Assessment of the Synergetic Performance of Nanostructured CeO2-SnO2/Al2O3 Mixed Oxides on Automobile Exhaust Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis Route
2.3. Catalyst Substrate Preparation
2.4. Sample Characterization
2.5. Catalyst Performance Testing
3. Results
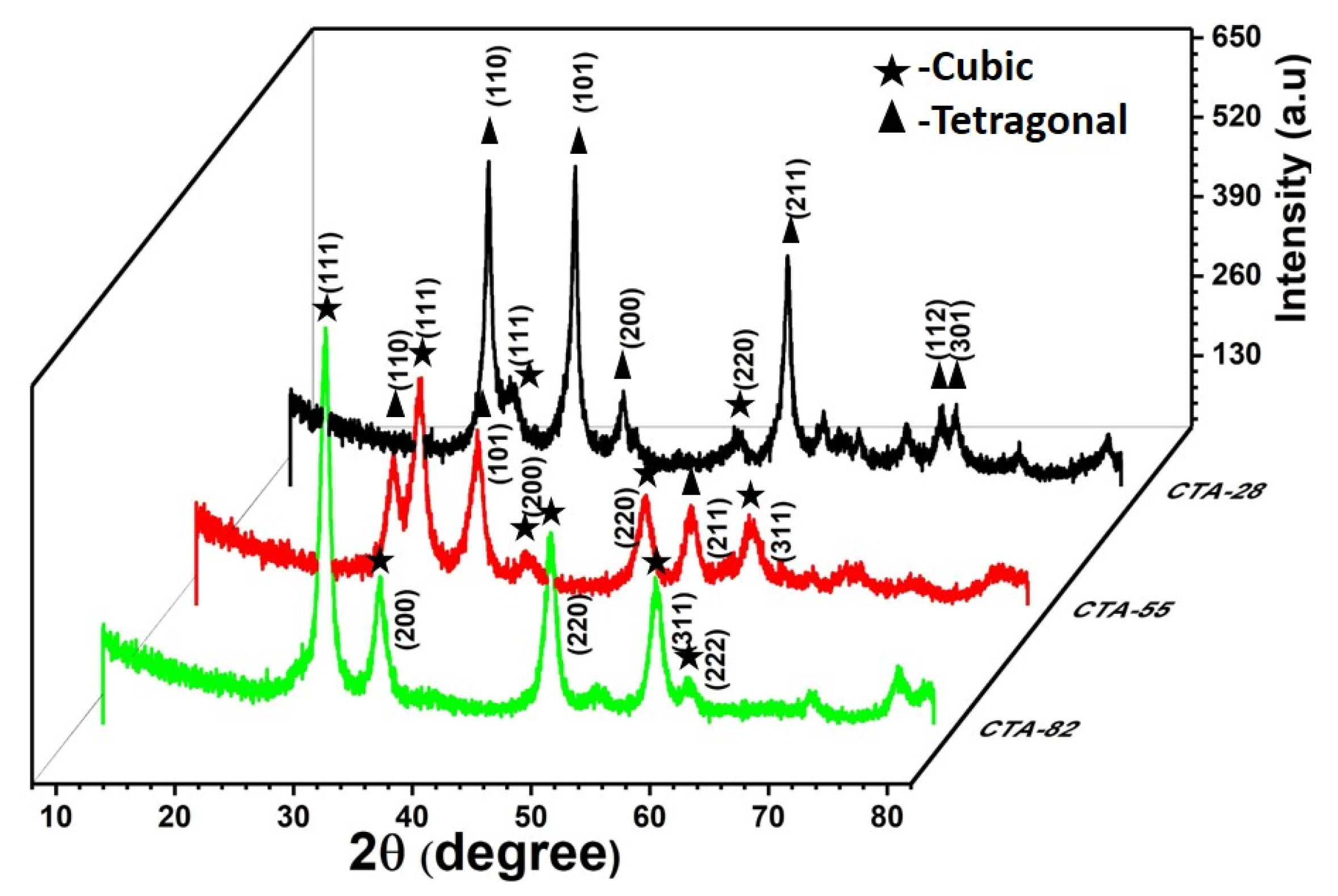
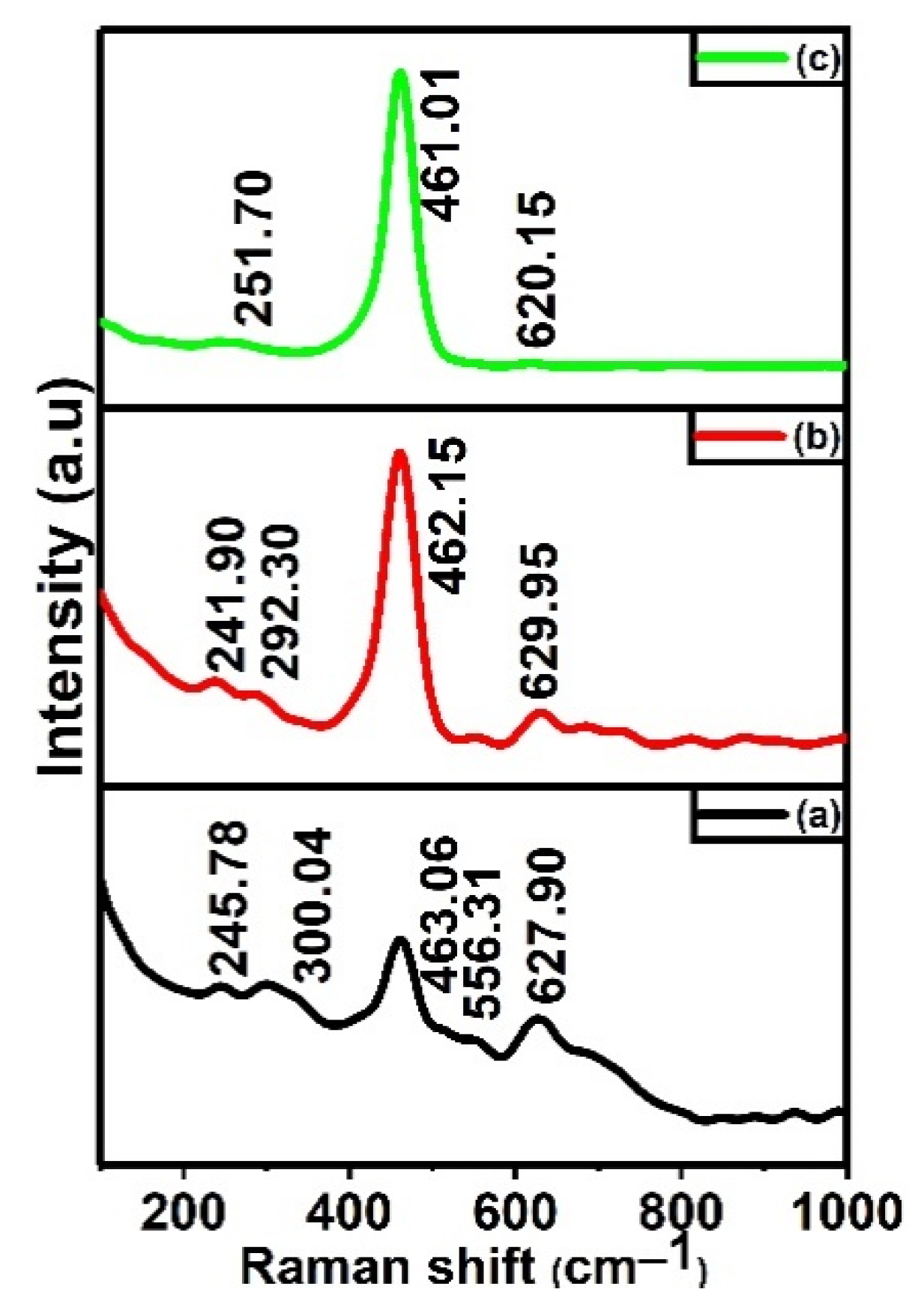
3.1. Structural Investigations (XRD, Raman, HRTEM)
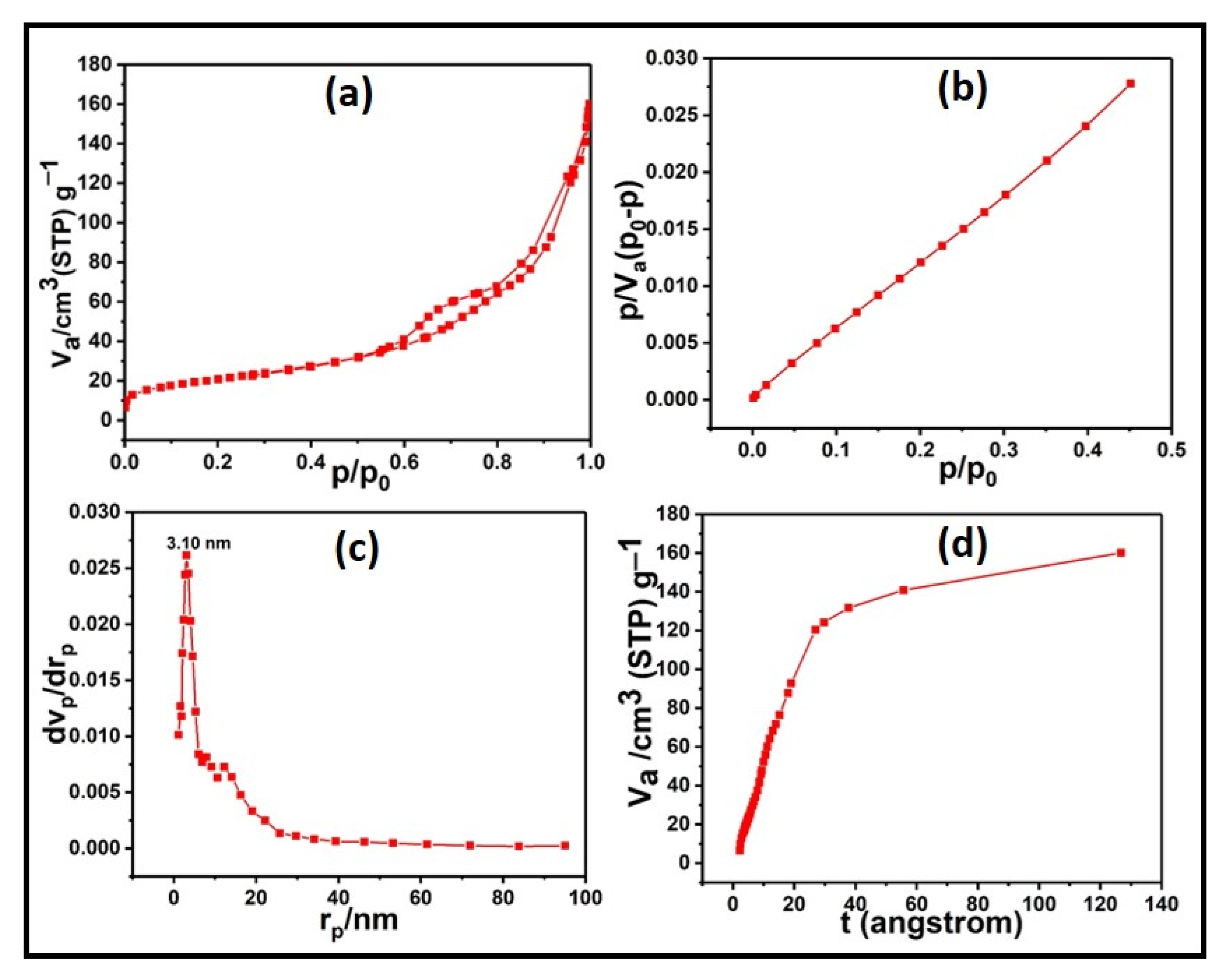
3.2. Textural Investigations
3.3. Chemical States Analysis (XPS)
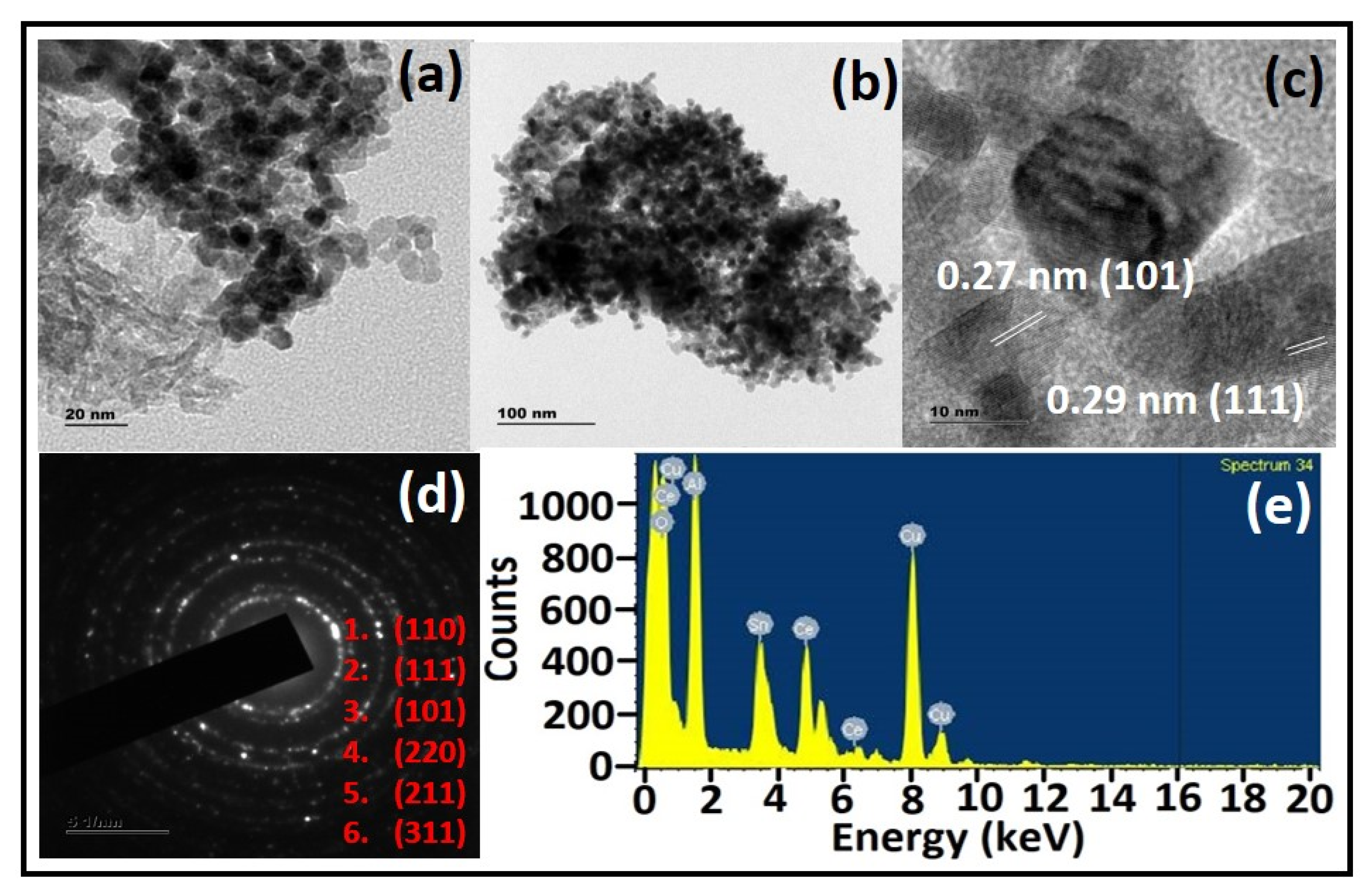
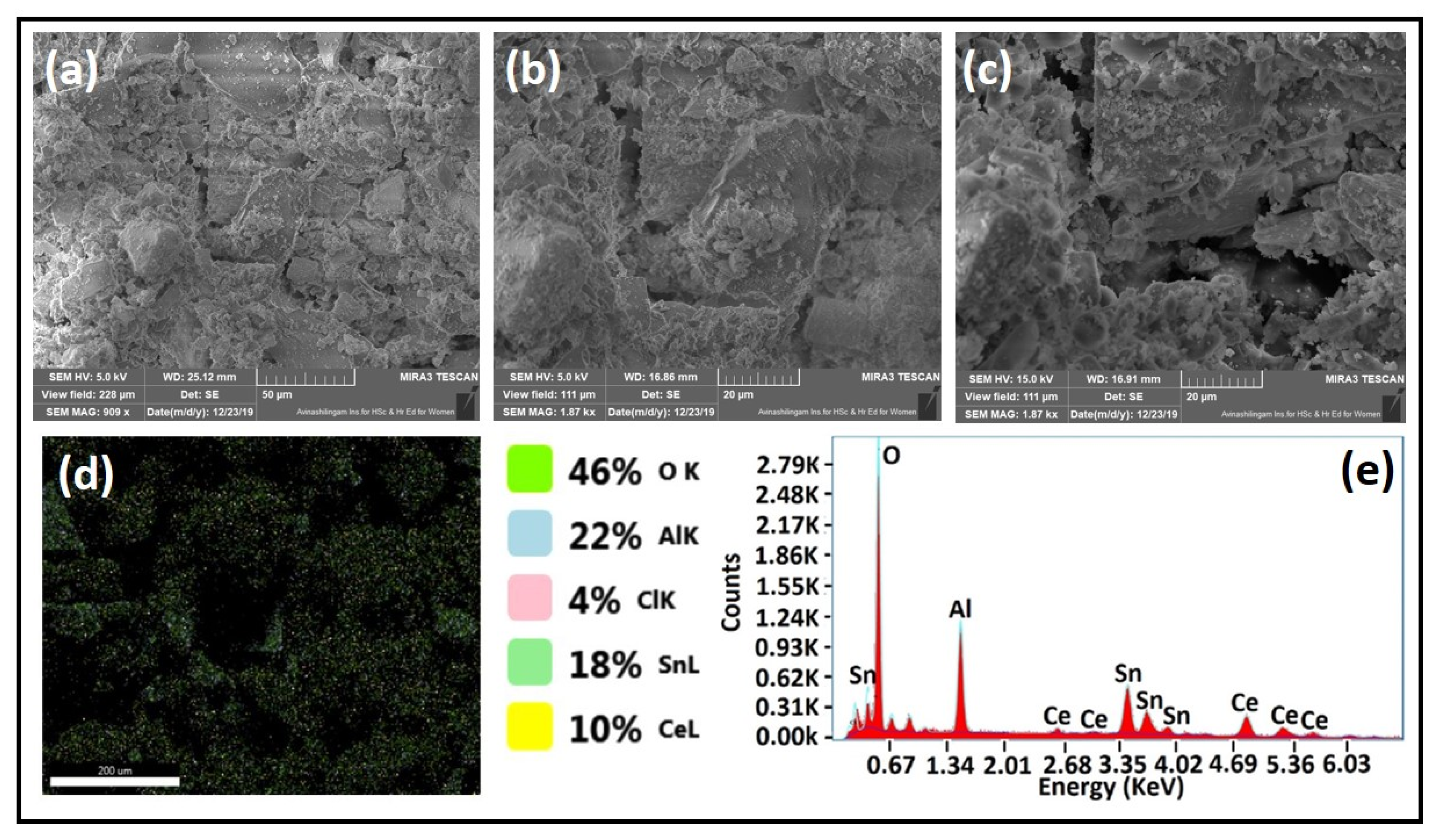
3.4. Morphological Analysis
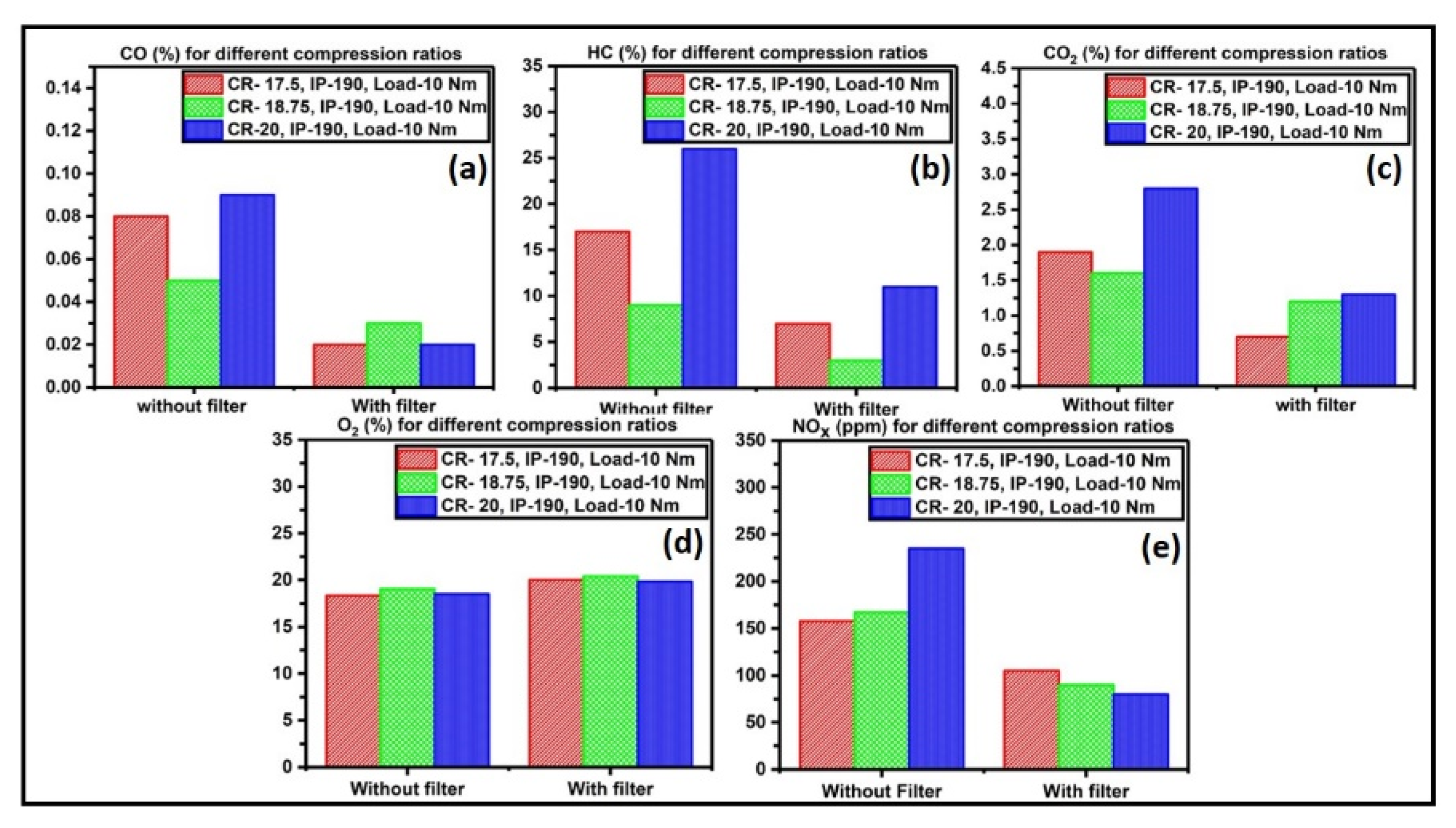
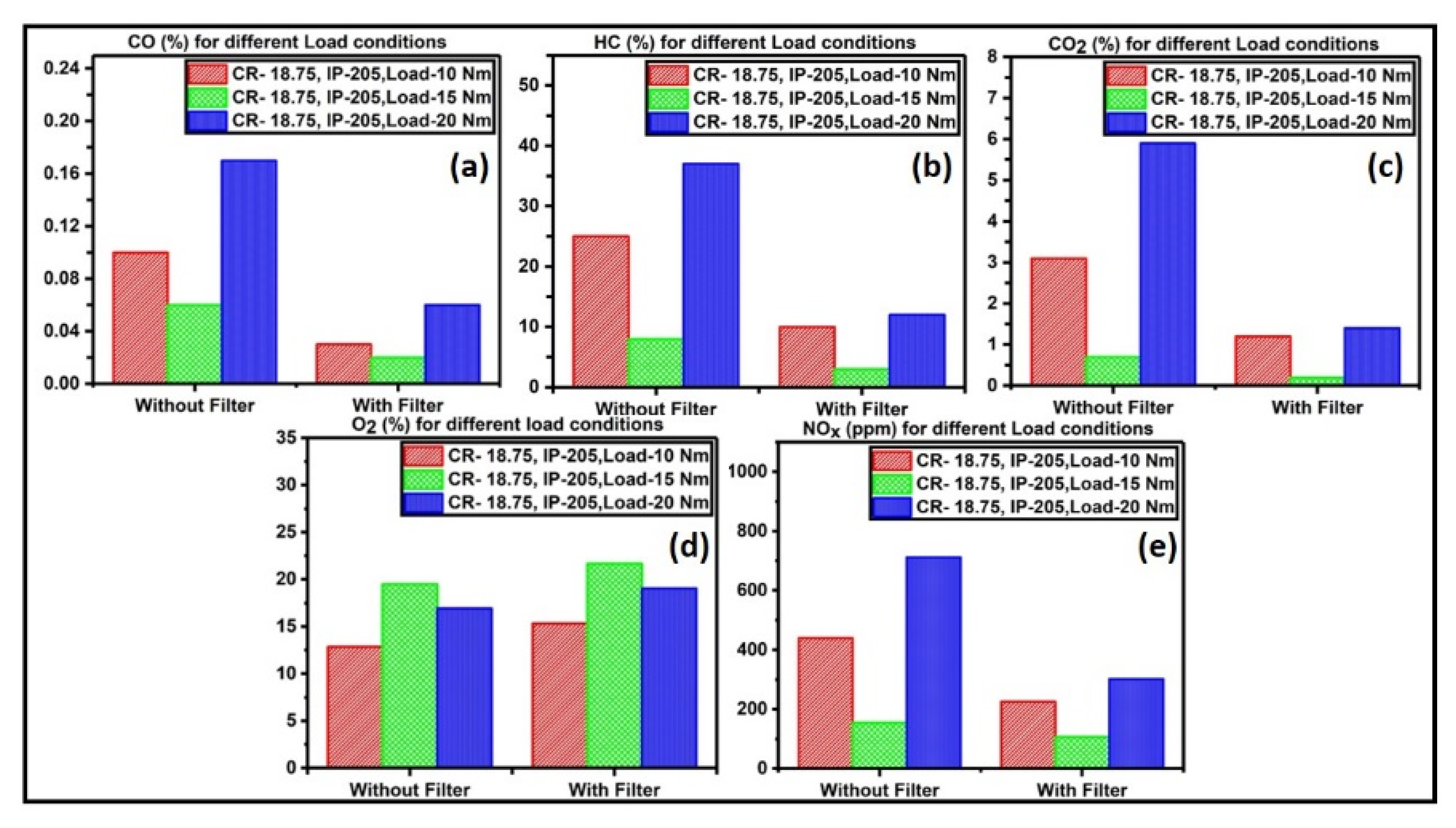
3.5. Catalyst Activity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balakrishnan, U.; Magda, T. Air pollution and academic performance Evidence from India. World Dev. 2021, 146, 105553. [Google Scholar] [CrossRef]
- Patil, A.A.; Joshi, R.R.; Dhavale, A.J.; Balwan, K.S. Review of Bharat Stage 6 emission norms. Int. J. Eng. Res. Technol. 2019, 6, 1359–1361. [Google Scholar]
- Guttikunda, S.K.; Rahul, G.; Pallavi, P. Nature of air pollution, emission sources, and management in the Indian cities. Atmos. Environ. 2014, 95, 501–510. [Google Scholar] [CrossRef]
- Shuang, L.I.U.; Xiaodong, W.U.; Duan, W.; Rui, R.A.N. Ceria-based catalysts for soot oxidation a review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Giuliano, M.; Ricchiardi, G.; Damin, A.; Sgroi, M.; Nicol, G.; Parussa, F. Thermal ageing effects in a commercial three-way catalyst Physical characterization of washcoat and active metal evolution. Int. J. Automot. Technol. 2020, 21, 329–337. [Google Scholar] [CrossRef]
- Shen, M.; Yang, M.; Wang, J.; Wen, J.; Zhao, M.; Wang, W. Pd/support interface-promoted Pd−Ce0.7Zr0.3O2−Al2O3 automobile three-way catalysts studying the dynamic oxygen storage capacity and CO, C3H8, and NO conversion. J. Phys. Chem. C 2009, 113, 3212–3322. [Google Scholar] [CrossRef]
- Morikawa, A.; Suzuki, T.; Kanazawa, T.; Kikuta, K.; Suda, A.; Shinjo, H. A new concept in high performance ceria–zirconia oxygen storage capacity material with Al2O3 as a diffusion barrier. Appl. Catal. B 2008, 78, 210–221. [Google Scholar] [CrossRef]
- Aneggi, E.; de Leitenburg, C.; Trovarelli, A. On the role of lattice/surface oxygen in ceria–zirconia catalysts for diesel soot combustion. Catal. Today 2012, 181, 108–115. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Zhou, R. Catalytic performance of manganese doped CuO–CeO2 catalysts for selective oxidation of CO in hydrogen-rich gas. Fuel 2016, 163, 56–64. [Google Scholar] [CrossRef]
- Ruiz, P.; Philomena Schlexer, A.; Tosoni, S.; Pacchioni, G. Increasing oxide reducibility the role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Liu, S.; Weng, D.; Lin, F.; Ran, R. MnOx–CeO2–Al2O3 mixed oxides for soot oxidation Activity and thermal stability. J. Hazard. Mater. 2011, 187, 283–290. [Google Scholar] [CrossRef]
- Hensgen, L.; Stöwe, K. Soot-catalyst contact studies in combustion processes using nano-scaled ceria as test material. Catal. Today 2011, 159, 100–107. [Google Scholar] [CrossRef]
- Bassou, B.; Guilhaume, N.; Lombaert, K.; Mirodatos, C.; Bianchi, D. Experimental microkinetic approach of the catalytic oxidation of diesel soot by ceria using temperature-programmed experiments. Part 1 impact and evolution of the ceria/soot contacts during soot oxidation. Energy Fuels 2010, 24, 4766–4780. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Zhao, M.; Gong, M.; Chen, Y. The effect of synthesis method on the properties and catalytic performance of Pd/Ce0.5Zr0.5O2-Al2O3 three-way catalyst. J. Mol. Catal. A Chem. 2014, 394, 10–21. [Google Scholar] [CrossRef]
- Habte, A.G.; Hone, F.G.; Dejene, F.B. Effect of solution pH on structural, optical and morphological properties of SnO2 nanoparticles. Phys. B Condens. Matter 2020, 580, 411832. [Google Scholar] [CrossRef]
- Phoka, S.; Laokul, P.; Swatsitang, E.; Promarak, V.; Seraphin, S.; Maensiri, S. Synthesis, structural and optical properties of CeO2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route. Mater. Chem. Phys. 2009, 115, 423–428. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Deng, S.; Rong, T.A. A Ce–Sn–Ox catalyst for the selective catalytic reduction of NOx with NH3. Catal. Commun. 2013, 40, 47–50. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, X.; Tang, C.; Qi, L.; Liu, B.; Gao, F.; Sun, K.; Dong, L.; Chen, Y. Textural, structural, and morphological characterizations and catalytic activity of nanosized CeO2–MOx (M = Mg2+, Al3+, Si4+) mixed oxides for CO oxidation. J. Colloid Interface Sci. 2011, 354, 341–352. [Google Scholar] [CrossRef]
- Lan, L.; Chen, S.; Cao, Y.; Wang, S.; Wu, Q.; Zhou, Y.; Huang, M.; Gong, M.; Chen, Y. Promotion of CeO2–ZrO2–Al2O3 composite by selective doping with barium and its supported Pd-only three-way catalyst. J. Mol. Catal. A Chem. 2015, 410, 100–109. [Google Scholar] [CrossRef]
- Fronzi, M.; Soon, A.; Delley, B.; Traversa, E.; Stampfl, C. Stability and morphology of cerium oxide surfaces in an oxidizing environment a first-principles investigation. J. Chem. Phys. 2009, 131, 104701. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Tang, C.; Yao, X.; Zhang, L.; Li, L.; Wang, X.; Deng, Y.; Gao, F.; Dong, L. Effect of metal ions doping (M = Ti4+, Sn4+) on the catalytic performance of MnOx/CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3. Appl. Catal. A Gen. 2015, 495, 206–216. [Google Scholar] [CrossRef]
- Vasile, A.; Bratan, V.; Hornoiu, C.; Caldararu, M.; Ionescu, N.I. Tatiana Yuzhakova, and ÁkosRédey. Electrical and catalytic properties of cerium–tin mixed oxides in CO depollution reaction. Appl. Catal. B Environ. 2013, 140, 25–31. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.; Zhang, Z.; Li, L. Improved reactivity for toluene oxidation on MnOx/CeO2-ZrO2 catalyst by the synthesis of cubic-tetragonal interfaces. Appl. Surf. Sci. 2021, 539, 148188. [Google Scholar] [CrossRef]
- Swatsitang, E.; Phokha, S.; Hunpratub, S.; Maensiri, S. Characterization of Sm-doped CeO2 nanoparticles and their magnetic properties. Phys. B Condens. Matter 2016, 485, 14–20. [Google Scholar] [CrossRef]
- Sellick, D.R.; Aranda, A.; García, T.; López, J.M.; Solsona, B.; Mastral, A.M.; Morgan, D.J.; Carley, A.F.; Taylor, S.H. Influence of the preparation method on the activity of ceria zirconia mixed oxides for naphthalene total oxidation. Appl. Catal. B 2013, 132, 98–106. [Google Scholar] [CrossRef]
- Dieguez, A.; Romano-Rodrıguez, A.; Vila, A.; Morante, J.R. The complete Raman spectrum of nanometric SnO2 particles. J. Appl. Phys. 2001, 90, 1550–1557. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M.; Bishop, S.R.; Rupp, J.L.M.; Tuller, H.L. Structural characterization and oxygen nonstoichiometry of ceria-zirconia (Ce1−xZrxO2−δ) solid solutions. Acta Mater. 2013, 61, 4277–4288. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Ji, Z.; Lv, Y.; Cao, Y.; Tang, C.; Gao, F.; Dong, L.; Chen, Y. A comparative study of different doped metal cations on the reduction, adsorption and activity of CuO/Ce0.67M0.33O2(M = Zr4+, Sn4+, Ti4+) catalysts for NO + CO reaction. Appl. Catal. B Environ. 2013, 130, 293–304. [Google Scholar] [CrossRef]
- Yao, X.; Tang, C.; Ji, Z.; Dai, Y.; Cao, Y.; Gao, F.; Dong, L.; Chen, Y. Investigation of the physicochemical properties and catalytic activities of Ce0.67M0.33O2 (M = Zr4+, Ti4+, Sn4+) solid solutions for NO removal by CO. Catal. Sci. Technol. 2013, 3, 688–698. [Google Scholar] [CrossRef]
- Vasilchenko, D.B.; Gulyaev, R.V.; Slavinskaya, E.M.; Stonkus, O.A.; Shubin, Y.V.; Korenev, S.V.; Boronin, A.I. Effect of Pd deposition procedure on activity of Pd/Ce0.5Sn0.5O2 catalysts for low-temperature CO oxidation. Catal. Commun. 2016, 73, 34–38. [Google Scholar] [CrossRef]
- Zhao, B.; Yang, C.; Wang, Q.; Li, G.; Zhou, R. Influence of thermal treatment on catalytic performance of Pd/(Ce, Zr) Ox–Al2O3 three-way catalysts. J. Alloy. Compd. 2010, 494, 340–346. [Google Scholar] [CrossRef]
- Motaung, D.E.; Mhlongo, G.H.; Makgwane, P.R.; Dhonge, B.P.; Cummings, F.R.; Swart, H.C.; Ray, S.S. Ultra-high sensitive and selective H2 gas sensor manifested by interface of n–n heterostructure of CeO2-SnO2 nanoparticles. Sens. Actuators B Chem. 2018, 254, 984–995. [Google Scholar] [CrossRef]
- Cao, J.L.; Wang, Y.; Zhang, T.Y.; Wu, S.H.; Yuan, Z.Y. Preparation, characterization and catalytic behavior of nanostructured mesoporous CuO/Ce0.8Zr0.2O2 catalysts for low-temperature CO oxidation. Appl. Catal. B 2008, 78, 120–128. [Google Scholar] [CrossRef]
- Liu, C.; Han, J.; Bi, Y.; Wang, J.; Guo, M.; Liu, Q. A novel Cerium-Tin composite oxide catalyst with high SO2 tolerance for selective catalytic reduction of NOx with NH3. Catal. Today 2021, 376, 65–72. [Google Scholar] [CrossRef]
- Wang, J.; Wen, J.; Shen, M. Effect of interaction between Ce0.7Zr0.3O2 and Al2O3 on structural characteristics, thermal stability, and oxygen storage capacity. J. Phys. Chem. C 2008, 112, 5113–5122. [Google Scholar] [CrossRef]
- Terribile, D.; Trovarelli, A.; Llorca, J.; de Leitenburg, C.; Dolcetti, G. The preparation of high surface area CeO2–ZrO2 mixed oxides by a surfactant-assisted approach. Catal. Today 1998, 43, 79–88. [Google Scholar] [CrossRef]
- Murugan, R.; Vijayaprasath, G.; Ravi, G. The influence of substrate temperature on the optical and micro structural properties of cerium oxide thin films deposited by RF sputtering. Superlattices Microstruct. 2015, 85, 321–330. [Google Scholar] [CrossRef]
- Ma, W.; Mashimo, T.; Tamura, S.; Tokuda, M.; Yoda, S.; Tsushida, M.; Koinuma, M.; Kubota, A.; Isobe, H.; Yoshiasa, A. Cerium oxide (CeO2−x) nanoparticles with high Ce3+ proportion synthesized by pulsed plasma in liquid. Ceram. Int. 2020, 46, 26502–26510. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Cheng, X.; Fang, D.; He, F.; Li, F.; Qi, K. Elucidate the promotional effects of Sn on Ce-Ti catalysts for NH3-SCR activity. J. Energy Inst. 2020, 93, 1053–1063. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, C.; Xie, Y.; Pan, Z.; Xue, X.; Zhang, R. A study on the catalytic oxidation of soot by Sn–Ce composite oxides: Adsorbed oxygen and defect sites synergistically enhance catalytic activity. New J. Chem. 2019, 43, 17423–17432. [Google Scholar] [CrossRef]
- Zhan, S.; Zhang, H.; Zhang, Y.; Shi, Q.; Li, Y.; Li, X. Efficient NH3-SCR removal of NOx with highly ordered mesoporous WO3 (χ)-CeO2 at low temperatures. Appl. Catal. B 2017, 203, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsan, A.; Vasanthakumar, V.; Karthikeyan, S.; Raj, V.; Shanavas, S.; Anbarasan, P.M. Multi-functional properties of ternary CeO2/SnO2/rGOnanocomposites visible light driven photocatalyst and heavy metal removal. J. Photochem. Photobiol. 2017, 346, 32–45. [Google Scholar] [CrossRef]
- Lu, W.; Iwasa, Y.; Ou, Y.; Jinno, D.; Kamiyama, S.; Petersen, P.M.; Ou, H. Effective optimization of surface passivation on porous silicon carbide using atomic layer deposited Al2O3. RSC Adv. 2017, 7, 8090–8097. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Yang, H. Investigation of the oxygen exchange property and oxygen storage capacity of CexZr1− xO2 nanocrystals. J. Phys. Chem. C 2009, 113, 6921–6928. [Google Scholar] [CrossRef]
- India Bharat Stage VI Emission Standards. Policy Update. International Council of Clean Transportation. 2016. Available online: https://theicct.org/sites/default/files/publications/India%20BS%20VI%20Policy%20Update%20vF.pdf (accessed on 24 December 2021).
- Chen, Y.-Z.; Liaw, B.-J.; Huang, C.-W. Selective oxidation of CO in excess hydrogen over CuO/CexSn1−xO2 catalysts. Appl. Catal. A Gen. 2006, 302, 168–176. [Google Scholar] [CrossRef]
- Papavasiliou, A.; Tsetsekou, A.; Matsouka, V.; Konsolakis, M.; Yentekakis, I.V.; Boukos, N. Development of a Ce–Zr–La modified Pt/γ-Al2O3 TWCs’ washcoat: Effect of synthesis procedure on catalytic behaviour and thermal durability. Appl. Catal. B Environ. 2009, 90, 162–174. [Google Scholar] [CrossRef]
- Dey, S.; Chandra Dhal, G. Controlling carbon monoxide emissions from automobile vehicle exhaust using copper oxide catalysts in a catalytic converter. Mater. Today Chem. 2020, 17, 100282. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Zhu, J.; Chen, C.; Lin, X.; Zheng, Q. The synergetic mechanism between copper species and ceria in NO abatement over Cu/CeO2 catalysts. Appl. Catal. A Gen. 2010, 377, 121–127. [Google Scholar] [CrossRef]
- Boaro, M.; Giordano, F.; Recchia, S.; Dal Santo, V.; Giona, M.; Trovarelli, A. On the mechanism of fast oxygen storage and release in ceria-zirconia model catalysts. Appl. Catal. B 2004, 52, 225–237. [Google Scholar] [CrossRef]
- Yao, X.; Xiong, Y.; Zou, W.; Zhang, L.; Wu, S.; Dong, X.; Gao, F.; Deng, Y.; Tang, C.; Chen, Z.; et al. Correlation between the physicochemical properties and catalytic performances of CexSn1–xO2 mixed oxides for NO reduction by CO. Appl. Catal. B Environ. 2014, 144, 152–165. [Google Scholar] [CrossRef]



| Engine Specifications | |
|---|---|
| Make/Model | Kirloskar |
| Engine type | Four stroke, multifuel variable compression ratio, water-cooled |
| No. of cylinders | Single |
| Combustion chamber | Direct injection (open chamber) |
| Type of fuel | Diesel |
| Bore diameter/stroke length | 87.5 mm/110 mm |
| Speed | 1500 rpm |
| Max torque | 235 Nm |
| Dynamometer | Eddy current dynamometer |
| Compression ratio | 17.5:1 to 20:1 |
| Rated power | 5 hp (5.2 kW) |
| Sample Compositions | Miller Planes | Crystallite Size (nm) a | Lattice Parameter (Å) a | Lattice Strain (ε) a | Position of Raman Active Band F2g (cm−1) b |
|---|---|---|---|---|---|
| CTA-28 | (110) (111) | 13.54 12.27 | a = b = 4.6825 c = 3.1699 a = b = c = 5.3626 | 3.10 × 10−3 | 463.06 |
| CTA-55 | (110) (111) | 12.32 10.22 | a = b = 4.7795 c = 3.1995 a = b = c = 5.3307 | 3.39 × 10−3 | 462.15 |
| CTA-82 | (111) | 11.41 | a = b = c = 5.4098 | 2.8 × 10−3 | 461.01 |
| Sample Composition | Relative Amount (%) a | Ce/Sn Ratio a | Ce3+ (%) a | Defective Oxygen (%) a | Surface Area, Pore Diameter, and Pore Volume b | |
|---|---|---|---|---|---|---|
| CTA-55 | Ce-3d Sn-3d Al-2p O-1s C-1s | 5.69 6.55 10.08 50.96 26.72 | 0.86 | 54.32 | 86.74 | 73 m2g−1 3.10 nm 0.2168 cm3g−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayachandran, V.; Dhandapani, V.S.; Muniappan, E.; Park, D.; Kim, B.; Arun, A.P.; Ayyappan, P.R. Assessment of the Synergetic Performance of Nanostructured CeO2-SnO2/Al2O3 Mixed Oxides on Automobile Exhaust Control. Materials 2022, 15, 8460. https://doi.org/10.3390/ma15238460
Jayachandran V, Dhandapani VS, Muniappan E, Park D, Kim B, Arun AP, Ayyappan PR. Assessment of the Synergetic Performance of Nanostructured CeO2-SnO2/Al2O3 Mixed Oxides on Automobile Exhaust Control. Materials. 2022; 15(23):8460. https://doi.org/10.3390/ma15238460
Chicago/Turabian StyleJayachandran, Varuna, Vishnu Shankar Dhandapani, Elango Muniappan, Dongkyou Park, Byungki Kim, A. P. Arun, and P. R. Ayyappan. 2022. "Assessment of the Synergetic Performance of Nanostructured CeO2-SnO2/Al2O3 Mixed Oxides on Automobile Exhaust Control" Materials 15, no. 23: 8460. https://doi.org/10.3390/ma15238460






