Effects of Cold Rolling on the Microstructure and Corrosion Resistance of the Double-Glow Plasma Ni-Cr Alloying Layer on Q235 Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
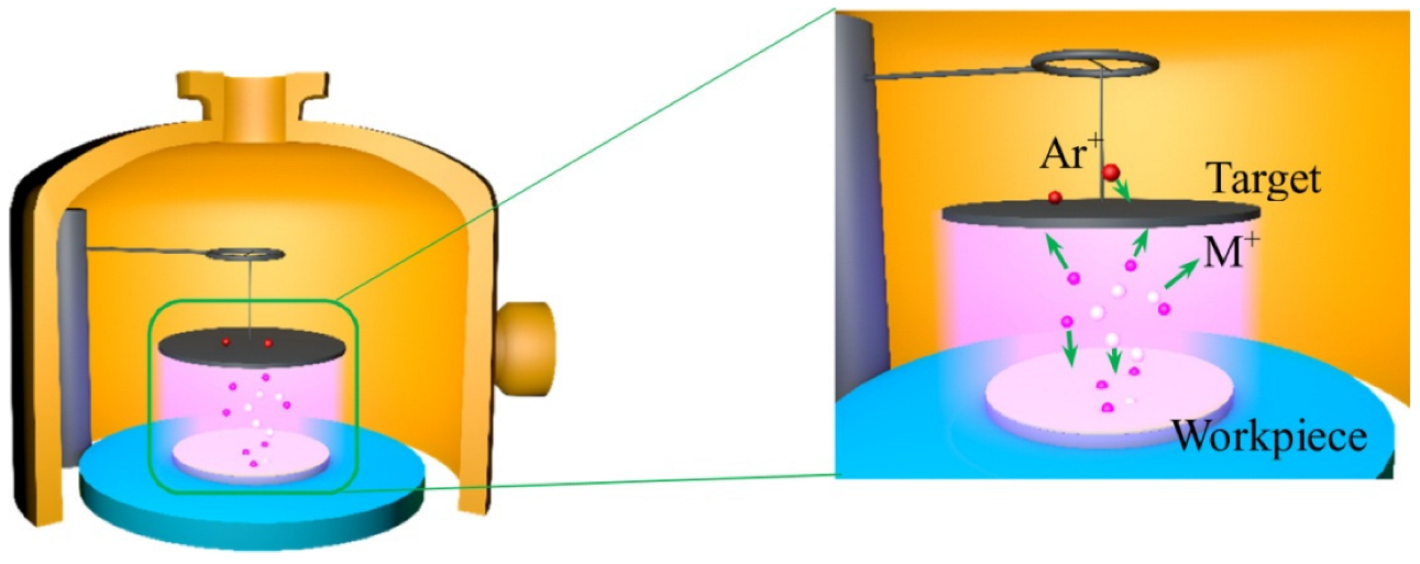
2.2. Test and Inspection Equipment
3. Result and Analysis
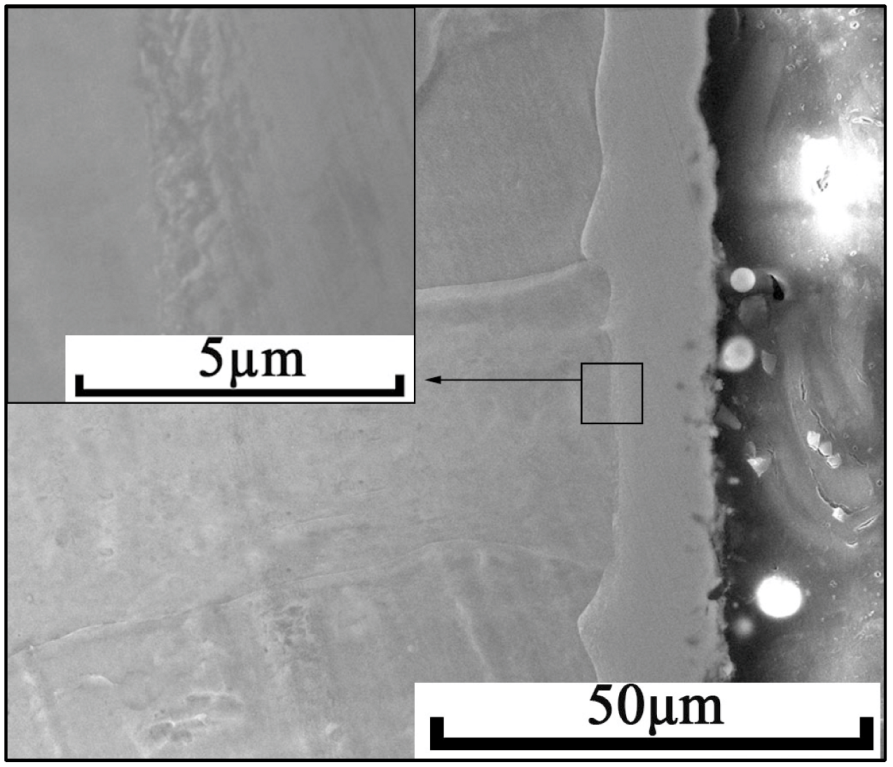
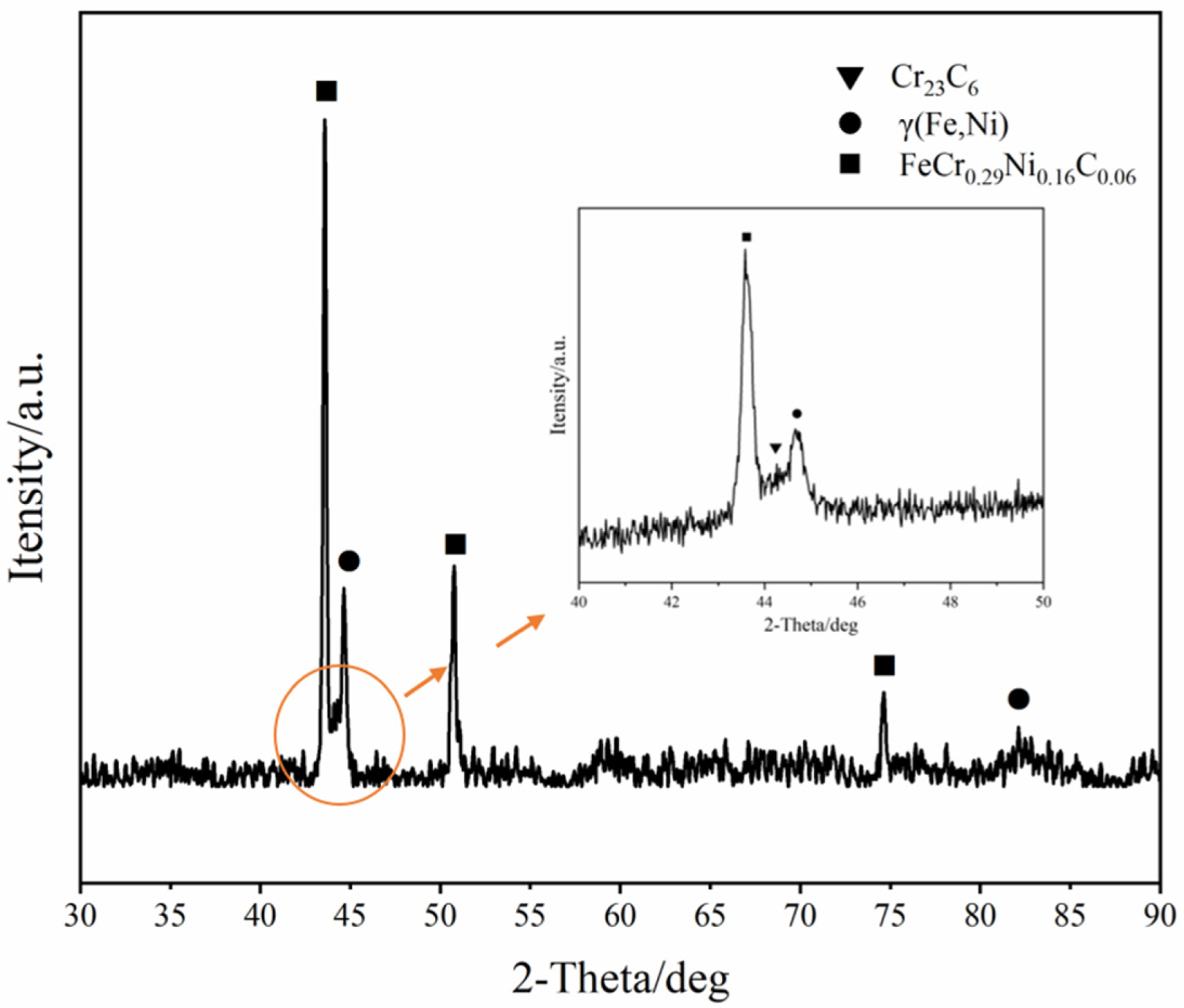
3.1. Morphology and Phase Analysis of the Alloyed Layer
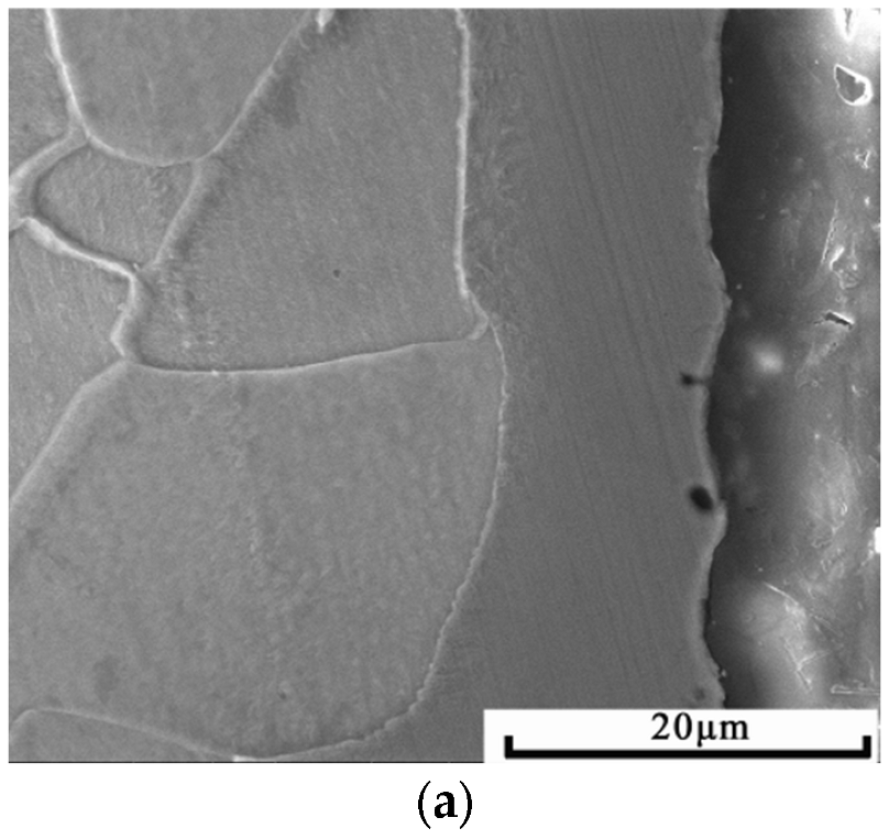
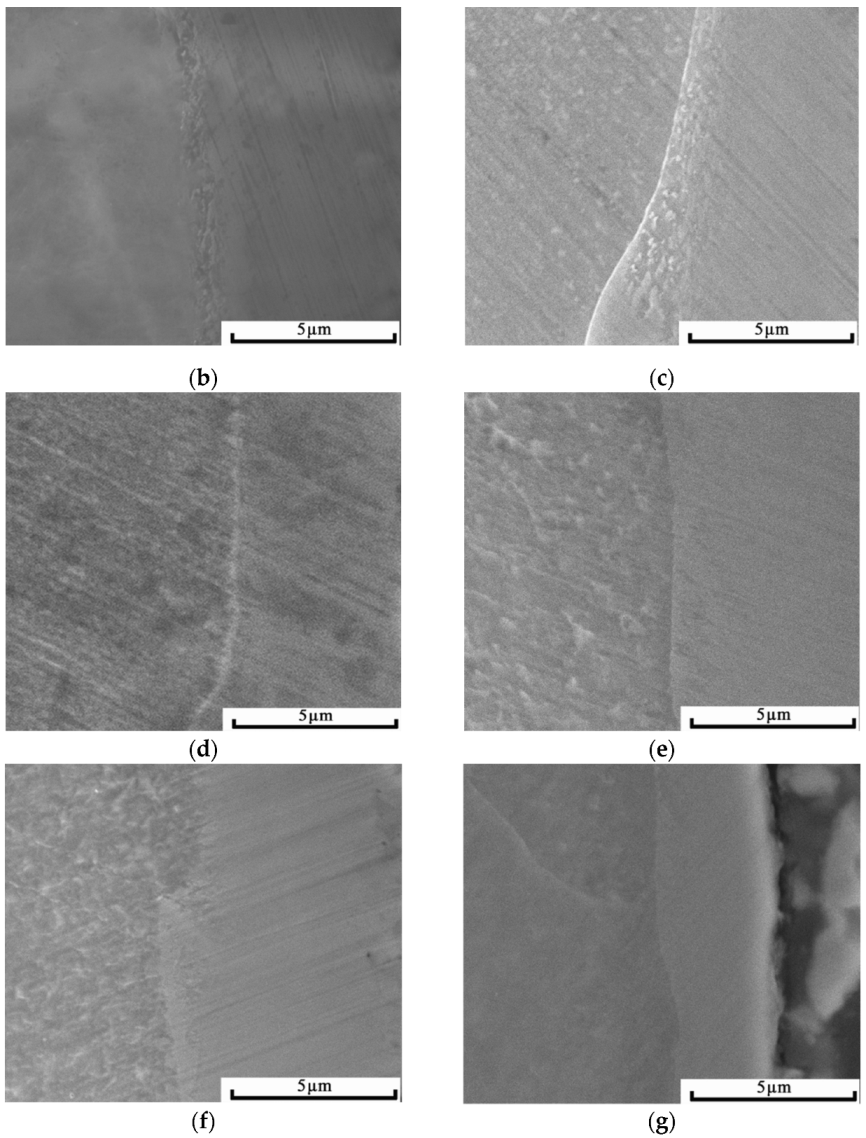
3.2. Effects of Cold Rolling on the Microstructure of the Alloyed Layer
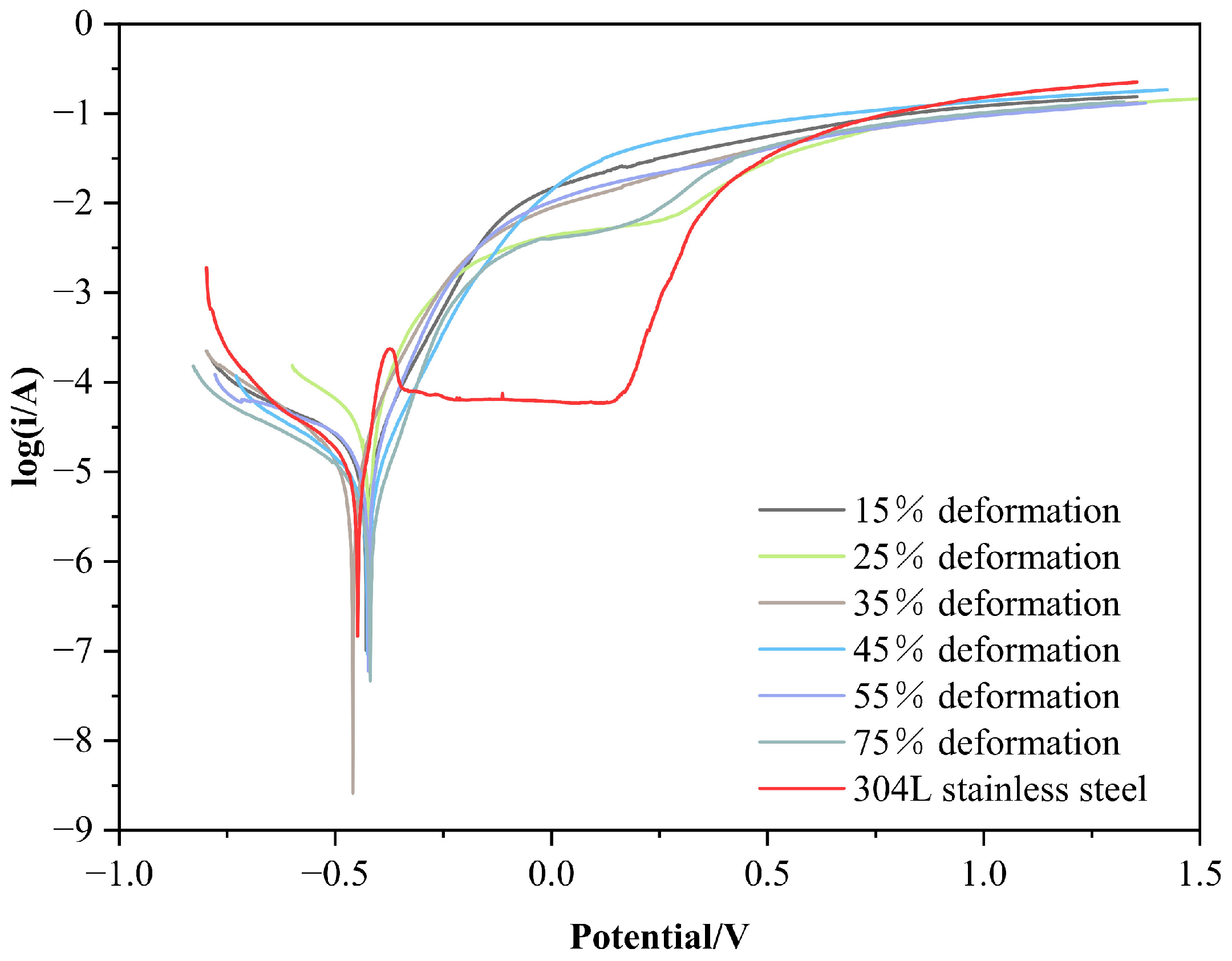
3.3. Electrochemical Corrosion Resistance of a Cold-Rolled Specimen in a 3.5% NaCl Solution
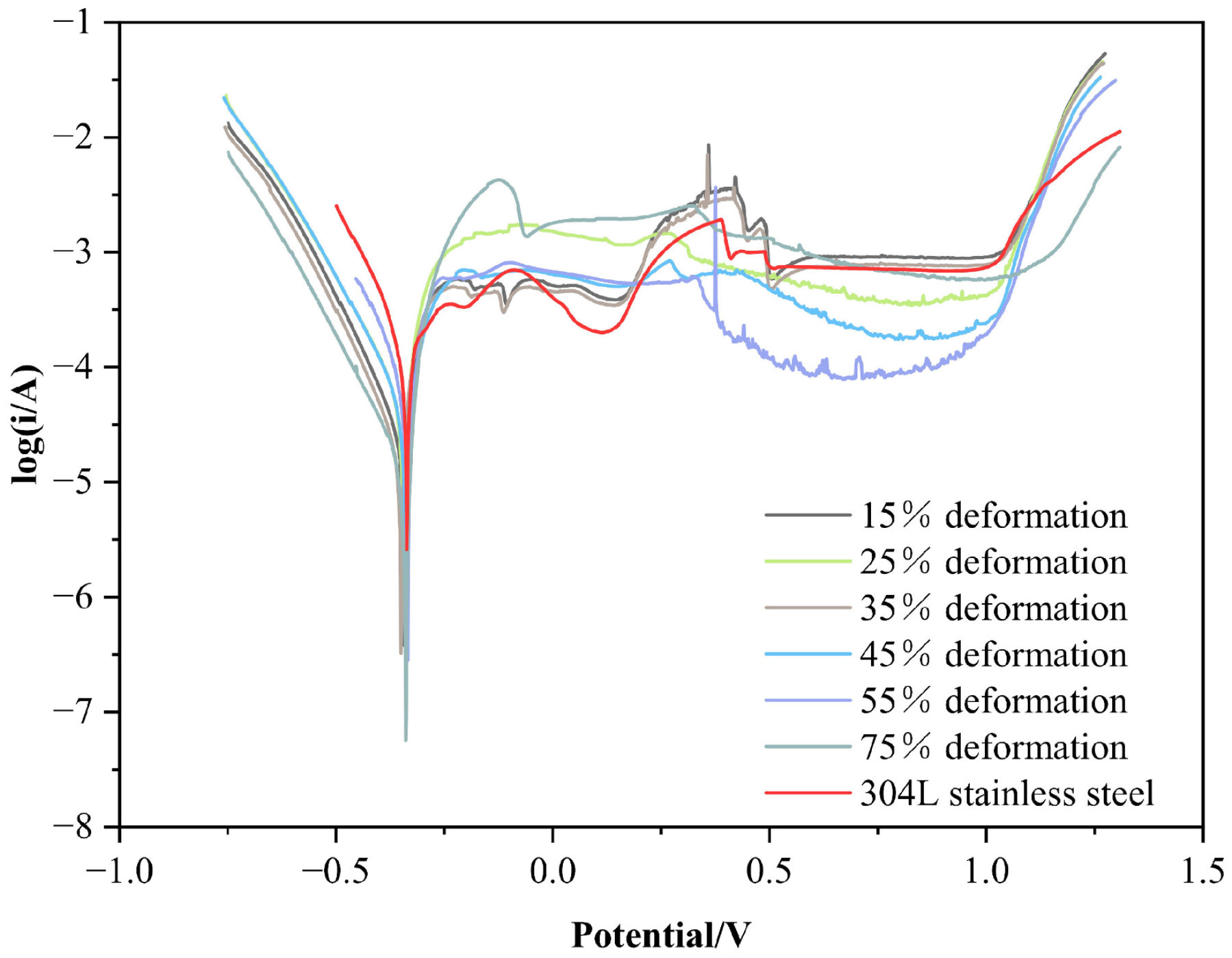
3.4. Electrochemical Corrosion Resistance of Cold-Rolled Specimen in 5% H2SO4 Solution
4. Conclusions
- (1)
- A Ni-Cr layer can be prepared on the surface of Q235 steel using DGPSA technology. This alloyed layer is uniform and dense without cracks and holes. Ni and Cr are distributed in gradient from the outside to the inside. The alloyed layer is metallurgically bonded with the matrix and its phase composition is FeCr0.29Ni0.16C0.06, Cr23C6 compound and γ solid solution.
- (2)
- The Ni-Cr layer cold-rolled with a deformation ranging from 15% to 75% has good plastic toughness and plastic deformation synchronous with the matrix, with no fracturing and spalling.
- (3)
- The self-corrosion potential of the cold-rolled specimens in 5% H2SO4 and 3.5% NaCl solutions is close to that of 304L stainless steel, and the corrosion currents are much lower. The corrosion resistance of the cold-rolled specimens is comparable to the original specimens, with no significant changes.
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, S.; Lin, N.M.; Zeng, Q.F.; Zhang, H.X.; Liu, X.P.; Wang, Z.H.; Wu, Y.C. Recent developments in research of double glow plasma surface alloying technology: A brief review. J. Mater. Res. Technol. 2020, 9, 6859–6882. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Liu, J.; Chen, B.L.; Duan, Y.P.; Hui, L.; Zhao, Y.P. Application of Low Temperature Plasma Technology for the Protection of Metals. Mater. Prot. 2019, 52, 116–120+124. [Google Scholar]
- Xu, Z.; Huang, J.; Xu, Z.F.; Liu, X.P.; Wu, H.Y. Plasma Surface Metallurgy of Materials Based on Double Glow Discharge Phenomenon. Am. J. Phys. Appl. 2021, 9, 70–87. [Google Scholar] [CrossRef]
- Zhu, X.L.; Yao, Z.J.; Gu, X.D.; Cong, W.; Zhang, P.Z. Microstructure and corrosion resistance of Fe-Al intermetallic coating on 45 steel synthesized by double glow plasma surface alloying technology. Trans. Nonferrous Met. Soc. China 2009, 19, 143–148. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Zhang, H.; Ma, Y.; Liu, X.; He, Z. Microstructure and Nanoindentation Behavior of Ni Surface Modified Ti6Al4V. Surf. Eng. 2015, 31, 923–929. [Google Scholar] [CrossRef]
- Xie, R.Z.; Zhou, H.W.; Zou, J.J.; Lin, N.M. Review on the Applications of Double Glow Plasma Surface Alloying for Improving Corrosion Resistance and Wear Resistance of Iron and Steel. Corros. Prot. 2015, 36, 1174–1179. [Google Scholar]
- Zhao, K.; Wu, H.Y.; Xiao, C.L.; Dong, J.Y.; Ren, J.Z.; Peng, Z.X. Study on Corrosion Resistance and Biological Properties of the Double Glow Plasma Nb-Zr Biological Implantation Alloying Layers. Coatings 2022, 12, 942. [Google Scholar] [CrossRef]
- He, J.; Pan, Y.; Wang, J.X.; Zhou, Z.F. Effect of Cold Rolling Deformation on the Corrosion Resistance of Electrodeposited Ni-Co Coating/Steel-Strip. Mater. Prot. 2008, 41, 54–56+90. [Google Scholar]
- Wang, L.; Lu, W.J.; Qin, J.N.; Zhang, F.; Zhang, D. Microstructure and mechanical properties of cold-rolled TiNbTaZr biomedical beta titanium alloy. Mater. Sci. Eng. A 2008, 490, 421–426. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhang, H.; Bi, H.Y.; Chang, E.; Li, M.C. Corrosion behavior of cold-rolled metastable Cr-Mn-Ni-N austenitic stainless steel in acidic NaCl solution. J. Mater. Res. Technol. 2022, 19, 278–288. [Google Scholar] [CrossRef]
- Guo, K.; Wang, T.S.; Meng, K.; Yao, C.; Wang, Q.F. Influence of cold rolling deformation on mechanical properties and corrosion behavior of Ti. Mater. Res. Express. 2020, 7, 066511. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Tao, J.; Liu, Z.; Chen, Z.; Zhu, W. A Novel Synthesis Method for Large Area Metallic Amorphous/Nanocrystal Film by The Glow-Discharge Plasma Technique. Scr. Mater. 2007, 57, 587–590. [Google Scholar] [CrossRef]
- Wei, D.B.; Zhang, P.Z.; Yan, Y.Q.; Chen, X.H.; Li, F.K.; Wang, S.Y.; Yao, Z.J. High-Temperature Oxidation of Double-Glow Plasma Tantalum Alloying on γ-TiAl. Oxid Met. 2019, 92, 337–351. [Google Scholar] [CrossRef]
- Luo, X.X.; Cao, J.; Meng, G.H.; Yu, F.L.; Jiang, Q.; Zhang, P.Z.; Xie, H. Double Glow Plasma Surface Metallurgy Technology Fabricated Fe-Al-Cr Coatings with Excellent Corrosion Resistance. Coatings 2020, 10, 575. [Google Scholar] [CrossRef]
- Wu, W.P.; Chen, Z.F.; Li, B.B.; Cong, X.N.; Chen, Q. Mechanical and electrochemical properties of platinum coating by double glow plasma on titanium alloy substrate. Russ. J. Electrochem. 2013, 49, 76–80. [Google Scholar] [CrossRef]
- Wang, J.Q.; Yang, B.; Zhang, B.; Lin, H.L.; Chen, B.; Jing, Q. Corrosion property of copperized layer on Zr formed by double glow plasma surface alloying technique. Rare Met. 2016, 35, 711–717. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, J.; Liu, L.L.; Jiang, S.Y. Electrochemical Corrosion Behavior of Ta2N Nanoceramic Coating in Simulated Body Fluid. Materials 2016, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, J.J.; Yang, X.; Guo, N.; Xu, H.B.; Li, H.; Jin, Y.; Yu, H.B. Microstructure and annealing behavior of Cr-coatings deposited by double glow plasma on AISI 5140 steel. Results Phys. 2019, 15, 102674. [Google Scholar] [CrossRef]



| C | Si | Mn | P | S | Cr | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.18 | 0.13 | 0.34 | 0.020 | 0.012 | 0.01 | 0.002 | 0.01 | Bal. |
| Test Specimen | Self-Corrosion PotentialEcorr/V | Self-Corrosion Current Densityicorr/mA·cm−2 | Initiating Passivity Current Densityi/mA·cm−2 | Maintaining Passivity Current Densityi/mA·cm−2 | Relative Corrosion Resistance |
|---|---|---|---|---|---|
| 15% | −0.428 | 0.0103 | - | - | 2.65 |
| 25% | −0.431 | 0.0158 | - | - | 1.73 |
| 35% | −0.419 | 0.0112 | - | - | 2.44 |
| 45% | −0.425 | 0.0100 | - | - | 2.73 |
| 55% | −0.423 | 0.0136 | - | - | 2.01 |
| 75% | −0.416 | 0.0153 | - | - | 1.78 |
| Original specimen | −0.423 | 0.0116 | - | - | 2.35 |
| 304L | −0.448 | 0.0273 | 0.0631 | 0.240 | 1.00 |
| Test Specimen | Self-Corrosion PotentialEcorr/V | Self-Corrosion Current Densityicorr/mA·cm−2 | Initiating Passivity Current Densityi/mA·cm−2 | Maintaining Passivity Current Densityi/mA·cm−2 | Relative Corrosion Resistance |
|---|---|---|---|---|---|
| 15% | −0.342 | 0.320 | 0.575 | 0.871 | 1.24 |
| 25% | −0.345 | 0.328 | 1.480 | 0.389 | 1.22 |
| 35% | −0.352 | 0.301 | 0.562 | 0.871 | 1.33 |
| 45% | −0.339 | 0.386 | 0.676 | 0.182 | 1.03 |
| 55% | −0.348 | 0.319 | 0.589 | 0.079 | 1.25 |
| 75% | −0.350 | 0.318 | 3.980 | 0.525 | 1.25 |
| Original specimen | −0.351 | 0.307 | 1.510 | 1.200 | 1.30 |
| 304L | −0.342 | 0.399 | 0.363 | 0.759 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Yao, Z.; Chen, X.; Yao, Q.; Zhang, P.; Huang, G.; Feng, B.; Xu, X. Effects of Cold Rolling on the Microstructure and Corrosion Resistance of the Double-Glow Plasma Ni-Cr Alloying Layer on Q235 Steel. Materials 2022, 15, 7882. https://doi.org/10.3390/ma15227882
Zhu X, Yao Z, Chen X, Yao Q, Zhang P, Huang G, Feng B, Xu X. Effects of Cold Rolling on the Microstructure and Corrosion Resistance of the Double-Glow Plasma Ni-Cr Alloying Layer on Q235 Steel. Materials. 2022; 15(22):7882. https://doi.org/10.3390/ma15227882
Chicago/Turabian StyleZhu, Xiaolin, Zhengjun Yao, Xiang Chen, Qiang Yao, Pingze Zhang, Guanxi Huang, Baodong Feng, and Xuebin Xu. 2022. "Effects of Cold Rolling on the Microstructure and Corrosion Resistance of the Double-Glow Plasma Ni-Cr Alloying Layer on Q235 Steel" Materials 15, no. 22: 7882. https://doi.org/10.3390/ma15227882





