Morphological Changes of Calcium Carbonate and Mechanical Properties of Samples during Microbially Induced Carbonate Precipitation (MICP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Bacterial Cultivation
2.1.2. Sand
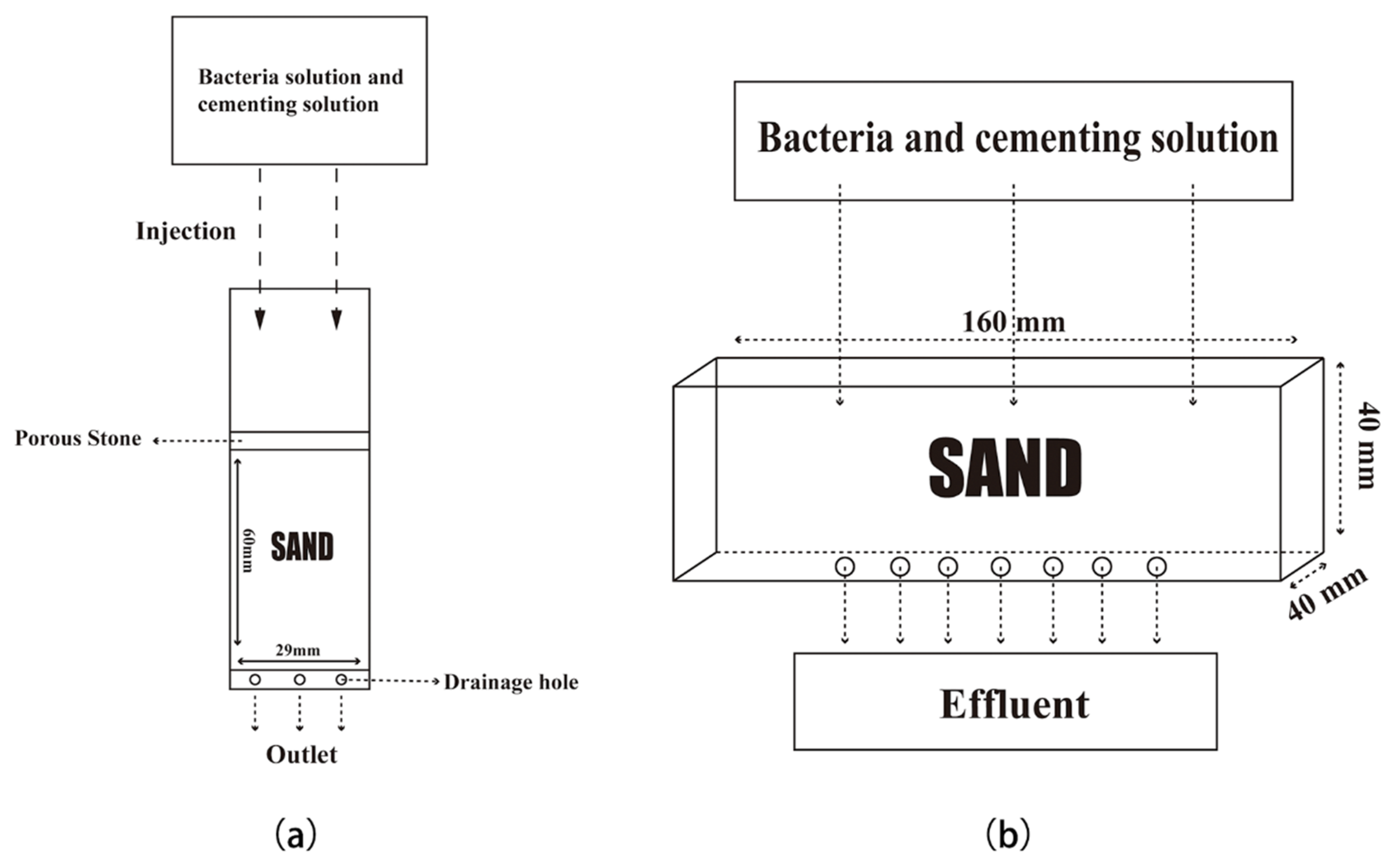
2.1.3. Specimen Preparation
2.2. Properties Tests
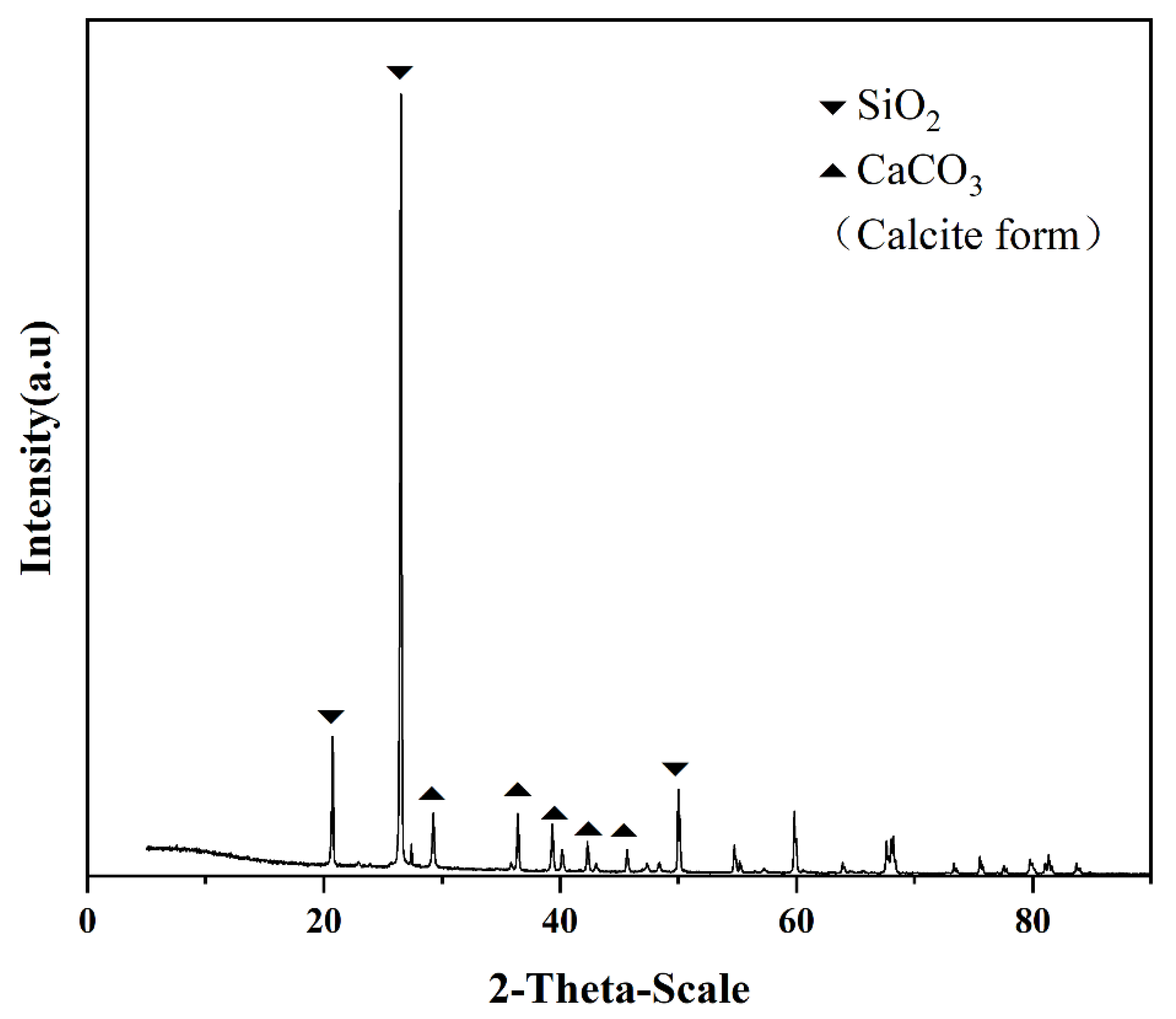
2.2.1. Microscale Observation and Spectroscopy Analysis
2.2.2. Unconfined Compressive Strength and Flexure Strength Test
2.2.3. Water Absorption Test
2.2.4. Calcite Content Tests
2.3. Statistical Analysis
3. Results and Discussions
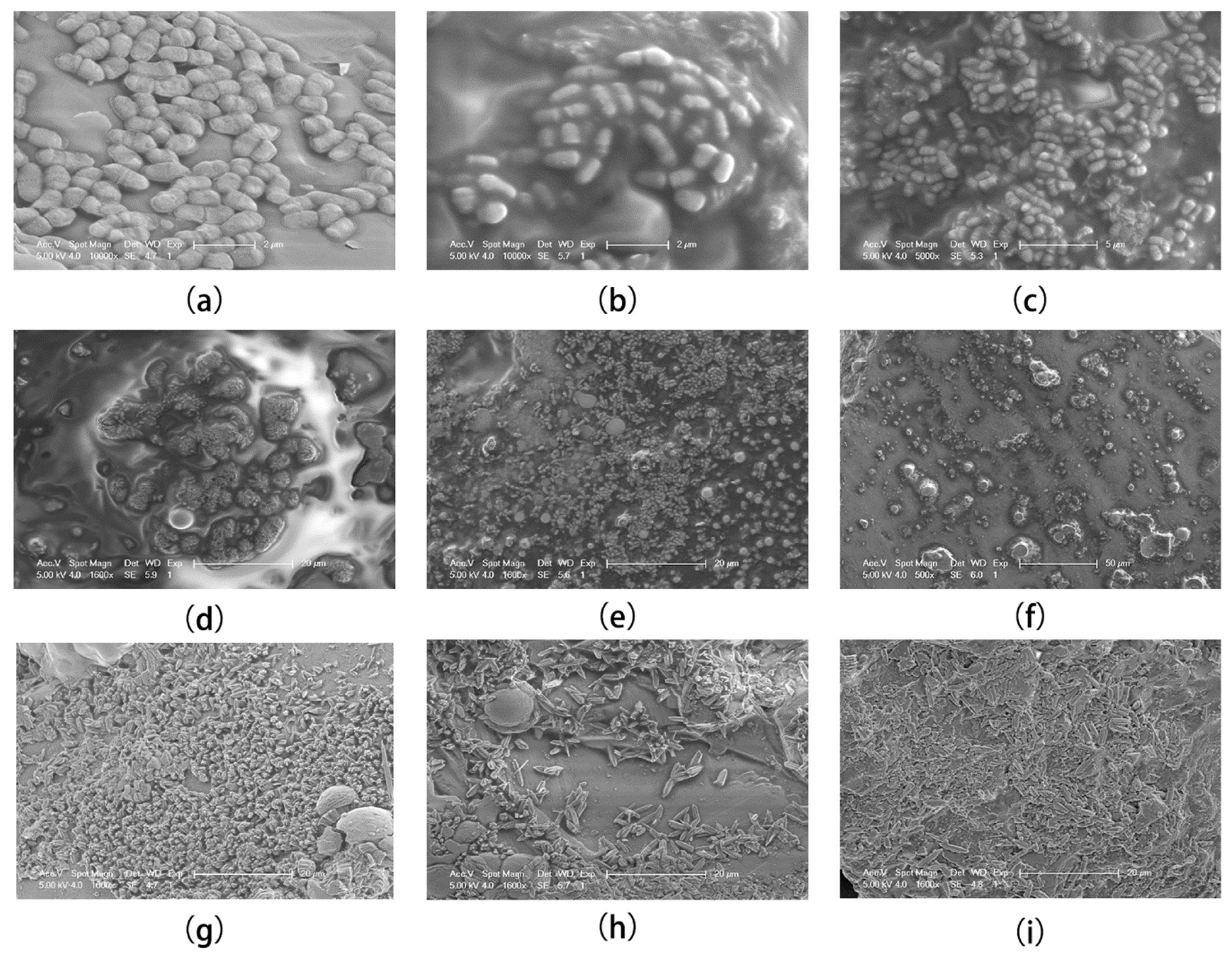
3.1. Morphology Alteration Process of Calcium Carbonate Crystals
3.2. Mechanical Performance of Sand Columns
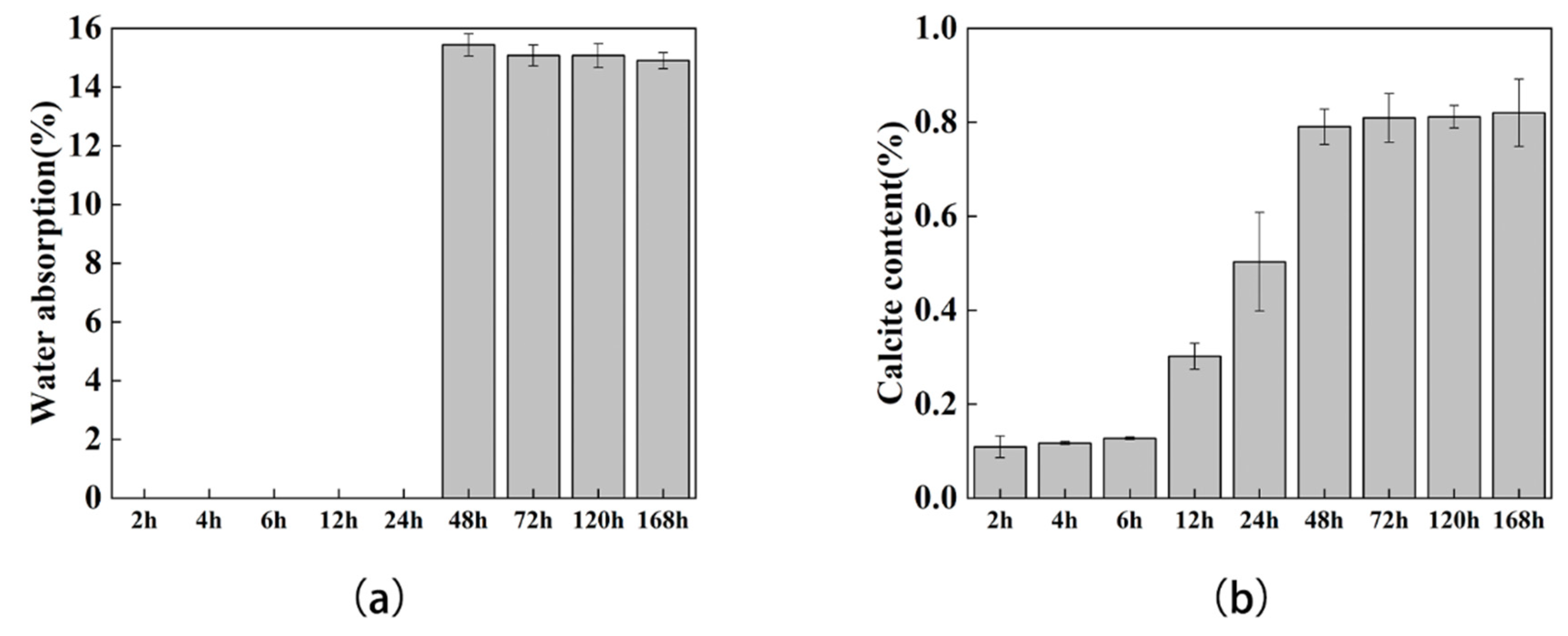
3.3. Water Absorption and Calcite Content Test
4. Conclusions
- (1)
- Bacteria are thought to play a central role in the MICP reaction as calcium carbonate nucleation sites; the calcium carbonate-encapsulated colonies within two hours of the start of MICP were captured, demonstrating that this is direct evidence for bacteria as calcium carbonate nucleation sites.
- (2)
- During the MICP process (7 days), the morphology alterations of calcium carbonate underwent the following processes: For the first two hours, the bacteria were encapsulated by calcium carbonate as nucleation sites. At this time, the calcium carbonate was an ellipsoid with a length of about 2 μm. From 4 h to 48 h, the ellipsoid calcium carbonate was wrapped by unknown colloids to form irregular lumps with protrusions. Notably, unidentified gelatinous substances have also been reported in the literature and have been considered to be bacterial secretions. From 72 h to 168 h, the calcium carbonate crystals changed from round to sharp, and they were densely distributed in clusters.This experiment only observed the change in trend of calcium carbonate morphology within 7 days, and did not follow up reports. In the next experiment, we will conduct experiments on calcium carbonate crystals in the long-term (more than 7 days) MICP reaction to explore further.
- (3)
- The mechanical experiments showed that, with the increase in calcium carbonate production, the corresponding flexural and compressive strengths also increased, which was consistent with the phenomenon reported in the literature. At the same time, with the increase of calcium carbonate, the permeability of the sand column also decreased, which indicates that calcium carbonate reduced the porosity of the soil.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Tenno, T.; Uiga, K.; Mashirin, A.; Zekker, I.; Rikmann, E. Modeling closed equilibrium systems of H2O–dissolved CO2–solid CaCO3. J. Phys. Chem. A 2017, 121, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Ghosh, A.D. Review on microbial induced calcite precipitation mechanisms leading to bacterial selection for microbial concrete. Constr. Build. Mater. 2019, 225, 67–75. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Wang, Y.; DeJong, J.; Kabla, A. Micro-Scale Visualization of Microbial-Induced Calcium Carbonate Precipitation (MICP) Processes; American Society of Civil Engineers: Reston, VI, USA, 2019. [Google Scholar]
- van Paassen, L.A.; Ghose, R.; van der Linden, T.J.; van der Star, W.R.; van Loosdrecht, M.C. Quantifying biomediated ground improvement by ureolysis: Large-scale biogrout experiment. J. Geotech. Geoenviron. Eng. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.-D.; Roncal-Herrero, T.; Shaw, S. Mechanistic insights into the crystallization of amorphous calcium carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors affecting efficiency of microbially induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2012, 138, 992–1001. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Chu, D.H.; Vinoba, M.; Bhagiyalakshmi, M.; Baek, I.H.; Nam, S.C.; Yoon, Y.; Kim, S.H.; Jeong, S.K. CO2 mineralization into different polymorphs of CaCO3 using an aqueous-CO2 system. RSC Adv. 2013, 3, 21722–21729. [Google Scholar] [CrossRef]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol. J. 2012, 29, 544–549. [Google Scholar] [CrossRef]
- Yao, D.; Wu, J.; Wang, G.; Wang, P.; Zheng, J.-J.; Yan, J.; Xu, L.; Yan, Y. Effect of wool fiber addition on the reinforcement of loose sands by microbially induced carbonate precipitation (MICP): Mechanical property and underlying mechanism. Acta Geotech. 2021, 16, 1401–1416. [Google Scholar] [CrossRef]
- Yao, D.; Wu, J.; Niu, S.; Gu, Z.; Zheng, J.-J.; Yan, J.; Xu, L.; Yang, M.; Yan, Y. An anionic biopolymer γ-polyglutamate enhanced the microbially induced carbonate precipitation for soil improvement: Mechanical behaviors and underlying mechanism. Acta Geotech. 2022, 17, 4485–4496. [Google Scholar] [CrossRef]
- Choi, S.-G.; Wang, K.; Chu, J. Properties of biocemented, fiber reinforced sand. Constr. Build. Mater. 2016, 120, 623–629. [Google Scholar] [CrossRef]
- Mountassir, G.E.; Lunn, R.J.; Moir, H.; MacLachlan, E. Hydrodynamic coupling in microbially mediated fracture mineralization: Formation of self-organized groundwater flow channels. Water Resour. Res. 2014, 50, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.-J.; Lai, H.-J.; Hoang, T.; Chu, J. One-phase-low-pH enzyme induced carbonate precipitation (EICP) method for soil improvement. Acta Geotech. 2021, 16, 481–489. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Ferris, F.G. The influence of Bacillus pasteurii on the nucleation and growth of calcium carbonate. Geomicrobiol. J. 2006, 23, 213–226. [Google Scholar] [CrossRef]
- Van Paassen, L.A. Biogrout, Ground Improvement by Microbial Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Freeze-thaw durability and shear responses of cemented slope soil treated by microbial induced carbonate precipitation. Soils Found. 2020, 60, 840–855. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Effect of wetting and drying cycles on the durability of bio-cemented soil of expressway slope. Int. J. Environ. Sci. Technol. 2022, 19, 2309–2322. [Google Scholar] [CrossRef]
- Minto, J.M.; Tan, Q.; Lunn, R.J.; El Mountassir, G.; Guo, H.; Cheng, X. ‘Microbial mortar’-restoration of degraded marble structures with microbially induced carbonate precipitation. Constr. Build. Mater. 2018, 180, 44–54. [Google Scholar] [CrossRef]
- Rong, H.; Qian, C.-X.; Li, L.-Z. Influence of molding process on mechanical properties of sandstone cemented by microbe cement. Constr. Build. Mater. 2012, 28, 238–243. [Google Scholar] [CrossRef]
- EN 196-1; Methods of testing cement-part 1: Determination of strength. European Committee for Standardization: Brussels, Belgium, 2016.
- Manzur, T.; Rahman, F.; Afroz, S.; Huq, R.S.; Efaz, I.H. Potential of a microbiologically induced calcite precipitation process for durability enhancement of masonry aggregate concrete. J. Mater. Civil Eng. 2017, 29, 4016290. [Google Scholar] [CrossRef]
- Cui, M.-J.; Zheng, J.-J.; Zhang, R.-J.; Lai, H.-J.; Zhang, J. Influence of cementation level on the strength behaviour of bio-cemented sand. Acta Geotech. 2017, 12, 971–986. [Google Scholar] [CrossRef]
- Rong, H.; Qian, C.X. Binding functions of microbe cement. Adv. Eng. Mater. 2015, 17, 334–340. [Google Scholar] [CrossRef]
- Kawano, J.; Shimobayashi, N.; Kitamura, M.; Shinoda, K.; Aikawa, N. Formation process of calcium carbonate from highly supersaturated solution. J. Cryst. Growth 2002, 237, 419–423. [Google Scholar] [CrossRef]
- Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Effects of Bacterial Density on Growth Rate and Characteristics of Microbial-Induced CaCO3 Precipitates: Particle-Scale Experimental Study. J. Geotech. Geoenviron. Eng. 2021, 147, 4021036. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Zhou, J. Influence of porosity on compressive and tensile strength of cement mortar. Constr. Build. Mater. 2013, 40, 869–874. [Google Scholar] [CrossRef]
- Sharmila, P.; Dhinakaran, G. Compressive strength, porosity and sorptivity of ultra fine slag based high strength concrete. Constr. Build. Mater. 2016, 120, 48–53. [Google Scholar] [CrossRef]
- Kwan, W.H.; Ramli, M.; Cheah, C.B. Flexural strength and impact resistance study of fibre reinforced concrete in simulated aggressive environment. Constr. Build. Mater. 2014, 63, 62–71. [Google Scholar] [CrossRef]
- Brocks, T.; Cioffi, M.O.H.; Voorwald, H.J.C. Effect of fiber surface on flexural strength in carbon fabric reinforced epoxy composites. Appl. Surf. Sci. 2013, 274, 210–216. [Google Scholar] [CrossRef]
- Qabany, A.A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. In Bio-and Chemo-Mechanical Processes in Geotechnical Engineering: Géotechnique Symposium in Print 2013; ICE Publishing: London, UK, 2014; pp. 107–115. [Google Scholar]
- Su, F.; Yang, Y.; Qi, Y.; Zhang, H. Combining microbially induced calcite precipitation (MICP) with zeolite: A new technique to reduce ammonia emission and enhance soil treatment ability of MICP technology. J. Environ. Chem. Eng. 2022, 10, 107770. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Chen, Q.; Wang, L.; Niu, S.; Zheng, J.; Yang, M.; Yan, Y. Morphological Changes of Calcium Carbonate and Mechanical Properties of Samples during Microbially Induced Carbonate Precipitation (MICP). Materials 2022, 15, 7754. https://doi.org/10.3390/ma15217754
Gu Z, Chen Q, Wang L, Niu S, Zheng J, Yang M, Yan Y. Morphological Changes of Calcium Carbonate and Mechanical Properties of Samples during Microbially Induced Carbonate Precipitation (MICP). Materials. 2022; 15(21):7754. https://doi.org/10.3390/ma15217754
Chicago/Turabian StyleGu, Zhaorui, Qing Chen, Lishuang Wang, Shuang Niu, Junjie Zheng, Min Yang, and Yunjun Yan. 2022. "Morphological Changes of Calcium Carbonate and Mechanical Properties of Samples during Microbially Induced Carbonate Precipitation (MICP)" Materials 15, no. 21: 7754. https://doi.org/10.3390/ma15217754




