Study on Carbonation Resistance of Polymer-Modified Sulphoaluminate Cement-Based Materials
Abstract
:1. Introduction
2. Raw Materials and Experimental Methods
2.1. Raw Materials
2.2. Experimental Methods
3. Results and Discussion
3.1. Cement Paste
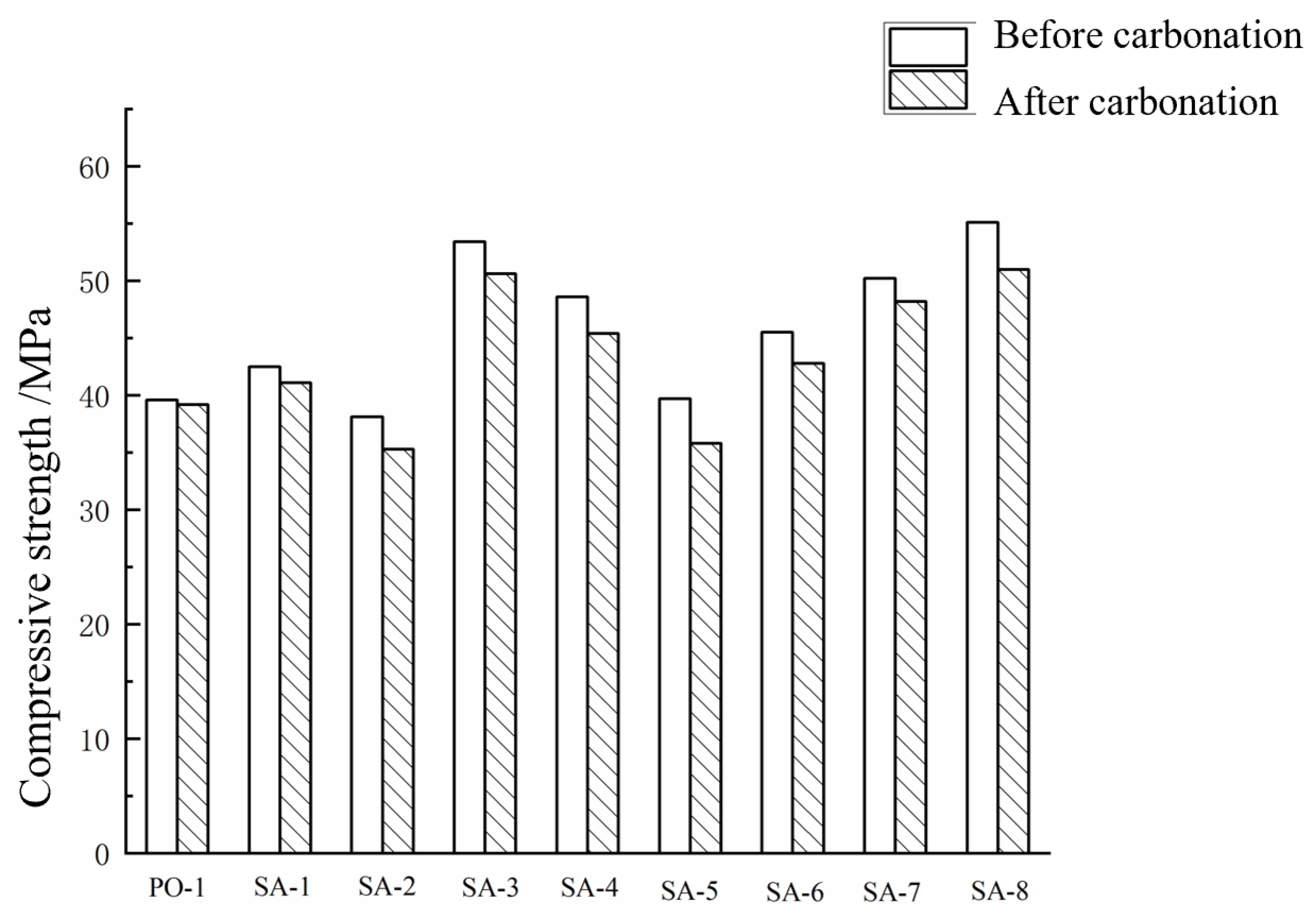
3.1.1. Compressive Strength of Cement Paste before and after Carbonation
3.1.2. Carbonation Depth/Area of Sulphoaluminate Cement Paste
3.2. Carbonation of Cement Mortar
3.2.1. Compressive Strength of Cement Mortar before and after Carbonation
3.2.2. Carbonation Depth/Area of Sulphoaluminate Cement Mortar
4. Conclusions and Outlook
- (1)
- For the cement paste, with the increase in polymer emulsion AMPS content, the 3-day strength of the sulphoaluminate cement with a water–cement ratio of 0.3 gradually decreased. When the addition amount is 5% and 10%, the carbonation resistance of sulphoaluminate cement can be improved. In contrast, the addition amount of 20% can reduce its carbonation resistance. The optimum amount of polymer emulsion for the sulphoaluminate cement paste system is 5%. The addition of polycarboxylic acid improves the carbonation resistance of sulphoaluminate cement, and the carbonation resistance is the best when the addition amount is 1.8%.
- (2)
- For the cement mortar, with the increase in the polymer emulsion content, the compressive strength of the sulphoaluminate cement mortar showed a downward trend, but it was higher than that of the blank sample (SA-9). The compressive strength of sulphoaluminate cement increases with the increase in acid content. After carbonation, the compressive strength of each sample decreased, and the changing trend of compressive strength among the groups was consistent with that before carbonation; with the increase in the amount of polymer emulsion incorporated, the carbonation depth/area gradually increased and the polymer emulsion carbonation resistance of sulphoaluminate cement mortar is the best when the addition amount is 5%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xue, W.; Liu, W.; Gao, C.; Fan, H.; Zhang, H. Experimental study of permeability evolution in modified desulfurization gypsum under complete stress–strain process. J. Clean. Prod. 2022, 359, 132185. [Google Scholar] [CrossRef]
- Vo, D.-H.; Do, N.-D.; Mamuye, Y.; Liao, M.-C.; Hwang, C.-L.; Tran, Q.-T. Engineering, properties and durability of concrete samples designed by densified mixture design algorithm (DMDA) method incorporating steel reducing slag aggregate. Constr. Build. Mater. 2022, 354, 129180. [Google Scholar] [CrossRef]
- Ho, L.S.; Huynh, T.-P. Recycled waste medical glass as a fine aggregate replacement in low environmental impact concrete: Effects on long-term strength and durability performance. J. Clean. Prod. 2022, 368, 133144. [Google Scholar] [CrossRef]
- Looney, T.; Leggs, M.; Volz, J.; Floyd, R. Durability and corrosion resistance of ultra-high performance concretes for repair. Constr. Build. Mater. 2022, 345, 128238. [Google Scholar] [CrossRef]
- Singh, P.; Roy, A.B.D.; Singh, H. Mechanical and durability properties of concrete incorporating weathered coarse Linz-Donawitz (LD) steel slag. J. Build. Eng. 2022, 61, 105301. [Google Scholar] [CrossRef]
- Praseeda, D.; Srinivasa Rao, K. Durability performance and microstructure analysis of nano engineered blended concrete. Clean. Mater. 2022, 6, 100155. [Google Scholar] [CrossRef]
- Kalyana Chakravarthy, P.R.; Namratha, K. Strength and durability properties of high strength self compacting concrete. Mater. Today Proc. 2022, 69, 896–900. [Google Scholar] [CrossRef]
- Taffese, W.Z.; Espinosa-Leal, L. Prediction of chloride resistance level of concrete using machine learning for durability and service life assessment of building structures. J. Build. Eng. 2022, 60, 105146. [Google Scholar] [CrossRef]
- Xue, W.; Mao, X.; Xu, W.; Zhang, H.; Gao, C. Macro-and meso-scale study on dynamic mechanical properties of shaft lining concrete exposed to high water pressure. Case Stud. Constr. Mater. 2022, 17, e1502. [Google Scholar] [CrossRef]
- Chen, D.; Liu, S.; Shen, J.; Sun, G.; Shi, J. Experimental study and modelling of concrete carbonation under the coupling effect of freeze-thaw cycles and sustained loads. J. Build. Eng. 2022, 52, 104390. [Google Scholar] [CrossRef]
- Ding, Z.; Quy, N.X.; Kim, J.; Hama, Y. Evaluations of frost and scaling resistance of fly ash concrete in terms of changes in water absorption and pore structure under the accelerated carbonation conditions. Constr. Build. Mater. 2022, 345, 128273. [Google Scholar] [CrossRef]
- Liu, Z.; van den Heede, P.; Zhang, C.; Shi, X.; Wang, L.; Li, J.; Yao, Y.; de Belie, N. Influence of sustained compressive load on the carbonation of concrete containing blast furnace slag. Constr. Build. Mater. 2022, 335, 127457. [Google Scholar] [CrossRef]
- Chang, J.; Jiang, T.; Cui, K. Influence on compressive strength and CO2 capture after accelerated carbonation of combination β-C2S with γ-C2S. Constr. Build. Mater. 2021, 312, 125359. [Google Scholar] [CrossRef]
- Ming, J.; Wu, M.; Shi, J. Passive film modification by concrete carbonation: Re-visiting a corrosion-resistant steel with Cr and Mo. Cem. Concr. Compos. 2021, 123, 104178. [Google Scholar] [CrossRef]
- Mei, K.; He, Z.; Yi, B.; Lin, X.; Wang, J.; Wang, H.; Liu, J. study on electrochemical characteristics of reinforced concrete corrosion under the action of carbonation and chloride. Case Stud. Constr. Mater. 2022, 17, e1351. [Google Scholar] [CrossRef]
- Andrade, H.D.; de Carvalho, J.M.F.; Costa, L.C.B.; Elói, F.P.D.F.; e Silva, K.D.D.C.; Peixoto, R.A.F. Mechanical performance and resistance to carbonation of steel slag reinforced concrete. Constr. Build. Mater. 2021, 298, 123910. [Google Scholar] [CrossRef]
- Wu, K.; Luo, S.; Zheng, J.; Yan, J.; Xiao, J. Influence of carbonation treatment on the properties of multiple interface transition zones and recycled aggregate concrete. Cem. Concr. Compos. 2022, 127, 104402. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.; Xu, F.; Meng, X.; Li, B.; Zhan, M. Study of the basic mechanical properties and degradation mechanism of recycled concrete with tailings before and after carbonation. J. Clean. Prod. 2020, 259, 120923. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J.; Sabri, M.M.S.; Huang, J. Influence of Graphene Nanoplates on Dispersion, Hydration Behavior of Sulfoaluminate Cement Composites. Materials 2022, 15, 5357. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J.; Sabri, M.M.S.; Huang, J. Study of Dispersion, Hydration, and Microstructure of Graphene Nanoplates-Modified Sulfoaluminate Cement Paste. Nanomaterials 2022, 12, 2708. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J.; Feo, L.; Chow, C.L.; Lau, D. Developments and Applications of Carbon Nanotube Reinforced Cement-Based Composites as Functional Building Materials. Front. Mater. 2022, 9, 146. [Google Scholar] [CrossRef]
- Dakwale, V.A.; Pachpor, P.D.; Mahesh, S.; Namdeo, D. Use of metakaolin with polymer modified concrete in repairing of structures. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Gwon, S.; Jang, S.Y.; Shin, M. Microstructure evolution and strength development of ultra rapid hardening cement modified with redispersible polymer powder. Constr. Build. Mater. 2018, 192, 715–730. [Google Scholar] [CrossRef]
- Chang, J.; Li, W.; Wang, D.; Zhang, Y. Effect of silicate modulus on tensile properties and microstructure of waterproof coating based on polymer and sodium silicate-activated GGBS. Constr. Build. Mater. 2020, 252, 119056. [Google Scholar] [CrossRef]
- Cui, K.; Lau, D.; Zhang, Y.; Chang, J. Mechanical properties and mechanism of nano-CaCO3 enhanced sulphoaluminate cement-based reactive powder concrete. Constr. Build. Mater. 2021, 309, 125099. [Google Scholar] [CrossRef]
- Cui, K.; Liang, K.; Chang, J.; Lau, D. Investigation of the macro performance, mechanism, and durability of multiscale steel fiber reinforced low-carbon ecological UHPC. Constr. Build. Mater. 2022, 327, 126921. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J. Hydration, reinforcing mechanism, and macro performance of multi-layer graphene-modified cement composites. J. Build. Eng. 2022, 57, 104880. [Google Scholar] [CrossRef]
- Ioannou, S.; Chowdhury, M.; Badr, A. Conformity of the performance of calcium sulfoaluminate cement based concretes to empirical models in current international design standards. Constr. Build. Mater. 2022, 326, 126748. [Google Scholar] [CrossRef]
- Kleib, J.; Aouad, G.; Benzerzour, M.; Zakhour, M.; Abriak, N. Effect of calcium sulfoaluminate cements composition on their durability. Constr. Build. Mater. 2021, 307, 124952. [Google Scholar] [CrossRef]
- Tambara, L.U.D., Jr.; de Matos, P.R.; Lima, G.S.; Silvestro, L.; Rocha, J.C.; Campos, C.E.M.; Gleize, P.J. effect of the nanosilica source on the rheology and early-age hydration of calcium sulfoaluminate cement pastes. Constr. Build. Mater. 2022, 327, 126942. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, G.; Liang, K. Development of fly ash and slag based high-strength alkali-activated foam concrete. Cem. Concr. Compos. 2022, 128, 104447. [Google Scholar] [CrossRef]


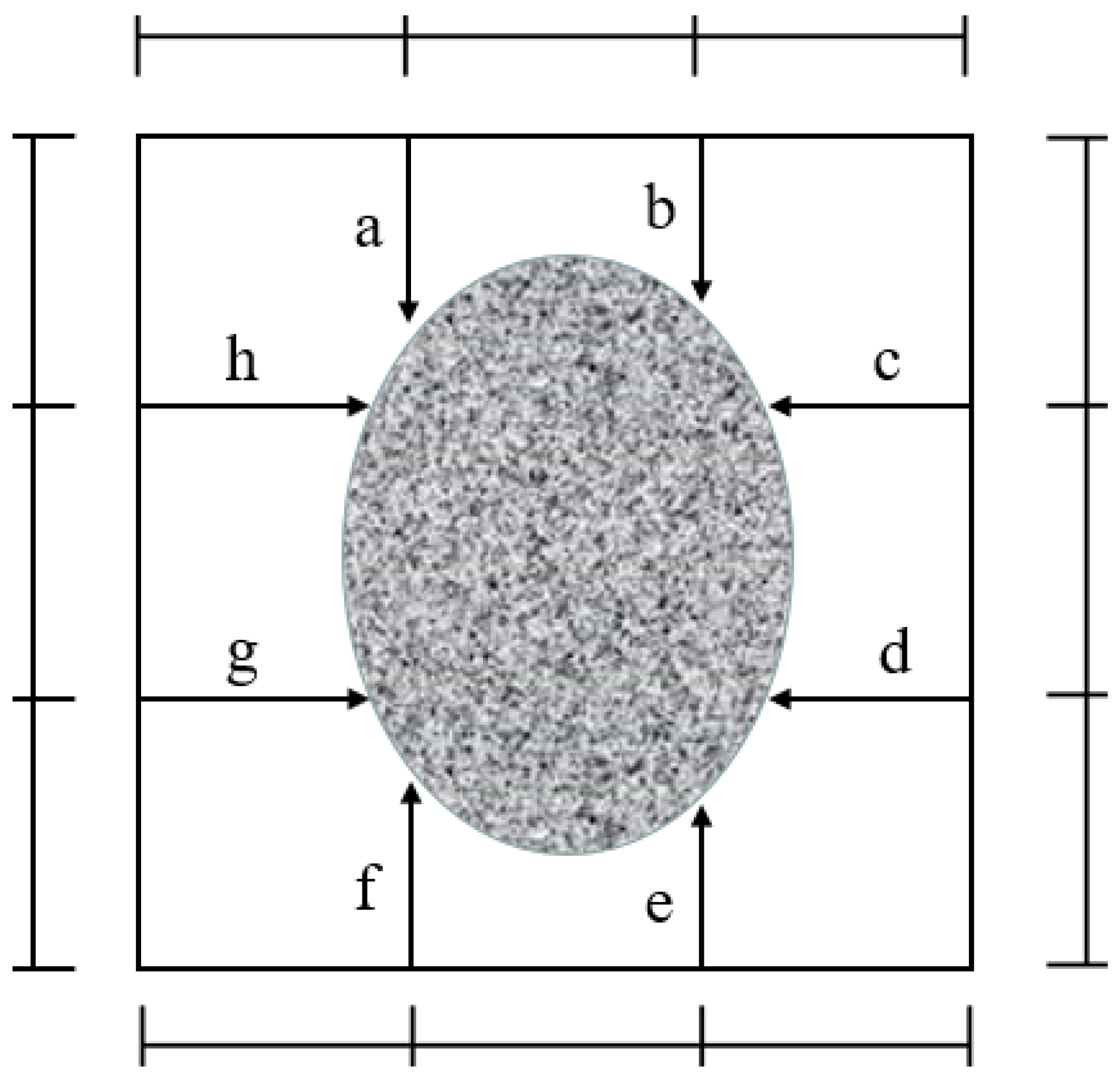


| Type | CaO (%) | SiO2 (%) | Al2O3 (%) | SO3 (%) | MgO (%) | Fe2O3 (%) | Specific Surface Area (m2/kg) |
|---|---|---|---|---|---|---|---|
| SAC | 43.85 | 16.15 | 18.53 | 12.91 | 2.86 | 2.62 | 350 |
| OPC | 64.1 | 20.9 | 4.8 | 3.3 | 1.8 | 2.6 | 430 |
| Abbreviation | Content | |||
|---|---|---|---|---|
| Cement/g | AMPS (wt%) | Polycarboxylic Acid (wt%) | W/C | |
| PO-1 (1) | OPC/140 g | 0 | 0 | 0.3 |
| SA-1 (8/17) | OPC/140 g | 0 | 0 | 0.3 |
| SA-2 (9/18) | SAC/140 g | 0 | 0 | 0.35 |
| SA-3 (5/14) | SAC/140 g | 5 | 0 | 0.3 |
| SA-4 (6/15) | SAC/140 g | 10 | 0 | 0.3 |
| SA-5 (7/16) | SAC/140 g | 20 | 0 | 0.3 |
| SA-6 (10) | SAC/140 g | 0 | 0.6 | 0.3 |
| SA-7 (11) | SAC/140 g | 0 | 1.2 | 0.3 |
| SA-8 (12) | SAC/140 g | 0 | 1.8 | 0.3 |
| Abbreviation | Content | ||||
|---|---|---|---|---|---|
| Cement/g | AMPS (wt%) | Polycarboxylic Acid (wt%) | W/C | L/S | |
| PO-2 (2) | OPC/450 g | 0 | 0 | 0.5 | 0.4 |
| SA-9 (4) | SAC/450 g | 0 | 0 | 0.5 | 0.4 |
| SA-10 (19) | SAC/450 g | 5 | 0 | 0.5 | 0.4 |
| SA-11 (20) | SAC/450 g | 10 | 0 | 0.5 | 0.4 |
| SA-12 (21) | SAC/450 g | 20 | 0 | 0.5 | 0.4 |
| SA-13 (22) | SAC/450 g | 0 | 0.6 | 0.5 | 0.4 |
| SA-14 (23) | SAC/450 g | 0 | 1.2 | 0.5 | 0.4 |
| SA-15 (24) | SAC/450 g | 0 | 1.8 | 0.5 | 0.4 |
| Number | PO-1 | SA-1 | SA-2 | SA-3 | SA-4 | SA-5 | SA-6 | SA-7 | SA-8 |
|---|---|---|---|---|---|---|---|---|---|
| The loss rate of compressive strength (%) | 1.01 | 3.06 | 7.34 | 5.24 | 6.58 | 9.82 | 5.93 | 3.98 | 7.44 |
| Number | PO-1 | SA-1 | SA-2 | SA-3 | SA-4 | SA-5 | SA-6 | SA-7 | SA-8 |
|---|---|---|---|---|---|---|---|---|---|
| h (mm) | 0 | 0.66 | 0.76 | 0.20 | 0.60 | 0.80 | 0.10 | 0.08 | 0.05 |
| S (mm2) | 0 | 51.06 | 58.49 | 15.84 | 46.56 | 61.44 | 7.96 | 6.37 | 3.99 |
| Carbonation (%) | 0 | 12.765 | 16.623 | 3.96 | 11.64 | 15.36 | 1.99 | 1.593 | 0.998 |
| Number | PO-2 | SA-9 | SA-10 | SA-11 | SA-12 | SA-13 | SA-14 | SA-15 |
|---|---|---|---|---|---|---|---|---|
| The loss rate of compressive strength (%) | 2.43 | 11.96 | 15.90 | 18.35 | 20.41 | 14.43 | 15.51 | 21.58 |
| Number | PO-2 | SA-9 | SA-10 | SA-11 | SA-12 | SA-13 | SA-14 | SA-15 |
|---|---|---|---|---|---|---|---|---|
| h (mm) | 0 | 2.9 | 2.2 | 2.4 | 3.10 | 1.6 | 0.80 | 0.42 |
| S (mm2) | 0 | 198.36 | 156.64 | 168.96 | 209.56 | 117.76 | 61.44 | 32.89 |
| Carbonation (%) | 0 | 49.59 | 39.16 | 42.24 | 52.39 | 29.44 | 15.36 | 8.223 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhang, B.; Fang, Y.; Chang, J. Study on Carbonation Resistance of Polymer-Modified Sulphoaluminate Cement-Based Materials. Materials 2022, 15, 8635. https://doi.org/10.3390/ma15238635
Zhang P, Zhang B, Fang Y, Chang J. Study on Carbonation Resistance of Polymer-Modified Sulphoaluminate Cement-Based Materials. Materials. 2022; 15(23):8635. https://doi.org/10.3390/ma15238635
Chicago/Turabian StyleZhang, Ping, Bingxin Zhang, Yanfeng Fang, and Jun Chang. 2022. "Study on Carbonation Resistance of Polymer-Modified Sulphoaluminate Cement-Based Materials" Materials 15, no. 23: 8635. https://doi.org/10.3390/ma15238635




