Polyether Ether Ketone Coated with Ultra-Thin Films of Titanium Oxide and Zirconium Oxide Fabricated by DC Magnetron Sputtering for Biomedical Application
Abstract
:1. Introduction
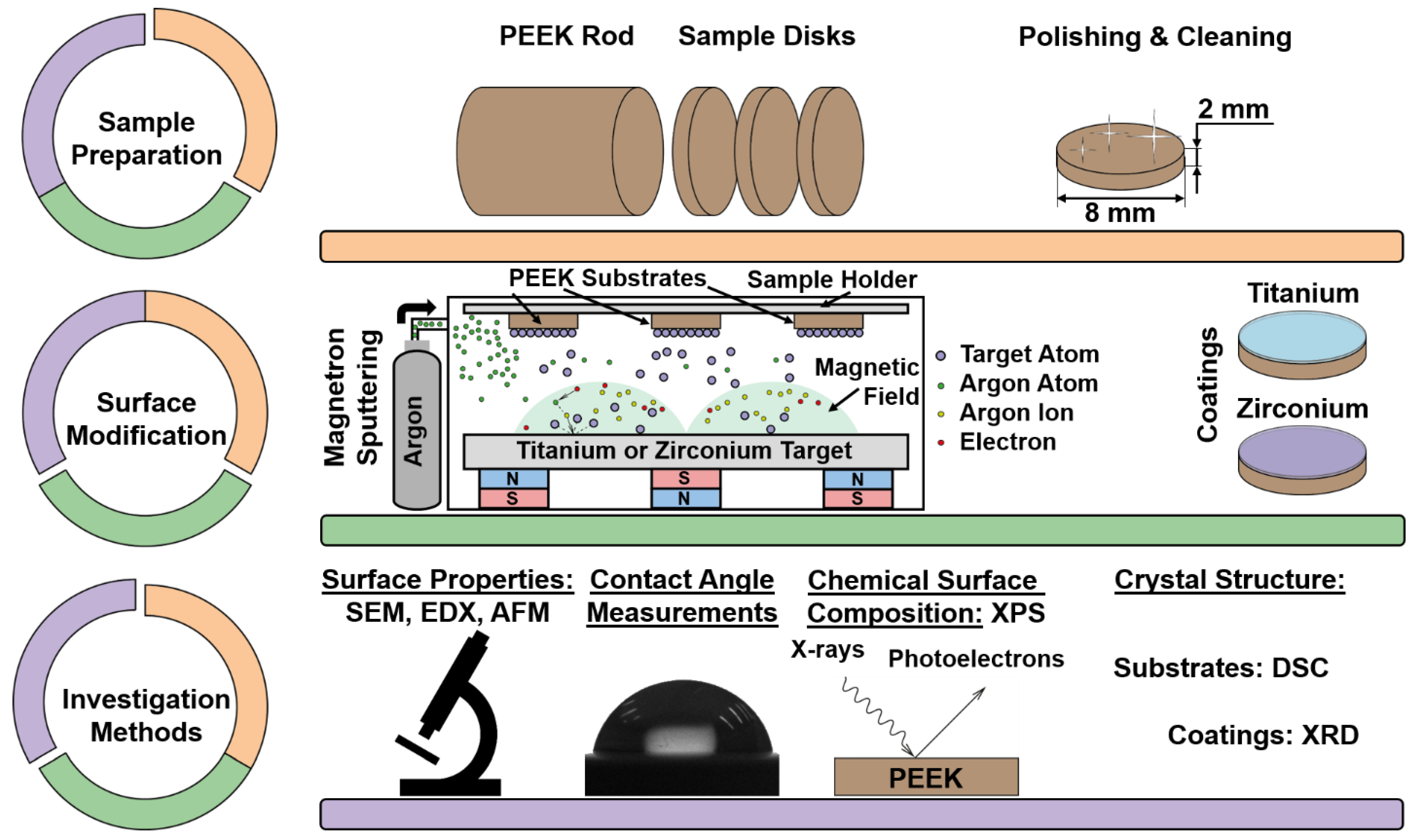
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Modification
2.3. Investigation Methods
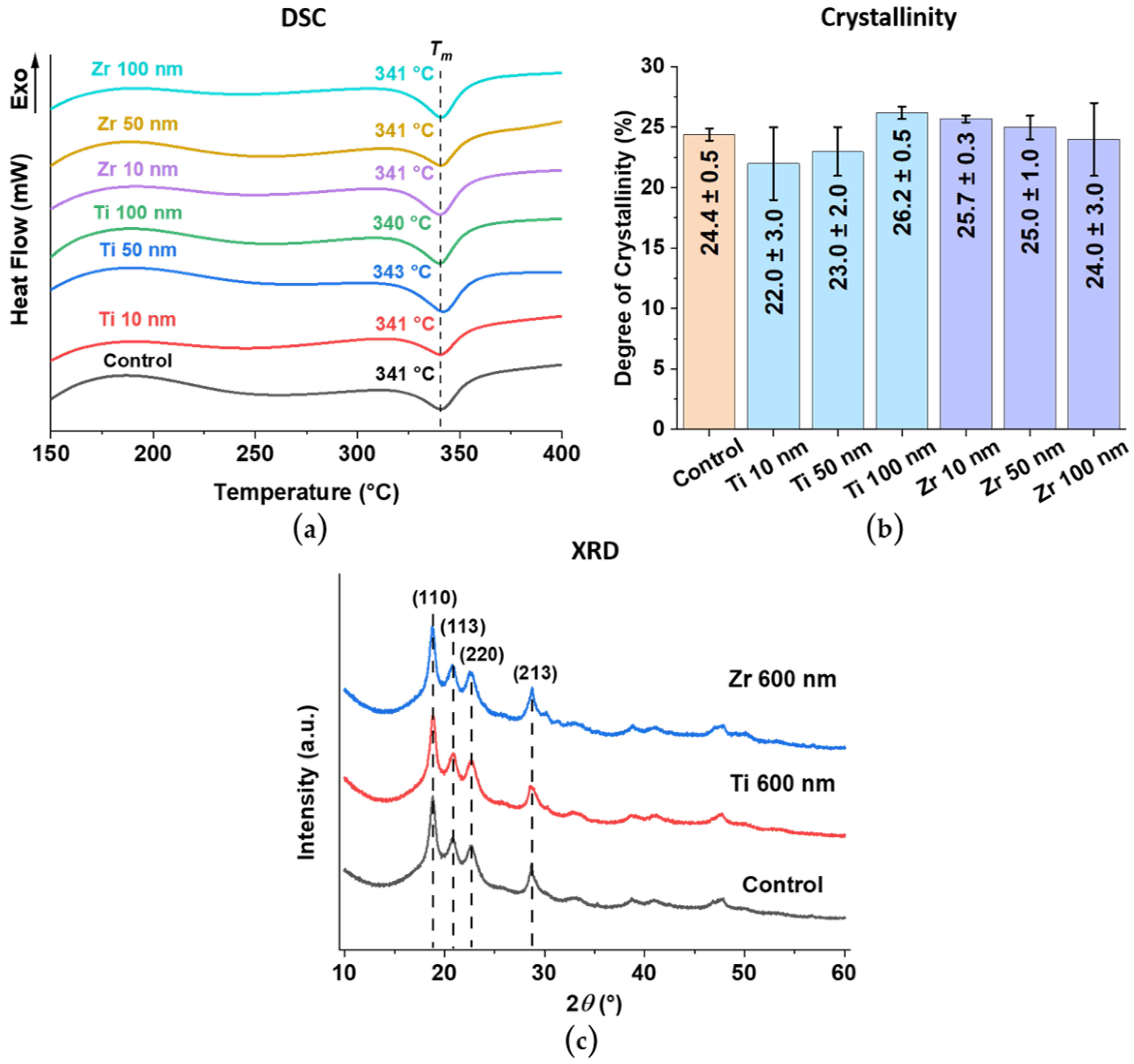
3. Results and Discussion
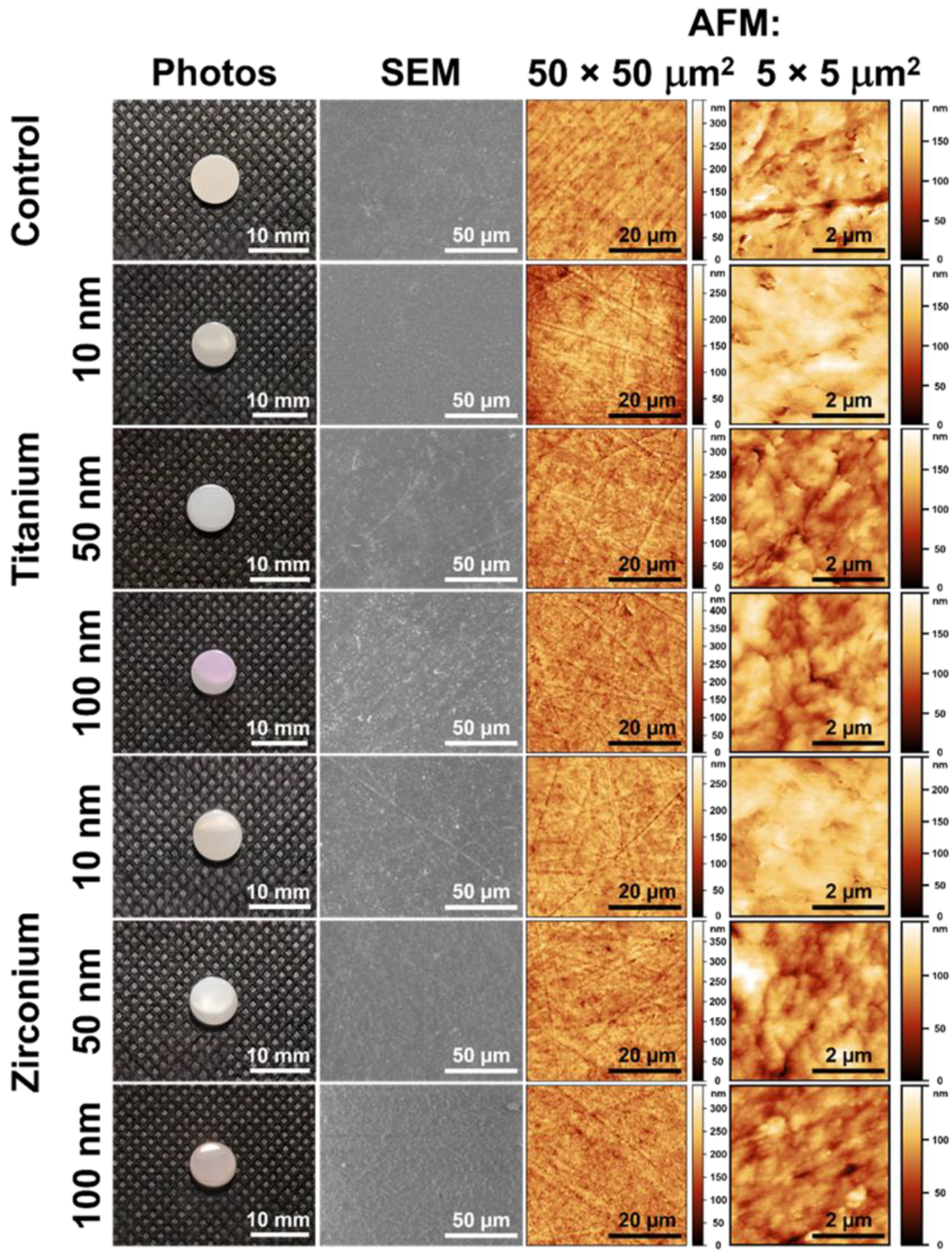
3.1. Coating Thickness and Surface Morphology
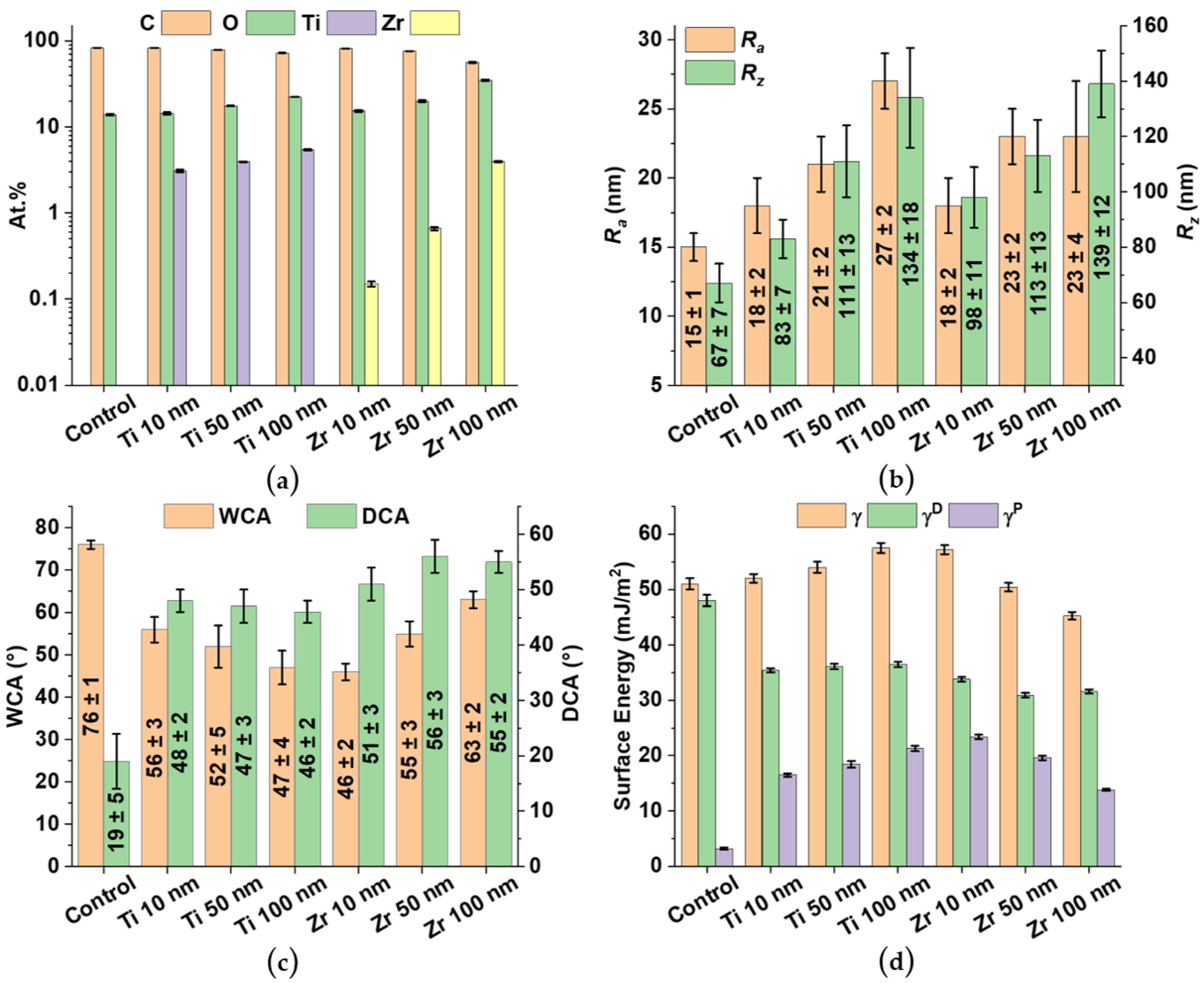
3.2. Elemental Composition of the Sample Surfaces
3.3. Surface Roughness
3.4. Surface Wettability
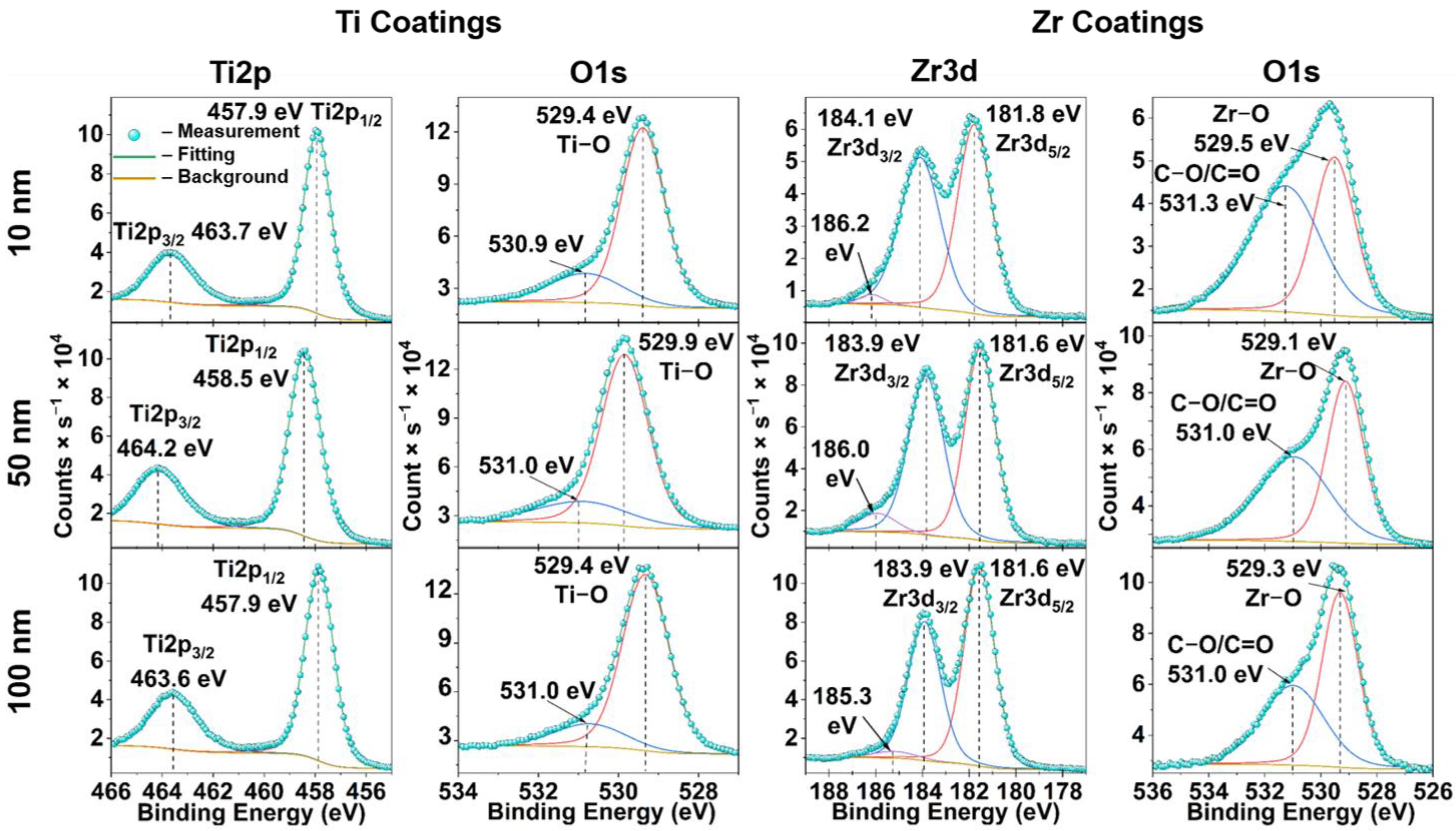
3.5. X-ray Photoelectron Spectroscopy
3.6. Crystal Structure of PEEK Substrate and Ti and Zr Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Y.H.; Liu, S.J. Polyetheretherketone for Orthopedic Applications: A Review. Curr. Opin. Chem. Eng. 2021, 32, 100687. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical Properties of Biomedical Titanium Alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Han, X.; Gao, W.; Zhou, Z.; Yang, S.; Wang, J.; Shi, R.; Li, Y.; Jiao, J.; Qi, Y.; Zhao, J. Application of Biomolecules Modification Strategies on PEEK and Its Composites for Osteogenesis and Antibacterial Properties. Colloids Surf. B. Biointerfaces 2022, 215, 112492. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, W.; Yuan, Z.; Chen, J.; Lyu, Z.; Wang, Y. A Partial Hemi-Resurfacing Preliminary Study of a Novel Magnetic Resonance Imaging Compatible Polyetheretherketone Mini-Prosthesis for Focal Osteochondral Defects. J. Orthop. Transl. 2021, 26, 67. [Google Scholar] [CrossRef]
- Kurtz, S.M. PEEK Biomaterials Handbook. A Volume in Plastics Design Library; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128125243. [Google Scholar]
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Zou, B.; Ding, S.; Li, L.; Huang, C. Effects of FDM-3D Printing Parameters on Mechanical Properties and Microstructure of CF/PEEK and GF/PEEK. Chin. J. Aeronaut. 2021, 34, 236–246. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Ismail, S.O.; Bowoto, O.K.; Omigbodun, F.T.; Olawumi, M.A.; Muhammad, M.A. Lattice Design and 3D-Printing of PEEK with Ca10(OH)(PO4)3 and in-Vitro Bio-Composite for Bone Implant. Int. J. Biol. Macromol. 2020, 165, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Poulsson, A.H.C.; Eglin, D.; Zeiter, S.; Camenisch, K.; Sprecher, C.; Agarwal, Y.; Nehrbass, D.; Wilson, J.; Richards, R.G. Osseointegration of Machined, Injection Moulded and Oxygen Plasma Modified PEEK Implants in a Sheep Model. Biomaterials 2014, 35, 3717–3728. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, P.; Zhang, X.; Xin, J.; Wang, Y.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to Improve Bioactive and Antibacterial Properties of Polyetheretherketone (PEEK) for Use as Orthopedic Implants. Mater. Today Bio 2022, 16, 100402. [Google Scholar] [CrossRef]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of Osteointegration Property between PEKK and PEEK: Effects of Surface Structure and Chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef]
- Han, C.M.; Lee, E.J.; Kim, H.E.; Koh, Y.H.; Kim, K.N.; Ha, Y.; Kuh, S.U. The Electron Beam Deposition of Titanium on Polyetheretherketone (PEEK) and the Resulting Enhanced Biological Properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.P.; Silva, S.N.; Sousa, J.A. Flexural Fatigue Behavior of Plasma-Sprayed Hydroxyapatite-Coated Polyether-Ether-Ketone (PEEK) Injection Moldings Derived from Dynamic Mechanical Analysis. Int. J. Fatigue 2018, 108, 1–8. [Google Scholar] [CrossRef]
- Vogel, D.; Dempwolf, H.; Baumann, A.; Bader, R. Characterization of Thick Titanium Plasma Spray Coatings on PEEK Materials Used for Medical Implants and the Influence on the Mechanical Properties. J. Mech. Behav. Biomed. Mater. 2018, 77, 600–608. [Google Scholar] [CrossRef]
- Novotná, Z.; Rimpelová, S.; Juřík, P.; Veselý, M.; Kolská, Z.; Hubáček, T.; Borovec, J.; Švorčík, V. Tuning Surface Chemistry of Polyetheretherketone by Gold Coating and Plasma Treatment. Nanoscale Res. Lett. 2017, 12, 424. [Google Scholar] [CrossRef] [Green Version]
- Xian, P.; Chen, Y.; Gao, S.; Qian, J.; Zhang, W.; Udduttula, A.; Huang, N.; Wan, G. Polydopamine (PDA) Mediated Nanogranular-Structured Titanium Dioxide (TiO2) Coating on Polyetheretherketone (PEEK) for Oral and Maxillofacial Implants Application. Surf. Coat. Technol. 2020, 401, 126282. [Google Scholar] [CrossRef]
- Hench, L.L.; Wilson, J. An Introduction to Bioceramics; World Scientific: Singapore, 1993. [Google Scholar] [CrossRef]
- Barkarmo, S.; Wennerberg, A.; Hoffman, M.; Kjellin, P.; Breding, K.; Handa, P.; Stenport, V. Nano-Hydroxyapatite-Coated PEEK Implants: A Pilot Study in Rabbit Bone. J. Biomed. Mater. Res. Part A 2013, 101, 465–471. [Google Scholar] [CrossRef]
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Kim, J.W.; Ahn, C.W.; Kim, H.E.; Yoon, B.H.; et al. Osteoconductive Hydroxyapatite Coated PEEK for Spinal Fusion Surgery. APSS 2013, 283, 6–11. [Google Scholar] [CrossRef]
- Fidancevska, E.; Ruseska, G.; Zafirovski, S.; Pavlovski, B. Thermal-Expansion and Mechanical Properties of the Ca10(P04)6(OH) 2-TiO2 Composite. Sci. Sinter. 2002, 34, 241–246. [Google Scholar] [CrossRef]
- Goyal, R.K.; Tiwari, A.N.; Mulik, U.P.; Negi, Y.S. Thermal Expansion Behaviour of High Performance PEEK Matrix Composites. J. Phys. D Appl. Phys. 2008, 41, 085403. [Google Scholar] [CrossRef]
- Yao, C.; Storey, D.; Webster, T.J. Nanostructured Metal Coatings on Polymers Increase Osteoblast Attachment. Int. J. Nanomed. 2007, 2, 487–492. [Google Scholar] [CrossRef]
- Han, C.M.; Jang, T.S.; Kim, H.E.; Koh, Y.H. Creation of Nanoporous TiO2 Surface onto Polyetheretherketone for Effective Immobilization and Delivery of Bone Morphogenetic Protein. J. Biomed. Mater. Res. Part A 2014, 102, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.B.; Swain, M.V. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is Zirconia a Viable Alternative to Titanium for Oral Implant? A Critical Review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef]
- Ozeki, K.; Masuzawa, T.; Aoki, H. Fabrication of Hydroxyapatite Thin Films on Polyetheretherketone Substrates Using a Sputtering Technique. Mater. Sci. Eng. C 2017, 72, 576–582. [Google Scholar] [CrossRef]
- Rabiei, A.; Sandukas, S. Processing and Evaluation of Bioactive Coatings on Polymeric Implants. J. Biomed. Mater. Res. Part A 2013, 101, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Kjellin, P.; Vikingsson, L.; Danielsson, K.; Johansson, P.; Wennerberg, A. A Nanosized Zirconium Phosphate Coating for PEEK Implants and Its Effect in Vivo. Materialia 2020, 10, 100645. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A Review of Hydroxyapatite-Based Coating Techniques: Sol–Gel and Electrochemical Depositions on Biocompatible Metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Su, J.; Wang, H.; Yang, L.; Li, M.; Li, Z.; Liu, J.; Wen, S.; Zhou, Y.; Yan, C.; et al. Aging Mechanism of Polyetheretherketone Powder during Layer-Wise Infrared Radiation of High-Temperature Laser Powder Bed Fusion. Mater. Des. 2022, 213, 110348. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural Evolution during Film Growth. J. Vac. Sci. Technol. A Vac. Surf. Film. 2003, 21, S117. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [Green Version]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A Review on the Wettability of Dental Implant Surfaces I: Theoretical and Experimental Aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [Green Version]
- Rosales-Leal, J.I.; Rodríguez-Valverde, M.A.; Mazzaglia, G.; Ramón-Torregrosa, P.J.; Díaz-Rodríguez, L.; García-Martínez, O.; Vallecillo-Capilla, M.; Ruiz, C.; Cabrerizo-Vílchez, M.A. Effect of Roughness, Wettability and Morphology of Engineered Titanium Surfaces on Osteoblast-like Cell Adhesion. Colloids Surf. A Physicochem. Eng. Asp. 2010, 365, 222–229. [Google Scholar] [CrossRef]
- Rawal, S.K.; Chawla, A.K.; Jayaganthan, R.; Chandra, R. The Influence of Various Sputtering Parameters on Structural, Wettability and Optical Properties of Zr2ON2 Thin Films. Mater. Sci. Eng. B 2014, 181, 16–23. [Google Scholar] [CrossRef]
- Hussain, S.; Rutledge, L.; Acheson, J.G.; Boyd, A.R.; Meenan, B.J. The Surface Characterisation of Polyetheretherketone (PEEK) Modified via the Direct Sputter Deposition of Calcium Phosphate Thin Films. Coatings 2020, 10, 1088. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Kalubarme, R.S.; Chikate, P.R.; Park, C.-J.; Ma, Y.-R.; Shirage, P.M.; Devan, R.S. An Investigation on the Effect of Li–Ion Cycling on the Vertically Aligned Brookite TiO2 Nanostructure. ChemistrySelec 2019, 4, 6620–6626. [Google Scholar] [CrossRef]
- Kondoh, J. Origin of the Hump on the Left Shoulder of the X-ray Diffraction Peaks Observed in Y2O3-Fully and Partially Stabilized ZrO. J. Alloy. Compd. 2004, 375, 270–282. [Google Scholar] [CrossRef]
- Obrosov, A.; Sutygina, A.N.; Manakhov, A.; Bolz, S.; Weiß, S.; Kashkarov, E.B. Oxidation Behavior of Zr–1Nb Corroded in Air at 400 °C after Plasma Immersion Titanium Implantation. Metals 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbé, K.; Denape, J.; Chabert, F. A Comparative Study of the Crystallinity of Polyetheretherketone by Using Density, DSC, XRD, and Raman Spectroscopy Techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Han, X.; Sharma, N.; Spintzyk, S.; Zhou, Y.; Xu, Z.; Thieringer, F.M.; Rupp, F. Tailoring the Biologic Responses of 3D Printed PEEK Medical Implants by Plasma Functionalization. Dent. Mater. 2022, 38, 1083–1098. [Google Scholar] [CrossRef]
- Li, H.; Khor, K.A.; Cheang, P. Adhesive and Bending Failure of Thermal Sprayed Hydroxyapatite Coatings: Effect of Nanostructures at Interface and Crack Propagation Phenomenon during Bending. Eng. Fract. Mech. 2007, 74, 1894–1903. [Google Scholar] [CrossRef]
- Aparicio-Razo, M.; Mongalo-Vázquez, J.L., Jr.; Yáñez Ramos, J.A.; Navarro-Zárate, A.; Hugo Santos-Enríquez, V.; Vivanco-Pérez, I.; Flores Méndez, J.; Alberto Paredes-Juárez, G. Comparison of Material Properties for Dental Implants: Titanium, Polyetheretherketone, Zirconium and Silicon Nitride. Adv. Mater. Lett. 2022, 13, 2201–1684. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Maryin, P.V.; Fedotkin, A.Y.; Bolbasov, E.N.; Kozelskaya, A.I.; Buldakov, M.A.; Evtina, A.A.; Cherdyntseva, N.V.; Rutkowski, S.; Tverdokhlebov, S.I. Surface Modification of PLLA Scaffolds via Reactive Magnetron Sputtering in Mixtures of Nitrogen with Noble Gases for Higher Cell Adhesion and Proliferation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129464. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujibayashi, S.; Yamaguchi, S.; Yamamoto, K.; Otsuki, B.; Takemoto, M.; Tsukanaka, M.; Kizuki, T.; Matsushita, T.; Kokubo, T.; et al. Bioactivity of Sol–Gel-Derived TiO2 Coating on Polyetheretherketone: In Vitro and in Vivo Studies. Acta Biomater. 2016, 35, 305–317. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akimchenko, I.O.; Rutkowski, S.; Tran, T.-H.; Dubinenko, G.E.; Petrov, V.I.; Kozelskaya, A.I.; Tverdokhlebov, S.I. Polyether Ether Ketone Coated with Ultra-Thin Films of Titanium Oxide and Zirconium Oxide Fabricated by DC Magnetron Sputtering for Biomedical Application. Materials 2022, 15, 8029. https://doi.org/10.3390/ma15228029
Akimchenko IO, Rutkowski S, Tran T-H, Dubinenko GE, Petrov VI, Kozelskaya AI, Tverdokhlebov SI. Polyether Ether Ketone Coated with Ultra-Thin Films of Titanium Oxide and Zirconium Oxide Fabricated by DC Magnetron Sputtering for Biomedical Application. Materials. 2022; 15(22):8029. https://doi.org/10.3390/ma15228029
Chicago/Turabian StyleAkimchenko, Igor O., Sven Rutkowski, Tuan-Hoang Tran, Gleb E. Dubinenko, Vsevolod I. Petrov, Anna I. Kozelskaya, and Sergei I. Tverdokhlebov. 2022. "Polyether Ether Ketone Coated with Ultra-Thin Films of Titanium Oxide and Zirconium Oxide Fabricated by DC Magnetron Sputtering for Biomedical Application" Materials 15, no. 22: 8029. https://doi.org/10.3390/ma15228029





