The In Situ Hydrothermal and Microwave Syntheses of Zinc Oxides for Functional Cement Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Zinc Oxide
2.3. Photocatalytic Properties of Zinc Oxide
2.4. Production and Characterization of Cement Composites
2.5. Antimicrobial Properties of Pristine Admixtures and Cement Composites
3. Results and Discussion
3.1. Characteristics of Powders
3.1.1. Crystal Structure
3.1.2. Morphology and Textural Properties
3.1.3. Thermal Stability
3.1.4. Optical Properties
3.1.5. Photocatalytic Activity
3.1.6. Antimicrobial Properties
3.2. Cement Composites Analysis
3.2.1. Cement Mortars Setting Time
3.2.2. Plasticity of Cement Mortars
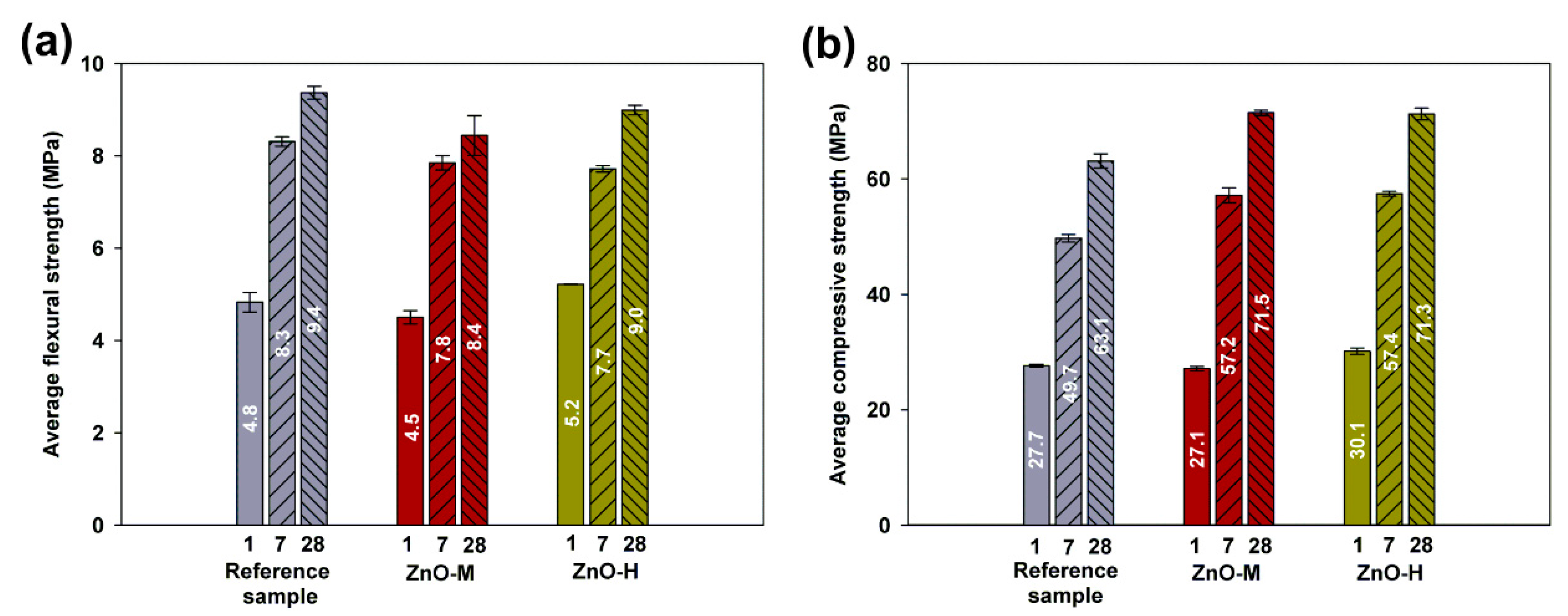
3.2.3. Flexural and Compressive Strength Tests
3.2.4. Microstructural Analysis Results
3.2.5. Antimicrobial Properties of Cement Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SCOPUS. Available online: www.scopus.com (accessed on 16 December 2021).
- Wang, Z.; Qin, W.; Zhang, L. Experimental study on mechanical properties of nano-modified cement paste. Arch. Civ. Eng. 2020, 66, 253–263. [Google Scholar]
- Akono, A.T. Effect of nano-TiO2 on C-S-H phase distribution within Portland cement paste. J. Mater. Sci. 2020, 55, 11106–11119. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Mechanical properties of cement mortar with Al2O3 nanoparticles. J. Am. Sci. 2010, 6, 94–97. [Google Scholar]
- Liu, J.; Li, Q.; Xu, S. Influence of nanoparticles on fluidity and mechanical properties of cement mortar. Constr. Build. Mater. 2015, 101, 892–901. [Google Scholar] [CrossRef]
- Oltulu, M.; Sahin, R. Effect of nano-SiO2, nano-Al2O3 and nano-Fe2O3 powders on compressive strengths and capillary water absorption of cement mortar containing fly ash: A comparative study. Energy Build. 2013, 58, 292–301. [Google Scholar] [CrossRef]
- Singh, L.P.; Karade, S.R.; Bhattacharyya, S.K.; Yousuf, M.M.; Ahalawat, S. Beneficial role of nanosilica in cement based materials—A review. Constr. Build. Mater. 2013, 47, 1069–1077. [Google Scholar] [CrossRef]
- Madandoust, R.; Mohseni, E.; Yasin Mousavi, S.; Namnevis, M. An experimental investigation on the durability of self-compacting mortar containing nano-SiO2, nano-Fe2O3 and nano-CuO. Constr. Build. Mater. 2015, 86, 44–50. [Google Scholar] [CrossRef]
- Carmo, R.; Costa, H.; Soldado, E.; Julio, E. Influence of nano-SiO2, nano-Al2O3 and nano-ZnO additions on cementitious matrixes with different powder and steel fibers content. J. Adv. Concr. Tech. 2021, 19, 40–52. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Khalaf, M.A.; Ban Cheah, C.; Ramli, M.; Ahmed, N.M.; Al-Shwaiter, A. Effect of nano zinc oxide and silica on mechanical, fluid transport and radiation attenuation properties of steel furnace slag heavyweight concrete. Constr. Build. Mater. 2021, 274, 121785. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Lu, Z.; Chen, J. Properties and hydration mechanism of cement pastes in presence of nano-ZnO. Constr. Build. Mater. 2021, 289, 123080. [Google Scholar] [CrossRef]
- Ataie, F.F.; Juenger, M.C.G.; Taylor-Lange, S.C.; Riding, K.A. Comparison of the retarding mechanisms of zinc oxide and sucrose on cement hydration and interactions with supplementary cementitious materials. Cem. Concr. Res. 2015, 72, 128–136. [Google Scholar] [CrossRef]
- Garg, R.; Garg, R. Effect of zinc oxide nanoparticles on mechanical properties of silica fume-based cement composites. Mat. Today Proc. 2021, 43, 778–783. [Google Scholar] [CrossRef]
- Ghafari, E.; Ghahari, S.A.; Feng, Y.; Severgnini, F.; Lu, N. Effect of Zinc oxide nanoparticles on the rheological properties of cement paste. Compos. B 2016, 105, 160–166. [Google Scholar] [CrossRef]
- Visali, C.; Priya, A.K.; Dharmaraj, R. Utilization of ecofriendly self-cleaning concrete using zinc oxide and polypropylene fibre. Mater. Today Proc. 2021, 37, 1083–1086. [Google Scholar] [CrossRef]
- Mu, B.; Ying, X.; Petropoulus, E.; He, S. Preparation of AgCl/ZnO nano-composite for effective antimicrobial protection of stone-made building elements. Mater. Lett. 2021, 285, 129143. [Google Scholar] [CrossRef]
- Kiran Babu, L.; Sarala, E.; Audiseshaiah, O.; Madhusudana, R.; Rami Reddy, Y.V. Synthesis, characterization of nanocrystalline ZnO via two different chemical methods and its antibacterial activity. Surf. Interfaces 2019, 16, 93–100. [Google Scholar] [CrossRef]
- Klapiszewska, I.; Parus, A.; Ławniczak, Ł.; Jesionowski, T.; Klapiszewski, Ł.; Ślosarczyk, A. Production of antibacterial cement composites containing ZnO/lignin and ZnO-SiO2/lignin hybrid admixtures. Cem. Concr. Compos. 2021, 124, 104250. [Google Scholar] [CrossRef]
- Alam, M.W.; Pandey, P.; Khan, F.; Souayeh, B.; Farhan, M. Study to investigate the potential of combined extract of leaves and seeds of Moringa oleifera in groundwater purification. Int. J. Environ. Res. Public Health 2020, 17, 7468. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, T.; Jayaprakash, R.; Pinna, N.; Singh, V.N.; Mehta, B.R.; Phani, A.R. Microwave-assisted synthesis and characterization of flower shaped zinc oxide nanostructures. Mater. Lett. 2009, 63, 242–245. [Google Scholar] [CrossRef]
- Kubiak, A.; Żółtowska, S.; Gabała, E.; Szybowicz, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Controlled microwave-assisted and pH-affected growth of ZnO structures and their photocatalytic performance. Powder Technol. 2021, 386, 221–235. [Google Scholar] [CrossRef]
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H. Microwave-assisted synthesis of zinc oxide nanoparticles. Proc. Mater. Sci. 2015, 11, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef] [PubMed]
- Droepenu, E.K.; Wee, B.S.; Chin, S.F.; Kok, K.Y.; Maligan, M.F. Zinc oxide nanoparticles synthesis methods and its effect on morphology: A review. Biointerface Res. Appl. Chem. 2022, 12, 4261–4292. [Google Scholar]
- Kubiak, A.; Żółtowska, S.; Bartkowiak, A.; Gabała, E.; Sacharczuk, N.; Zalas, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. The TiO2-ZnO systems with multifunctional applications in photoactive processes—Efficient photocatalyst under UV-LED light and electrode materials in DSSCs. Materials 2021, 14, 6063. [Google Scholar] [CrossRef] [PubMed]
- EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- PN-EN 1015-3:1999/A2; Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table). European Committee for Standardization: Brussels, Belgium, 2006.
- EN 196-3; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. European Committee for Standardization: Brussels, Belgium, 2016.
- M7-A7; M100S, Performance Standards for Antimicrobial Susceptibility Testing, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. 26th ed. Clinical and Laboratory Standard Institute: Malvern, PA, USA, 2016.
- Esteban-Tejeda, L.; Prado, C.; Cabal, B.; Sanz, J.; Torrecillas, R.; Moya, J.S. Antibacterial and antifungal activity of ZnO containing glasses. PLoS ONE 2015, 10, e0132709. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Song, M.; Shin, H.S. The role of pH variation on the growth of zinc oxide nanostructures. Appl. Surf. Sci. 2009, 255, 4891–4896. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphoslogies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Vian, M.A.; Chemat, F. Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. TrAC Trends Anal. Chem. 2013, 47, 1–11. [Google Scholar] [CrossRef]
- Mehnane, H.F.; Wang, C.; Kondamareddy, K.K.; Yu, W.; Sun, W.; Liu, H.; Bai, S.; Liu, W.; Guo, S.; Zhao, X.Z. Hydrothermal synthesis of TiO2 nanoparticles doped with trace amounts of strontium, and their application as working electrodes for dye sensitized solar cells: Tunable electrical properties & enhanced photo-conversion performance. RSC Adv. 2017, 7, 2358–2364. [Google Scholar]
- Mohammadi, E.; Aliofkhazraei, M.; Hasanpoor, M.; Chipara, M. Hierarchical and complex ZnO nanostructure by microwave-assisted synthesis: Morphologies, growth mechanism and classification. Crit. Rev. Solid. State Mater. Sci. 2018, 43, 475–541. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Microwave processing: Fundamentals and applications. Compos. Part A Appl. Sci. Manuf. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Dac Dien, N. Preparation of various morphologies of ZnO nanostructure through wet chemical methods. Adv. Mater. Sci. 2019, 4, 1–5. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhu, Y.J.; Wang, S.W. Sonochemical and microwave-assisted synthesis of linked single-crystalline ZnO rods. Mater. Chem. Phys. 2004, 88, 421–426. [Google Scholar] [CrossRef]
- Tsuji, M. Microwave-assisted synthesis of metallic nanomaterials in liquid phase. ChemistrySelect 2017, 2, 805–819. [Google Scholar] [CrossRef]
- Horikoshi, S.; Abe, H.; Torigoe, K.; Abe, M.; Serpone, N. Access to small size distributions of nanoparticles by microwave-assisted synthesis. Formation of Ag nanoparticles in aqueous carboxymethylcellulose solutions in batch and continuous-flow reactors. Nanoscale 2010, 2, 1441–1447. [Google Scholar] [CrossRef]
- Kim, S.K.; Reddy, B.M.; Park, S.E. High-Performance microwave synthesized mesoporous TS-1 zeolite for catalytic oxidation of cyclic olefins. Ind. Eng. Chem. Res. 2018, 57, 3567–3574. [Google Scholar] [CrossRef]
- Bhuyan, T.; Khanuja, M.; Sharma, R.; Patel, S.; Reddy, M.R.; Anand, S.; Varma, A. A comparative study of pure and copper (Cu)-doped ZnO nanorods for antibacterial and photocatalytic applications with their mechanism of action. J. Nanoparticle Res. 2015, 17, 288. [Google Scholar] [CrossRef]
- Khamthong, P.; Lumdubwong, N. Effects of heat-moisture treatment on normal and waxy rice flours and production of thermoplastic flour materials. Carbohydr. Polym. 2012, 90, 340–347. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bin Hussein, M.Z.; Taufiq-Yap, Y.H. Hydrothermal synthesis of zinc oxide nanoparticles using rice as soft biotemplate. Chem. Cent. J. 2013, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Van Werde, K.; Mondelaers, D.; Vanhoyland, G.; Nelis, D.; Van Bael, M.K.; Mullens, J.; Van Poucke, L.C.; Van Der Veken, B.; Desseyn, H.O. Thermal decomposition of the ammonium zinc acetate citrate precursor for aqueous chemical solution deposition of ZnO. J. Mater. Sci. 2002, 37, 81–88. [Google Scholar] [CrossRef]
- Mueller, R.; Kammler, H.K.; Wegner, K.; Pratsinis, S.E. OH surface density of SiO2 and TiO2 by thermogravimetric analysis. Langmuir 2003, 19, 160–165. [Google Scholar] [CrossRef]
- Baruah, S.; Rafique, F.F.; Dutta, J. Visible light photocatalysis by tailoring crystal defections in zinc oxide nanostructures. Nano 2008, 3, 399–407. [Google Scholar] [CrossRef]
- Lotus, A.F.; Tacastacas, S.N.; Pinti, M.J.; Britton, L.A.; Stojilovic, N.; Ramsier, R.D.; Chase, G.G. Fabrication and characterization of TiO2-ZnO composite nanofibers. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 43, 857–861. [Google Scholar] [CrossRef]
- Goodman, G. Pentachlorophenol. In Handbook of Pesticide Toxicology; Krieger, R.I., Krieger, W.C., Eds.; Academic Press: Cambridge, UK, 2001; pp. 1480–1481. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Chlorophenols; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 1999.
- Abnet, C.C. Carcinogenic Substances in Food: Mechanisms. Cancer Invest. 2003, 25, 189–196. [Google Scholar] [CrossRef]
- Theurich, J.; Lindner, M.; Bahnemann, D.W. Photocatalytic degradation of 4-chlorophenol in aerated aqueous titanium dioxide suspensions: A kinetic and mechanistic study. Langmuir 1996, 12, 6368–6376. [Google Scholar] [CrossRef]
- Kubiak, A.; Bielan, Z.; Kubacka, M.; Gabała, E.; Zgoła-Grześkowiak, A.; Janczarek, M.; Zalas, M.; Zielińska-Jurek, A.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Microwave-assisted synthesis of a TiO2-CuO heterojunction with enhanced photocatalytic activity against tetracycline. Appl. Surf. Sci. 2020, 520, 146344. [Google Scholar] [CrossRef]
- Benhebal, H.; Chaib, M.; Salmon, T.; Geens, J.; Leonard, A.; Lambert, S.D.; Crine, M.; Heinrichs, B. Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol-gel process. Alex. Eng. J. 2013, 52, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Stafford, U.; Gray, K.A.; Kamat, P.V. Radiolytic and TiO2-assisted photocatalytic degradation of 4-chlorophenol. A comparative study. J. Phys. Chem. 1994, 98, 6343–6351. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C: Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cubbage, J.W.; Tetzlaff, T.A.; Jenks, W.S. Photocatalytic degradation of 4-chlorophenol. 1. The hydroquinone pathway. J. Org. Chem. 1999, 64, 8509–8524. [Google Scholar] [CrossRef]
- Kumaresan, N.; Ramamurthi, K. Synthesis of ZnO/rGO nanocomposites by wet impregnation method for photocatalytic performance against RhB dye and 4-chlorophenol under UV light irradiation. J. Mater. Sci. Mater. Electron. 2020, 31, 3361–3374. [Google Scholar] [CrossRef]
- Guo, J.F.; Ma, B.; Yin, A.; Fan, K.; Dai, W.L. Photodegradation of rhodamine B and 4-chlorophenol using plasmonic photocatalyst of Ag-AgI/Fe3O4@SiO2 magnetic nanoparticle under visible light irradiation. Appl. Catal. B Environ. 2011, 101, 580–586. [Google Scholar] [CrossRef]
- Pozan, G.S.; Kambur, A. Significant enhancement of photocatalytic activity over bifunctional ZnO-TiO2 catalysts for 4-chlorophenol degradation. Chemosphere 2014, 105, 152–159. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H.; Zainal, Z.; Hussein, M.Z. Photocatalytic treatment of 4-chlorophenol in aqueous ZnO suspensions: Intermediates, influence of dosage and inorganic anions. J. Hazard. Mater. 2009, 168, 57–63. [Google Scholar] [CrossRef]
- Ismael, M. The photocatalytic performance of the ZnO/g-C3N4 composite photocatalyst toward degradation of organic pollutants and its inactivity toward hydrogen evolution: The influence of light irradiation and charge transfer. Chem. Phys. Lett. 2020, 739, 136992. [Google Scholar] [CrossRef]
- Bordoloi, D.; Baruah, A.C.; Barkakati, P.; Borthakur, P.C. Influence of ZnO on clinkerization and properties of VSK cement. Cem. Concr. Res. 1998, 28, 329–333. [Google Scholar] [CrossRef]
- Thangapandi, K.; Anuradha, R.; Archana, N.; Muthuraman, P.; Awoyera Paul, O.; Gobinath, R. Experimental study on performance of hardened concrete using nano materials. KSCE J. Civ. Eng. 2020, 24, 596–602. [Google Scholar] [CrossRef]
- Paul, S.C.; Babafemi, A.J.; Miah, M.J. A comparative study of Al2O3, ZnO and bentonite effect on structural grade mortar. In Proceedings of the 6th World Congress on Civil, Structural, and Environmental Engineering (CSEE’21), Lisbon, Portugal, 21–23 June 2021. [Google Scholar]
- Arefi, M.R.; Rezaei-Zarchi, S. Synthesis of zinc oxide nanoparticles and their effect on the compressive strength and setting time of self-compacted concrete paste as cementitious composites. Int. J. Mol. Sci. 2012, 13, 4340–4350. [Google Scholar] [CrossRef]
- Kantharia, M.; Kumar Mishra, P.; Jani, S.; Trivedi, M.K. Experimental assessment of cement mortar using nano oxide compounds. Mater. Today Proc. 2020, 29, 456–461. [Google Scholar] [CrossRef]
- Kumar, M.; Bansal, M.; Garg, R. An overview of beneficiary aspects of zinc oxide nanoparticles on performance of cement composites. Mater. Today Proc. 2021, 43, 892–898. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Chuo Ann, L.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro. Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Caetano, B.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf. B Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Liu, H.; Samal, M.; Yun, K. Synthesis of ZnO nanoparticles-decorated spindle-shaped graphene oxide for application in synergistic antibacterial activity. J. Photochem. Photobiol. B 2018, 183, 293–301. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Salem, J.; Anbar, R.; Kodeh, F.S.; Elmanama, A. Preparation and antimicrobial activity of ZnO-NPs coated cotton/starch and their functionalized ZnO-Ag/cotton and Zn(II) curcumin/cotton materials. Sci. Rep. 2020, 10, 5410. [Google Scholar] [CrossRef]










| Synthesis Method | Process Conditions |
|---|---|
| hydrothermal | T = 160 °C T = 6 h |
| microwave | T = 160 °C t = 1 min * P = 300 W |
| Sample | Cement (g) | Aggregate (g) | Water (mL) | Admixture (g) |
|---|---|---|---|---|
| CEM I | 450 | 1350 | 225 | - |
| ZnO-H | 0.45 | |||
| ZnO-M | 0.45 |
| Sample | The Average Size of Crystallites (nm) | Lattice Parameters (Å) | |
|---|---|---|---|
| a | c | ||
| ZnO-H | 48.5 (±0.2) | 3.24408 | 5.19691 |
| ZnO-M | 27.3 (±0.3) | 3.25611 | 5.21687 |
| Sample | ABET (m2/g) | Vp (cm3/g) | Sp (nm) |
|---|---|---|---|
| ZnO-H | 2 | 0.005 | 12.1 |
| ZnO-M | 8 | 0.020 | 24.2 |
| Material | Degradation Conditions | Degradation Efficiency | Ref. | ||
|---|---|---|---|---|---|
| Concentration | Amount of Photocatalyst | Type of Light Source | |||
| ZnO-M | 20 mg/dm3 | 0.1 g/dm3 | UV-LED (50 W) | 90% (in 120 min) | This work |
| ZnO-rGO | 5 mg/dm3 | 0.1 g/dm3 | Hg–Xe lamp (200 W) | 94% (in 60 min) | [59] |
| Ag–AgI/Fe3O4@SiO2 | 10 mg/dm3 | 0.1 g/dm3 | Hg–Xe lamp (250 W) | 90% (in 30 min) | [60] |
| ZnO-TiO2 | 25 mg/dm3 | 0.1 g/dm3 | Xe-lamp UV-B (16 W) | 95% (in 80 min) | [61] |
| ZnO | 50 mg/dm3 | 0.2 g/dm3 | Xe-lamp UV-A (6 W) | 95% (in 180 min) | [62] |
| ZnO/g-C3N4 | 42 mg/dm3 | 0.1 g/dm3 | Hg–Xe lamp (150 W) | 80% (in 360 min) | [63] |
| Compounds | Pseudomonas putida G− | Pseudomonas aeruginosa G− | Bacillus cereus G+ | Escherichia coli G− | Staphylococus aureus G+ | Candida albicans Yeast | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC * | MBC * | MIC * | MBC * | MIC * | MBC * | MIC * | MBC * | MIC * | MBC * | MIC * | MFC * | |
| ZnO-M | 10 | 20 | 10 | Above 20 | 5 | 20 | 0.63 | 2.5 | 10 | Above 20 | 20 | Above 20 |
| ZnO-H | 20 | Above 20 | 20 | Above 20 | 20 | Above 20 | Above 20 | Above 20 | 20 | Above 20 | Above 20 | Above 20 |
| Cement Mortar | Initial Setting Time (min) |
|---|---|
| Reference sample | 170 |
| Mortar doped with ZnO-H | 600 |
| Mortar doped with ZnO-M | 550 |
| Sample | Microbial Growth |
|---|---|
| Pure cement (reference) | +++ (extensive growth) |
| Cement composite with ZnO-H | ++ (high growth) |
| Cement composite with ZnO-M | − (lack of growth) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klapiszewska, I.; Kubiak, A.; Parus, A.; Janczarek, M.; Ślosarczyk, A. The In Situ Hydrothermal and Microwave Syntheses of Zinc Oxides for Functional Cement Composites. Materials 2022, 15, 1069. https://doi.org/10.3390/ma15031069
Klapiszewska I, Kubiak A, Parus A, Janczarek M, Ślosarczyk A. The In Situ Hydrothermal and Microwave Syntheses of Zinc Oxides for Functional Cement Composites. Materials. 2022; 15(3):1069. https://doi.org/10.3390/ma15031069
Chicago/Turabian StyleKlapiszewska, Izabela, Adam Kubiak, Anna Parus, Marcin Janczarek, and Agnieszka Ślosarczyk. 2022. "The In Situ Hydrothermal and Microwave Syntheses of Zinc Oxides for Functional Cement Composites" Materials 15, no. 3: 1069. https://doi.org/10.3390/ma15031069







