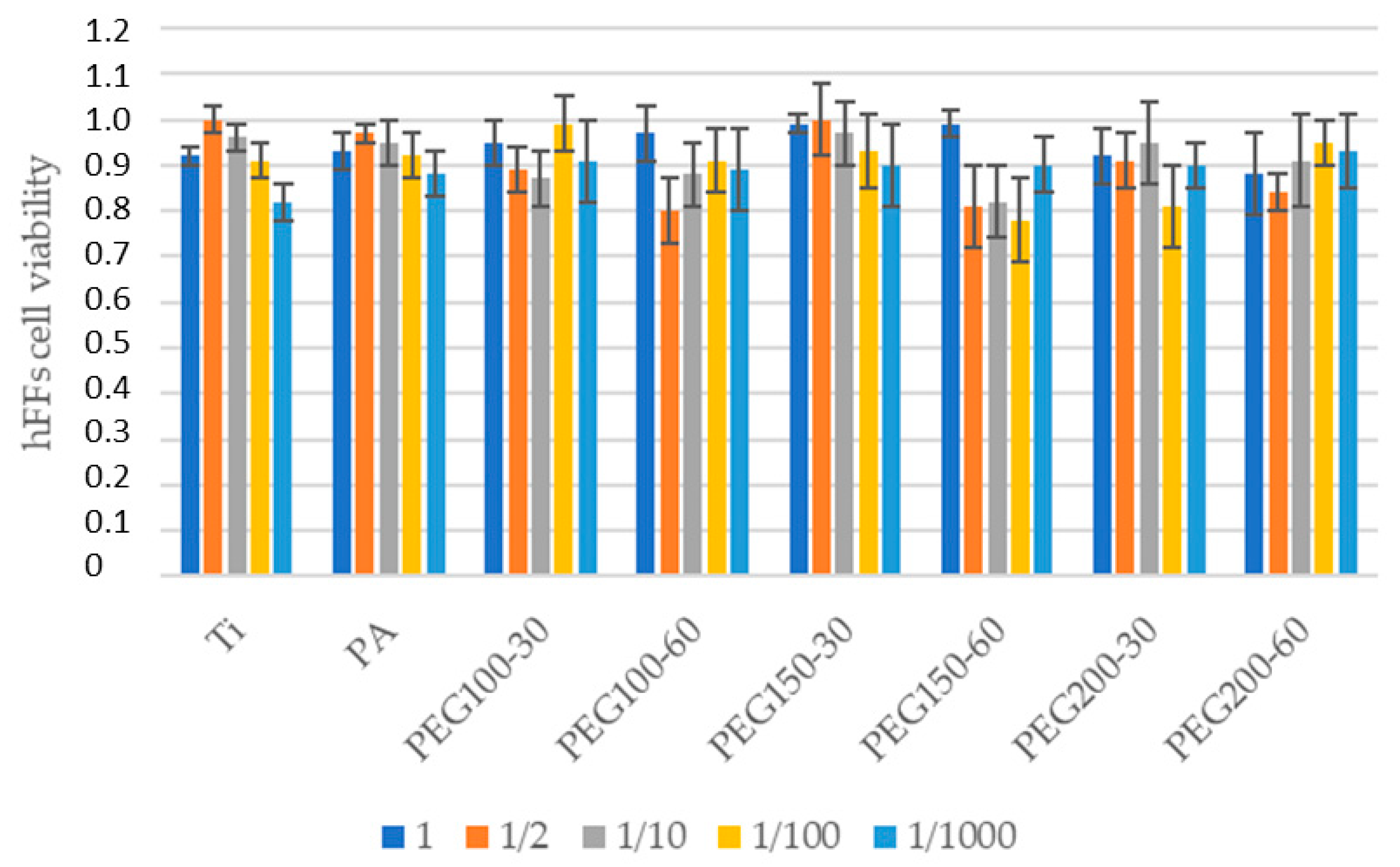
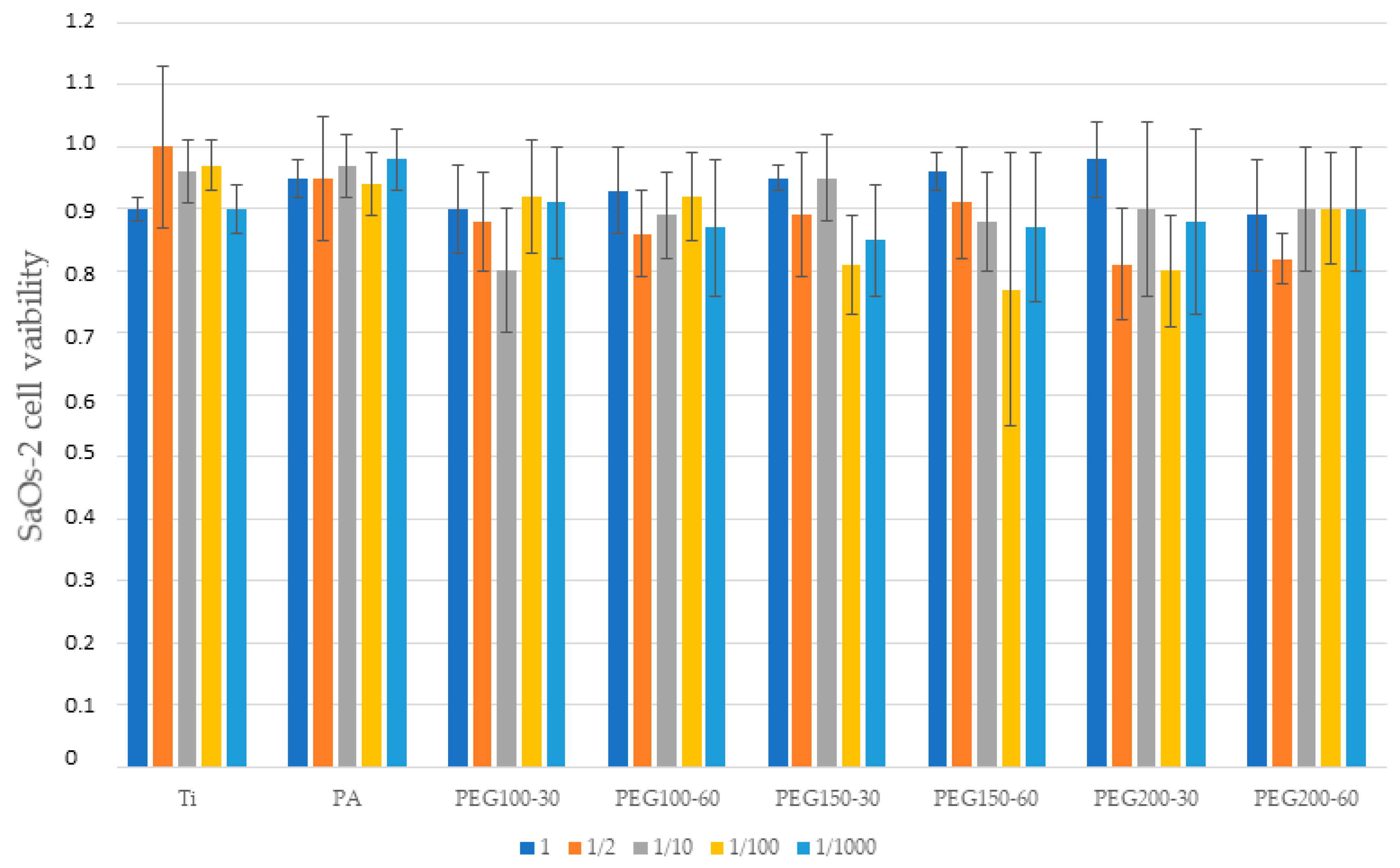
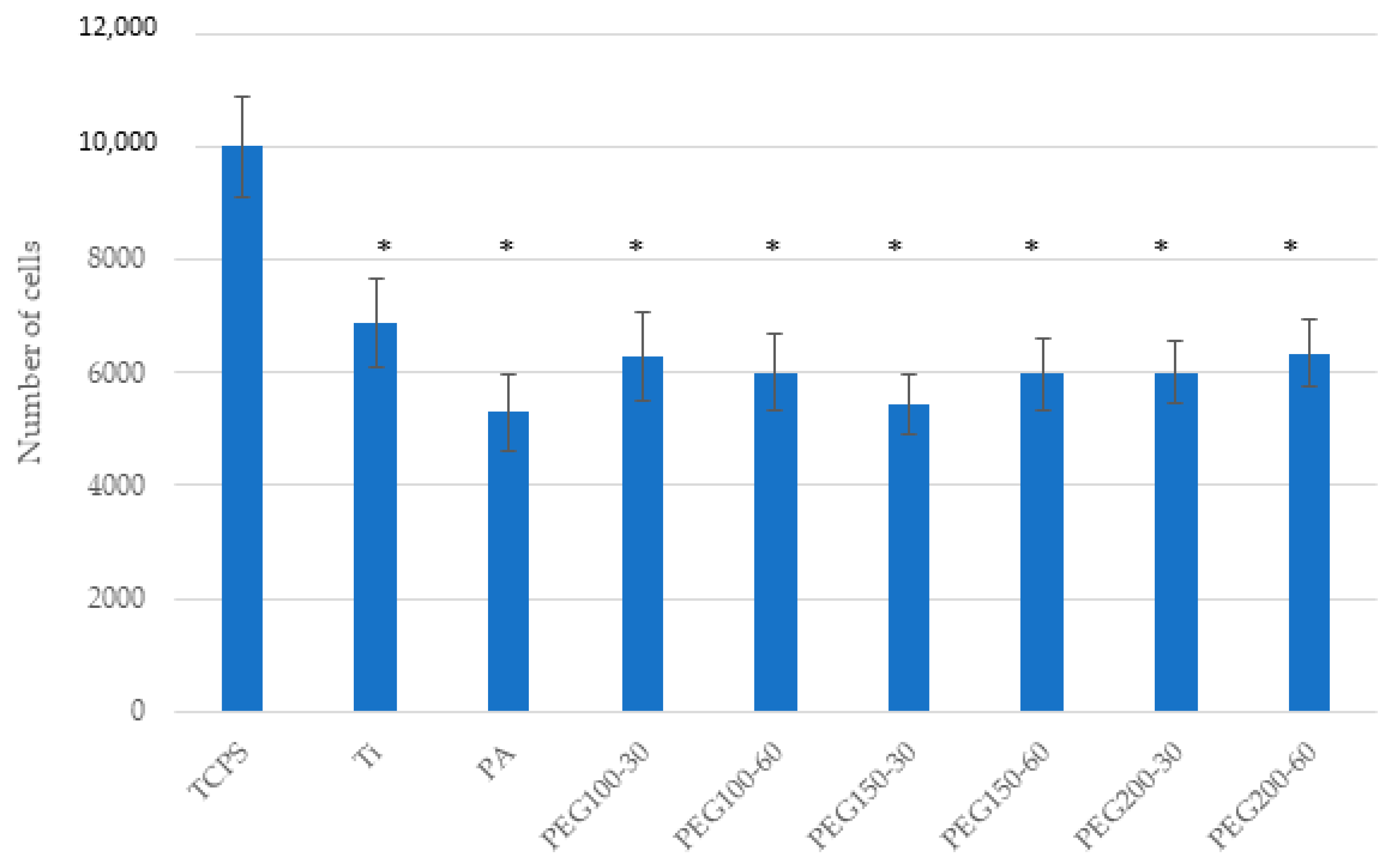
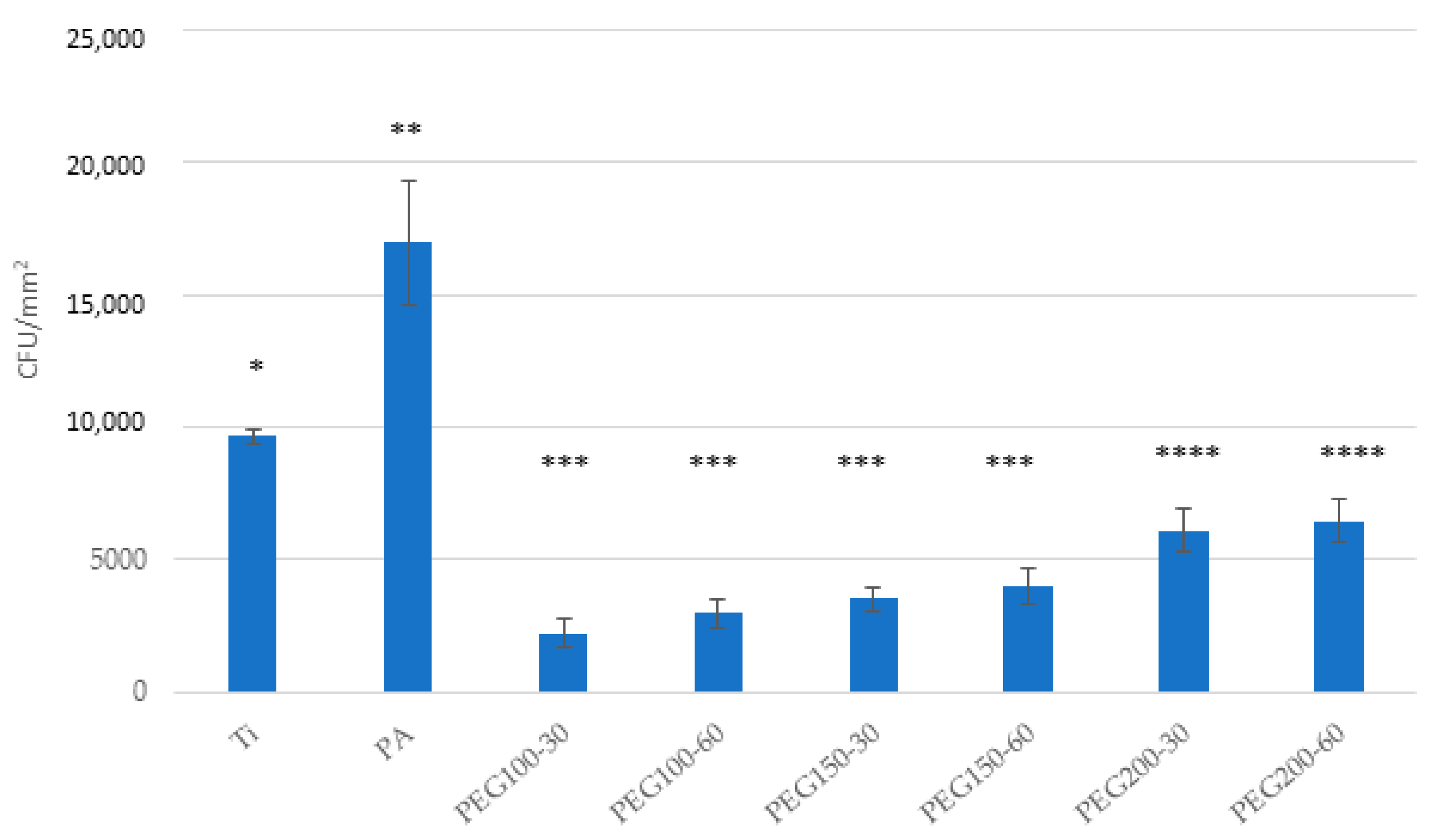
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.H.; Chang, C.S.; Hsieh, C.H.; Tseng, Y.C.; Shen, Y.S.; Huang, I.Y.; Yang, C.F.; Chen, C.M. The Use of Microimplants in Orthodontic Anchorage. J. Oral Maxillofac. Surg. 2006, 64, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Raffainl, M.; Melsen, B. Miniscrews as orthodontic anchorage: A preliminary report. Int. J. Adult Orthod. Orthognath. Surg. 1998, 13, 201. [Google Scholar]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Jamari, J. Tresca stress evaluation of Metal-on-UHMWPE total hip arthroplasty during peak loading from normal walking activity. Mater. Today Proc. 2022, 63, S143–S146. [Google Scholar] [CrossRef]
- Park, H.S.; Bae, S.M.; Kyung, H.M.; Sung, J.H. Simultaneous incisor retraction and distal molar movement with microimplant anchorage. World J. Orthod. 2004, 5, 164. [Google Scholar]
- Puigdollers, A.; Espinar, E. Ortodoncia., Clases de Ortodoncia. Mini Implantes en Ortodoncia; Puigdollers, A., Ed.; UIC Ed.: Barcelona, Spain, 2022. [Google Scholar]
- Miyawaki, S.; Koyama, I.; Inoue, M.; Mishima, K.; Sugahara, T.; Takano-Yamamoto, T. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 373–378. [Google Scholar] [CrossRef]
- Lee, A.; Wang, H.-L. Biofilm Related to Dental Implants. Implant Dent. 2010, 19, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Shahin, K.; Zhang, L.; Bao, H.; Hedayatkhah, A.; Soleimani-Delfan, A.; Komijani, M.; He, T.; Barazandeh, M.; Mansoorianfar, M.; Bouzari, M.; et al. An in-vitro study on a novel six-phage cocktail against multi-drug resistant-ESBL Shigella in aquatic environment. Lett. Appl. Microbiol. 2021, 72, 231–237. [Google Scholar] [CrossRef]
- Nisol, B.; Oldenhove, G.; Preyat, N.; Monteyne, D.; Moser, M.; Perez-Morga, D.; Reniers, F. Atmospheric plasma synthesized PEG coatings: Non-fouling biomaterials showing protein and cell repulsion. Surf. Coat. Technol. 2014, 252, 126–133. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Gil, F.J.; Mas-Moruno, C.; Canal, C.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloids Surf. B Biointerfaces 2017, 152, 367–375. [Google Scholar] [CrossRef]
- Zeng, G.; Ogaki, R.; Meyer, R.L. Non-proteinaceous bacterial adhesins challenge the antifouling properties of polymer brush coatings. Acta Biomater. 2015, 24, 64–73. [Google Scholar] [CrossRef]
- Saldarriaga Fernández, I.C.; Busscher, H.J.; Metzger, S.W.; Grainger, D.W.; van der Mei, H.C. Competitive Time- and Density-Dependent Adhesion of Staphylococci and Osteoblasts on Crosslinked Poly (ethylene Glycol)-Based Polymer Coatings in Co-Culture Flow Chambers. Biomaterials 2011, 32, 979–984. [Google Scholar] [CrossRef]
- Finke, B.; Luethen, F.; Schroeder, K.; Mueller, P.D.; Bergemann, C.; Frant, M.; Ohl, A.; Nebe, B.J. The effect of positively charged plasma polymerization on initial osteoblastic focal adhesion on titanium surfaces. Biomaterials 2007, 28, 4521–4534. [Google Scholar] [CrossRef] [PubMed]
- Özgüzar, H.F.; Meydan, A.E.; Göçmen, J.S.; Mutlu, M. Single-step amphoteric surface modification through plasma polymerization: Antifouling coating for titanium substrate. MRS Commun. 2021, 11, 523–531. [Google Scholar] [CrossRef]
- Hickok, N.J.; Shapiro, I.M. Immobilized antibiotics to prevent orthopaedic implant infections. Adv. Drug Deliv. Rev. 2012, 64, 1165–1176. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Anderson, M.; Bernards, M.T.; Hunt, H.K. PEG Functionalization of Whispering Gallery Mode Optical Microresonator Biosensors to Minimize Non-Specific Adsorption during Targeted, Label-Free Sensing. Sensors 2015, 15, 18040–18060. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Miao, J.; Yan, J.; Yang, K.; Mao, C.; Ju, J.; Shen, J. Applications of antibiofouling PEG-coating in electrochemical biosensors for determination of glucose in whole blood. Electrochim. Acta 2013, 89, 549–554. [Google Scholar] [CrossRef]
- Chen, X.; Su, Y.; Shen, F.; Wan, Y. Antifouling ultrafiltration membranes made from PAN-b-PEG copolymers: Effect of copolymer composition and PEG chain length. J. Membr. Sci. 2011, 384, 44–51. [Google Scholar] [CrossRef]
- Wang, P.; Tan, K.L.; Kang, E.T.; Neoh, K.G. Plasma-induced immobilization of poly (ethylene glycol) onto poly (vinylidene fluoride) microporous membrane. J. Membr. Sci. 2002, 195, 103–114. [Google Scholar] [CrossRef]
- Zou, L.; Vidalis, I.; Steele, D.; Michelmore, A.; Low, S.; Verberk, J. Surface hydrophilic modification of RO membranes by plasma polymerization for low organic fouling. J. Membr. Sci. 2011, 369, 420–428. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Canal, C.; Torrent-Camarero, S.; Garrido, B.; Gil, F.J.; Rodríguez, D. Antifouling coatings for dental implants: Polyethylene glycol-like coatings on titanium by plasma polymerization. Biointerphases 2015, 10, 029505. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Fernández-Calderón, M.C.; Pérez-Giraldo, C.; Manero, J.M.; Albericio, F.; Gil, F.J.; Rodríguez, D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014, 10, 3522–3534. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Tavoosi, M.; Mozafarinia, R.; Ghasemi, A.; Doostmohammadi, A. Preparation and characterization of TiO2 nanotube arrays on Ti6Al4V surface for enhancement of cell treatment. Surf. Coat. Technol. 2017, 321, 409–415. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Khataee, A.; Riahi, Z.; Shahin, K.; Asadnia, M.; Razmjou, A.; Hojjati-Najafabadi, A.; Mei, C.; Orooji, Y.; Li, D. Scalable fabrication of tunable titanium nanotubes via sonoelectrochemical process for biomedical applications. Ultrason. Sonochem. 2020, 64, 104783. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Gil, J.; Sandino, C.; Cerrolaza, M.; Pérez, R.; Herrero-Climent, M.; Rios-Carrasco, B.; Rios-Santos, J.V.; Brizuela, A. Influence of Bone-Level Dental Implants Placement and of Cortical Thickness on Osseointegration: In Silico and In Vivo Analyses. J. Clin. Med. 2022, 11, 1027. [Google Scholar] [CrossRef]
- Dávila, E.; Ortiz-Hernández, M.; Perez, R.A.; Herrero-Climent, M.; Cerrolaza, M.; Gil, F.J. Crestal module design optimization of dental implants: Finite element analysis and in vivo studies. J. Mater. Sci. Mater. Med. 2019, 30, 90. [Google Scholar] [CrossRef]
- Gil, F.J.; Manero, J.M.; Ginebra, M.P.; Planell, J.A. The effect of cooling rate on the cyclic deformation of β-annealed Ti-6Al-4V. Mater. Sci. Eng. A 2003, 349, 150–155. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Monsalve-Guil, L.; Jimenez-Guerra, A.; Ortiz, I.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pegueroles, M.; Pérez, R.A.; Gil, F.J. Importance of the Roughness and Residual Stresses of Dental Implants on Fatigue and Osseointegration Behavior. In Vivo Study in Rabbits. J. Oral Implant. 2016, 42, 469–476. [Google Scholar] [CrossRef]
- Gil, J.; Pérez, R.; Herrero-Climent, M.; Rizo-Gorrita, M.; Torres-Lagares, D.; Gutierrez, J.L. Benefits of Residual Aluminum Oxide for Sand Blasting Titanium Dental Implants: Osseointegration and Bactericidal Effects. Materials 2020, 15, 178. [Google Scholar] [CrossRef]
- Vara, J.C.; Delgado, J.; Estrada-Martínez, A.; Pérez-Pevida, E.; Brizuela, A.; Bosch, B.; Pérez, R.; Gil, J. Effect of the Nature of the Particles Released from Bone Level Dental Implants: Physicochemical and Biological Characterization. Coatings 2022, 12, 219. [Google Scholar] [CrossRef]
- Pegueroles, M.; Tonda-Turo, C.; Planell, J.A.; Gil, F.-J.; Aparicio, C. Adsorption of Fibronectin, Fibrinogen, and Albumin on TiO2: Time-Resolved Kinetics, Structural Changes, and Competition Study. Biointerphases 2012, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Drelich, J. Static contact angles for liquids at heterogeneous rigid solid surfaces. Pol. J. Chem. 1997, 71, 525–549. [Google Scholar]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y.F. Effect of surface roughness of the titanium alloy Ti–6Al–4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Tanaka, Y.; Matin, K.; Gyo, M.; Okada, A.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Tagami, J.; Hanawa, T. Effects of electrodeposited poly(ethylene glycol) on biofilm adherence to titanium. J. Biomed. Mater. Res. Part A 2010, 95, 1105–1113. [Google Scholar] [CrossRef]
- Ferraris, S.; Giachet, F.T.; Miola, M.; Bertone, E.; Varesano, A.; Vineis, C.; Cochis, A.; Sorrentino, R.; Rimondini, L.; Spriano, S. Nanogrooves and keratin nanofibers on titanium surfaces aimed at driving gingival fibroblasts alignment and proliferation without increasing bacterial adhesion. Mater. Sci. Eng. C 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Rodríguez-Hernández, A.G.; Delgado, L.M.; Manero, J.M.; Javier Gil, F.; Rodríguez, D. Silver deposition on titanium surface by electrochemical anodizing process reduces bacterial adhesion of Streptococcus sanguinis and Lactobacillus salivarius. Clin. Oral Impl. Res. 2015, 26, 1170–1179. [Google Scholar] [CrossRef] [Green Version]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial Properties of hLf1–11 Peptide onto Titanium Surfaces: A Comparison Study Between Silanization and Surface Initiated Polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, S.; Spriano, S.; Pan, G.; Venturello, A.; Bianchi, C.L.; Chiesa, R.; Faga, M.G.; Maina, G.; Vernè, E. Surface modification of Ti-6Al-4V alloy for biomineralization and specific biological response: Part I, inorganic modification. J. Mater. Sci. Mater. Med. 2011, 22, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Weinman, C.J.; Ober, C.K. Advances in polymers for anti-biofouling surfaces. J. Mater. Chem. 2008, 18, 3405–3413. [Google Scholar] [CrossRef]
- Neoh, K.G.; Hu, X.; Zheng, D.; Kang, E.T. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012, 33, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Ngo, K.X.; Vebert-Nardin, C. Anti-Infectious Surfaces Achieved by Polymer Modification. Macromol. Mater. Eng. 2014, 299, 648–668. [Google Scholar] [CrossRef]
- Aparicio, C.; Gil, F.J.; Planell, J.A.; Engel, E. Human-osteoblast proliferation and differentiation on grit-blasted and bioactive titanium for dental applications. J. Mater. Sci. Mater. Electron. 2002, 13, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, M.; Jones, J.E.; Ritts, A.C.; Yu, Q.; Sun, H. Inhibition of Staphylococcus epidermidis Biofilm by Trimethylsilane Plasma Coating. Antimicrob. Agents Chemother. 2012, 56, 5923–5937. [Google Scholar] [CrossRef] [Green Version]
- Michiardi, A.; Aparicio, C.; Planell, J.A.; Gil, F.J. New oxidation treatment of NiTi shape memory alloys to obtain Ni-free surfaces and to improve biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77, 249–256. [Google Scholar] [CrossRef]
- Bollen, C.M.L.; Papaioanno, W.; Van Eldere, J.; Schepers, E.; Quirynen, M.; Van Steenberghe, D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin. Oral Implant. Res. 1996, 7, 201–211. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Fraioli, R.; Albericio, F.; Manero, J.M.; Gil, F.J. Novel Peptide-Based Platform for the Dual Presentation of Biologically Active Peptide Motifs on Biomaterials. ACS Appl. Mater. Interfaces 2014, 6, 6525–6536. [Google Scholar] [CrossRef]
- Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Manero, J.M.; Gil, J.; Kessler, H.; Mas-Moruno, C. Mimicking bone extracellular matrix: Integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation, and differentiation on titanium. Colloids Surf. B Biointerfaces 2015, 128, 191–200. [Google Scholar] [CrossRef]
- Marguier, A.; Poulin, N.; Soraru, C.; Vonna, L.; Hajjar-Garreau, S.; Kunemann, P.; Airoudj, A.; Mertz, G.; Bardon, J.; Delmée, M.; et al. Bacterial Colonization of Low-Wettable Surfaces is Driven by Culture Conditions and Topography. Adv. Mater. Interfaces 2020, 7, 2000179. [Google Scholar] [CrossRef]

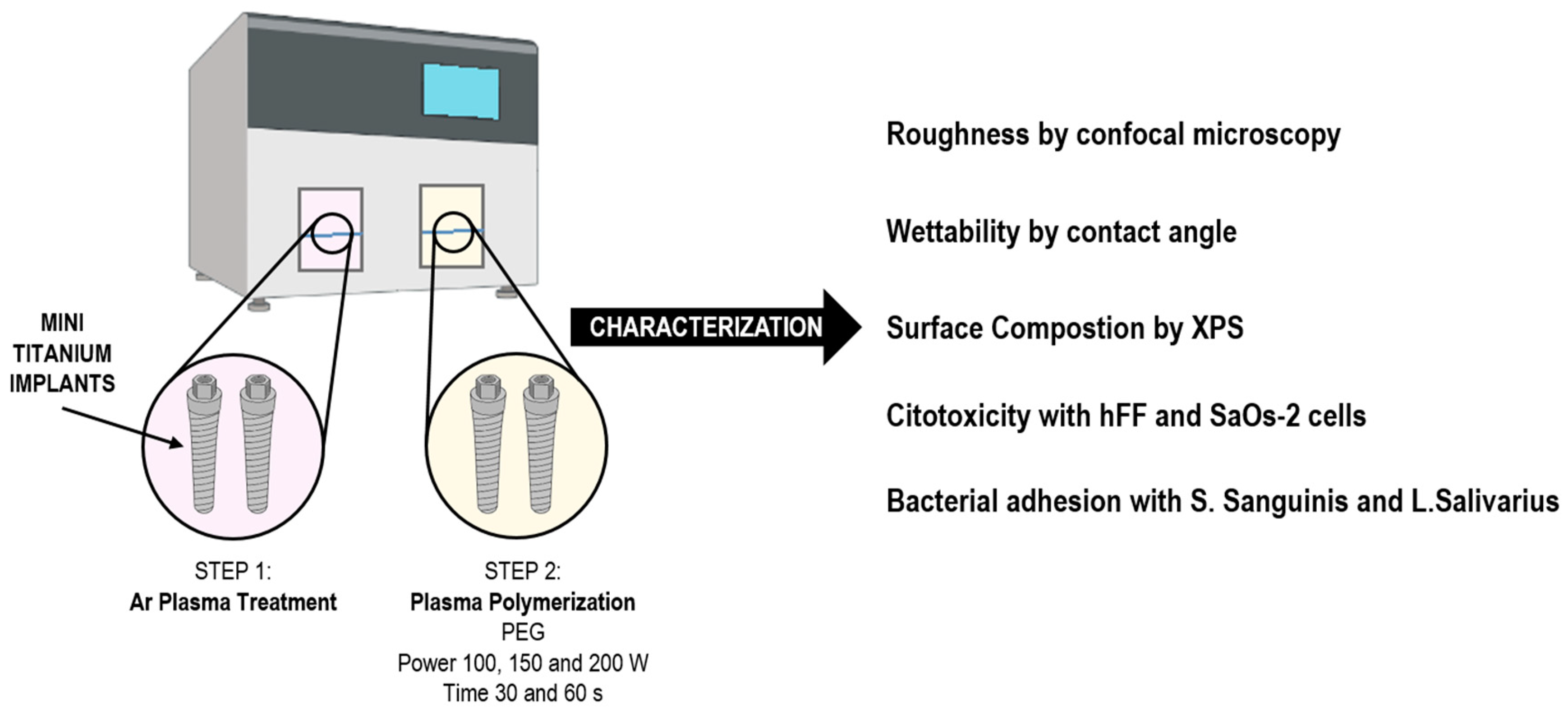



| Sample | Peak Power (W) | Time (min) |
|---|---|---|
| PEG100-30 | 100 | 30 |
| PEG100-60 | 100 | 60 |
| PEG150-30 | 150 | 30 |
| PEG150-60 | 150 | 60 |
| PEG200-30 | 200 | 30 |
| PEG200-60 | 200 | 60 |
| Sample | Ra (μm) | Pc (cm−1) |
|---|---|---|
| Ti | 0.33 ± 0.10 | 150.9 ± 69 |
| PA | 0.35 ± 0.20 | 153.4 ± 56 |
| PEG100-30 | 0.36 ± 0.30 | 152.8 ± 59 |
| PEG100-60 | 0.33 ± 0.21 | 146.9 ± 60 |
| PEG150-30 | 0.43 ± 0.12 | 150.9 ± 69 |
| PEG150-60 | 0.37 ± 0.09 | 150.9 ± 69 |
| PEG200-30 | 0.39 ± 0.09 | 150.9 ± 69 |
| PEG200-60 | 0.32 ± 0.12 | 150.9 ± 69 |
| Sample | Contact Angle (°) |
|---|---|
| PEG100-30 | 12.3 ± 0.9 * |
| PEG100-60 | 13.2 ± 1.3 * |
| PEG150-30 | 18.2 ± 1.2 ** |
| PEG150-60 | 19.1 ± 0.8 ** |
| PEG200-30 | 25.0 ± 2.2 *** |
| PEG200-60 | 25.2 ± 2.9 *** |
| Sample | O 1s | C 1s | Ti 2p |
|---|---|---|---|
| Ti | 55 ± 1 | 24 ± 1 | 20 ± 1 |
| PA | 63 ± 2 | 10 ± 1 | 26 ± 1 |
| PEG100-30 | 45 ± 2 | 41 ± 1 | 2 ± 1 |
| PEG100-60 | 54 ± 3 | 37 ± 2 | 5 ± 1 |
| PEG150-30 | 42 ± 1 | 47 ± 1 | 8 ± 1 |
| PEG150-60 | 45 ± 1 | 52 ± 1 | 8 ± 1 |
| PEG200-30 | 48 ± 2 | 44 ± 2 | 7 ± 1 |
| PEG200-60 | 40 ± 1 | 52 ± 3 | 6 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Fernandez, J.C.; Pastor, F.; Barrera Mora, J.M.; Brizuela, A.; Puigdollers, A.; Espinar, E.; Gil, F.J. Bacteriostatic Poly Ethylene Glycol Plasma Coatings for Orthodontic Titanium Mini-Implants. Materials 2022, 15, 7487. https://doi.org/10.3390/ma15217487
Rodriguez-Fernandez JC, Pastor F, Barrera Mora JM, Brizuela A, Puigdollers A, Espinar E, Gil FJ. Bacteriostatic Poly Ethylene Glycol Plasma Coatings for Orthodontic Titanium Mini-Implants. Materials. 2022; 15(21):7487. https://doi.org/10.3390/ma15217487
Chicago/Turabian StyleRodriguez-Fernandez, Juan Carlos, Francisco Pastor, Jose Maria Barrera Mora, Aritza Brizuela, Andreu Puigdollers, Eduardo Espinar, and F. Javier Gil. 2022. "Bacteriostatic Poly Ethylene Glycol Plasma Coatings for Orthodontic Titanium Mini-Implants" Materials 15, no. 21: 7487. https://doi.org/10.3390/ma15217487









