A Multilayer Paste Based on Ag Nanoparticles with Cu@Sn for Die Attachment in Power Device Packaging
Abstract
:1. Introduction
2. Experimental Materials and Method
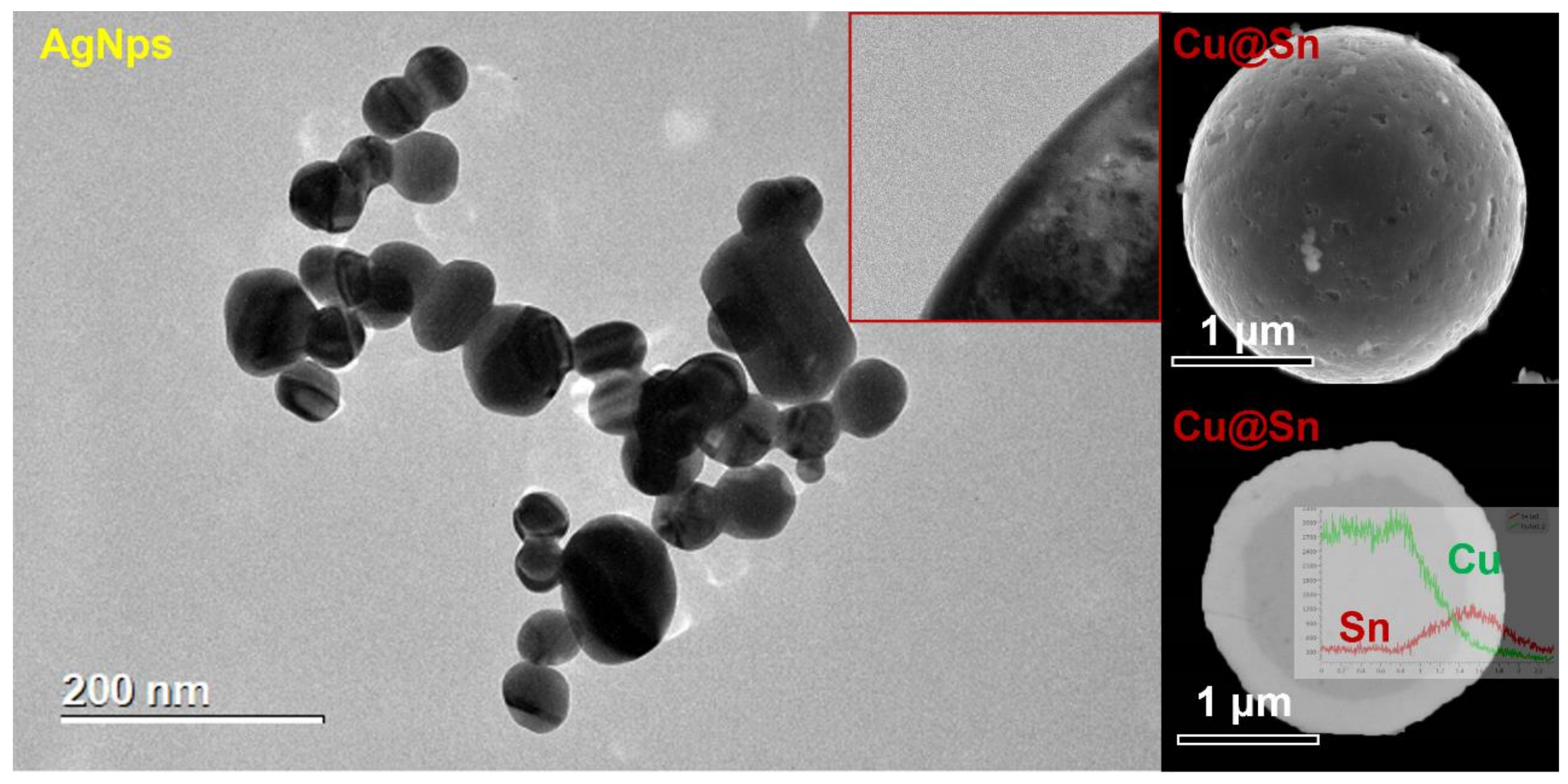
2.1. Cu@Sn and Ag NPs Preparation
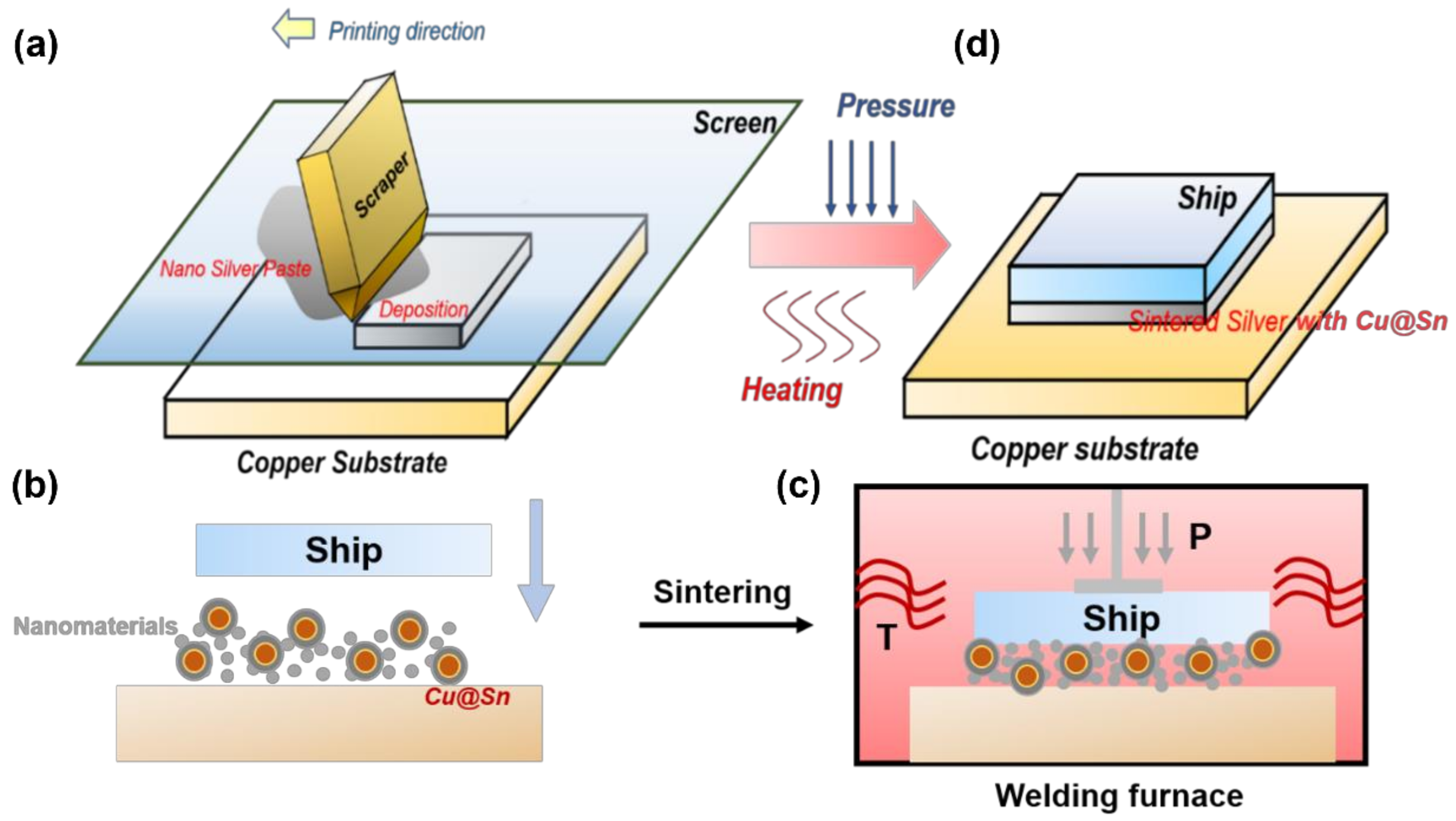
2.2. Soldering
2.3. Testing
3. Experimental Results and Discussion
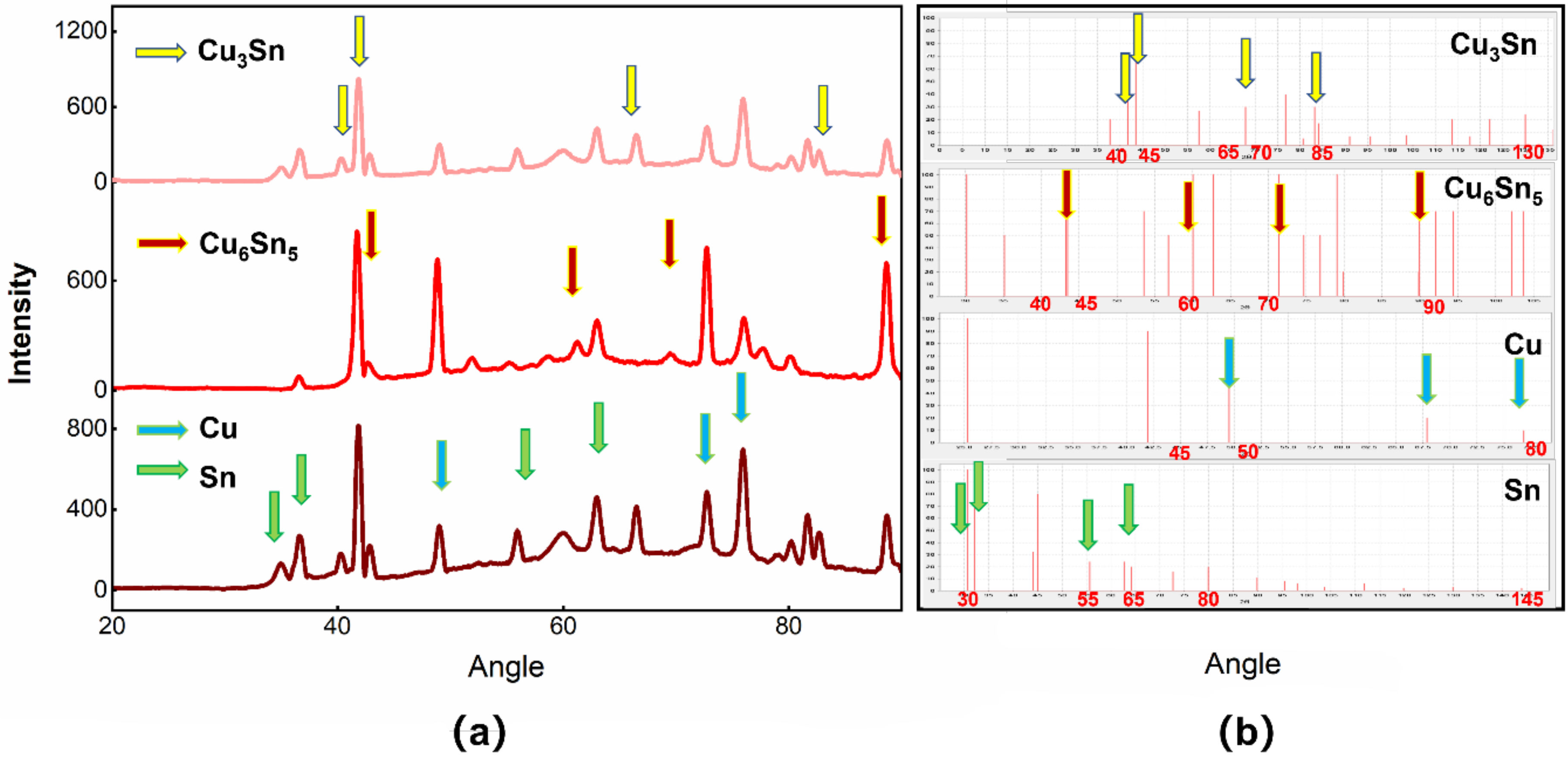
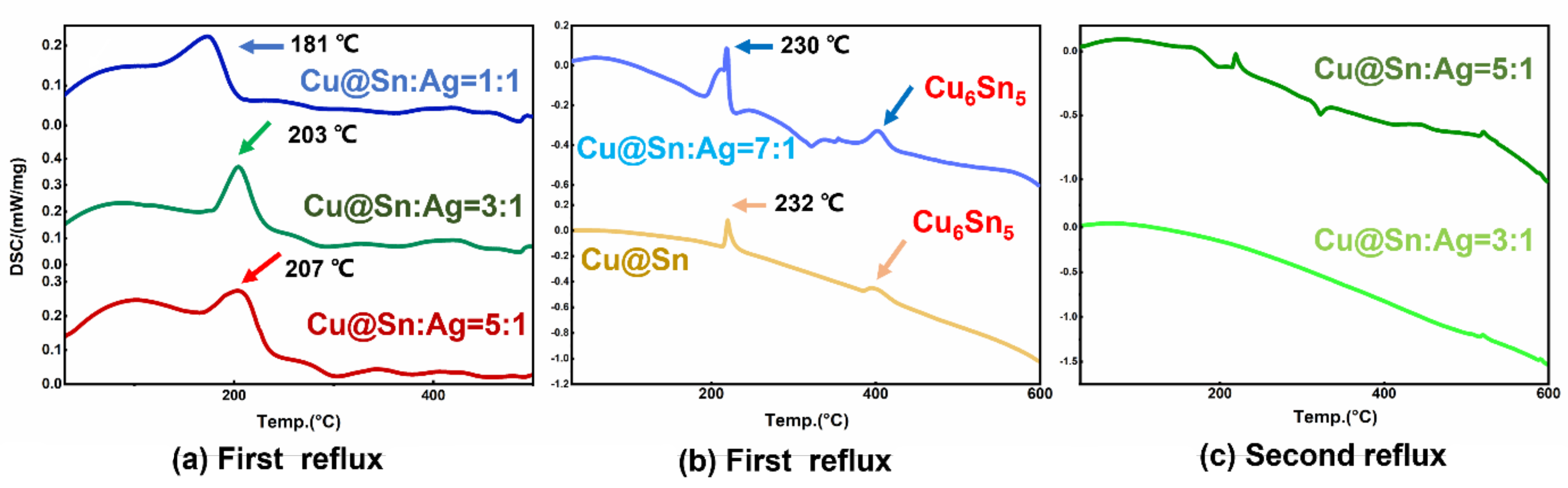
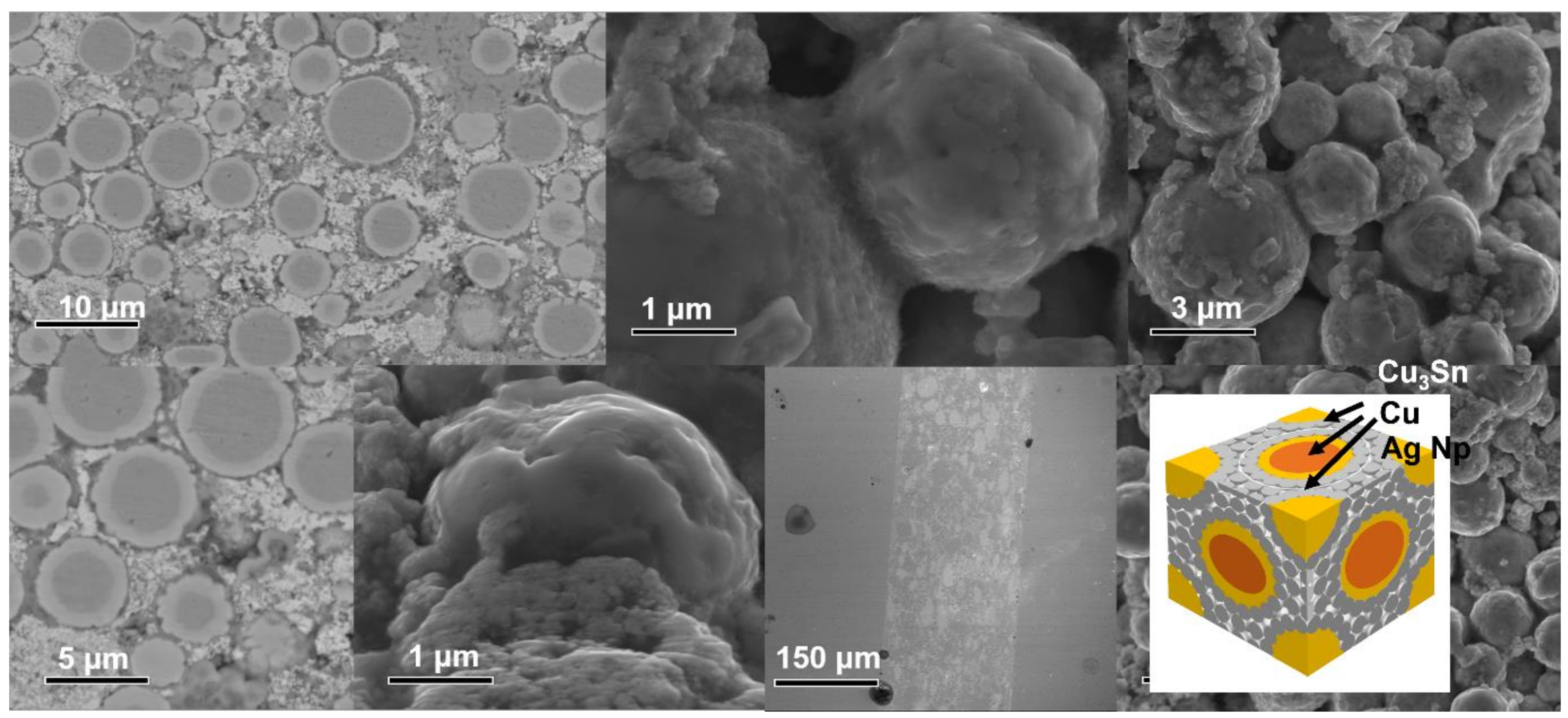
3.1. Cu@Sn Particles and Ag NPs of 3.0–5.0 μm
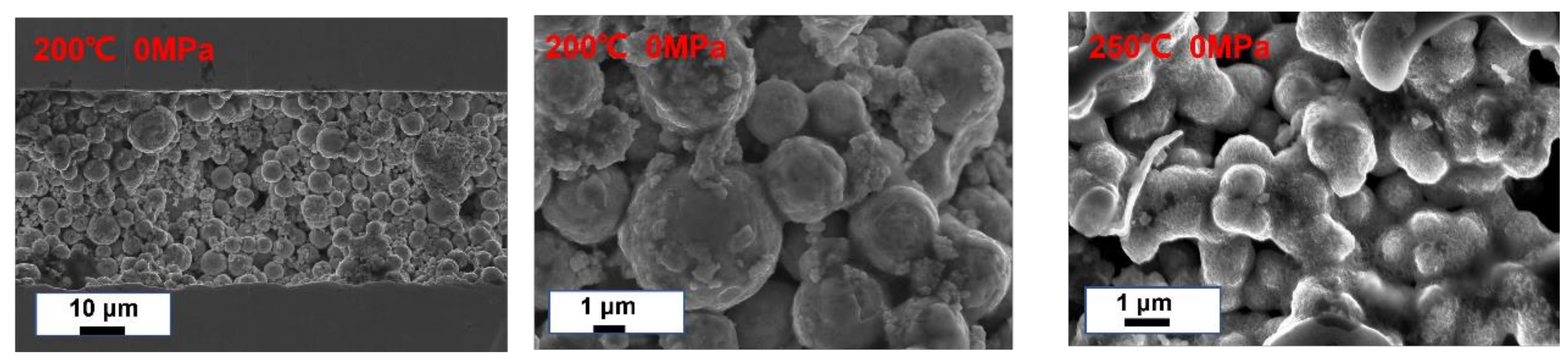
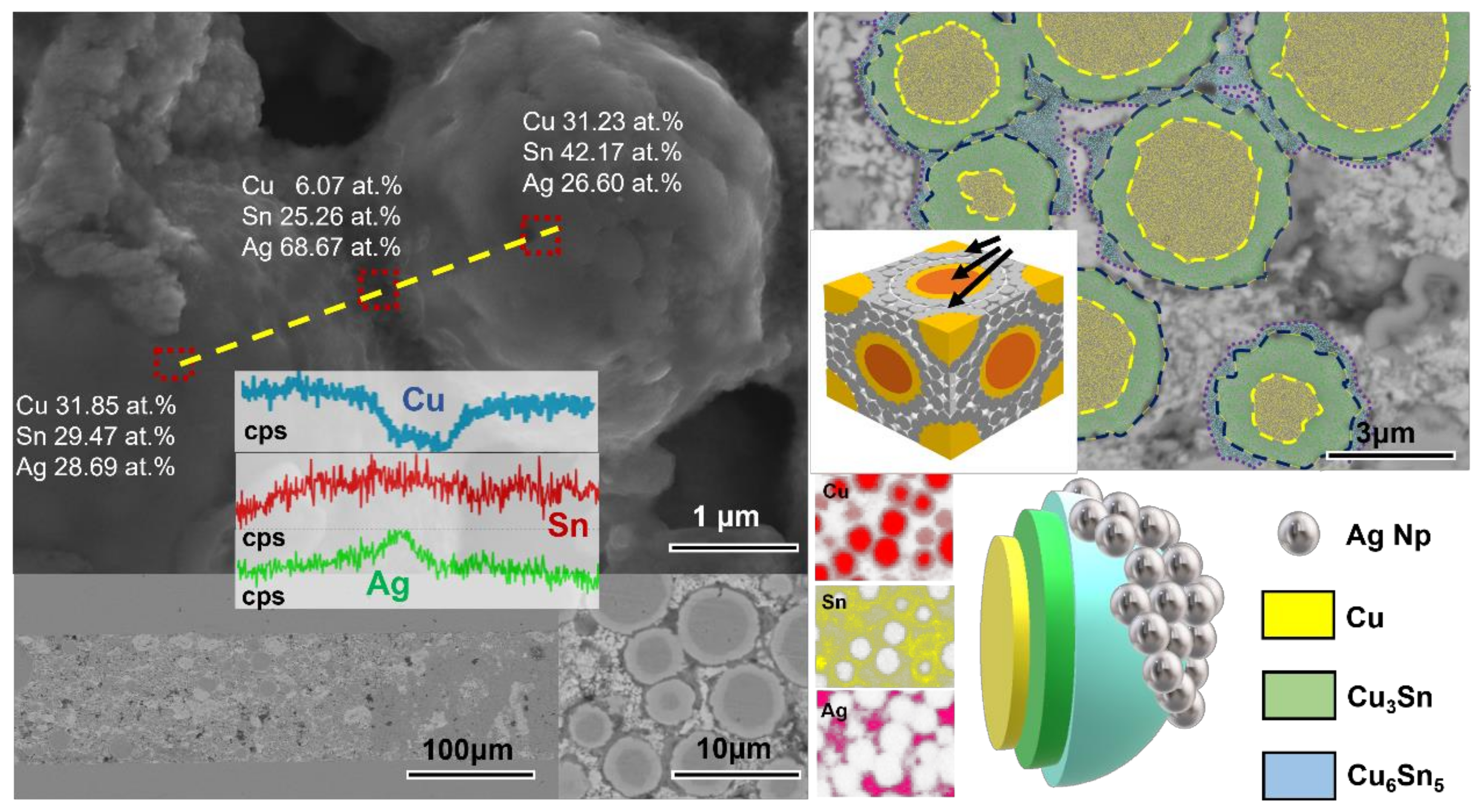
3.2. Joints
3.3. Thermal Cycling
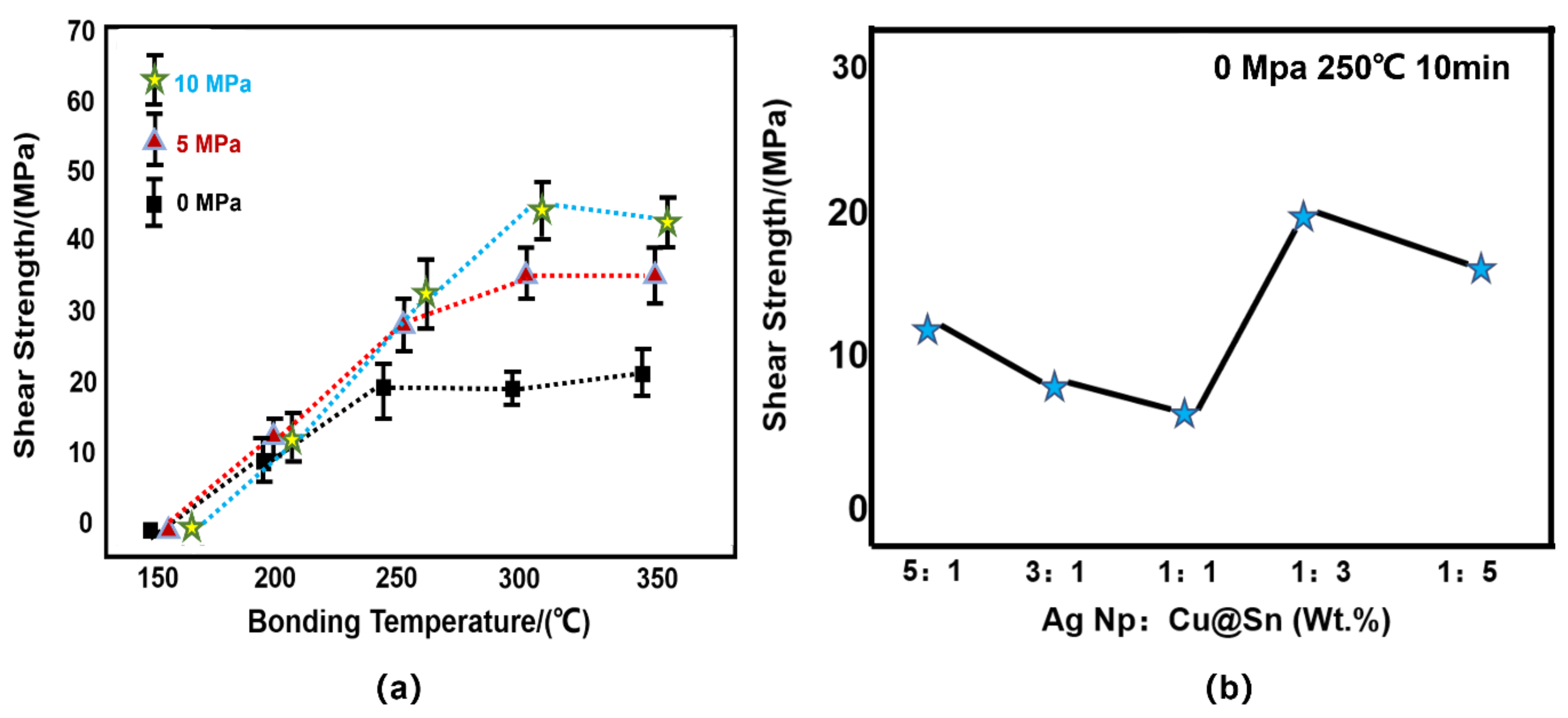
3.4. Shear Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, F.; Liu, H.; Hang, C.; Chen, H.; Li, M. Rapid Formation of Full Intermetallic Bondlines for Die Attachment in High-Temperature Power Devices Based on Micro-sized Sn-Coated Ag Particles. JOM 2019, 71, 3049–3056. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Howes, P.D.; Mannan, S.H. A review: On the development of low melting temperature Pb-free solders. Microelectron. Reliab. 2014, 54, 1253–1273. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, H.; Zhang, X.; Liu, L.; Peng, P.; Zou, G.; Zhou, Y.N. Preparation of nanoparticle and nanowire mixed pastes and their low temperature sintering. J. Alloy. Compd. 2017, 690, 86–94. [Google Scholar] [CrossRef]
- Rajaguru, P.; Lu, H.; Bailey, C. Sintered silver finite element modelling and reliability based design optimisation in power electronic module. Microelectron. Reliab. 2015, 55, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Wang, Y.; Mei, Y.; Xie, H.; Zhang, K.; Feng, S.; S Siow, K.; Li, X.; Lu, G.-Q. Rapid sintering of nano-Ag paste at low current to bond large area (>100 mm2) power chips for electronics packaging. J. Mater. Process. Technol. 2018, 255, 644–649. [Google Scholar] [CrossRef]
- Wang, J.; Fu, X.; Zhang, L.; Zhang, Z.; Liu, J.; Chen, S. A short review on laser welding/brazing of aluminum alloy to steel. Int. J. Adv. Manuf. Technol. 2021, 112, 2399–2411. [Google Scholar] [CrossRef]
- Wang, S.; Ji, H.; Li, M.; Wang, C. Fabrication of interconnects using pressureless low temperature sintered Ag nanoparticles. Mater. Lett. 2012, 85, 61–63. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Ji, H.; Wang, C. Rapid pressureless low-temperature sintering of Ag nanoparticles for high-power density electronic packaging. Scr. Mater. 2013, 69, 789–792. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Lin, T.; He, P. Recent advances in nano-materials for packaging of electronic devices. J. Mater. Sci. Mater. Electron. 2019, 30, 13855–13868. [Google Scholar] [CrossRef]
- Yang, D.; Huang, Y.; Tian, Y. Microstructure of Ag Nano Paste Joint and Its Influence on Reliability. Crystals 2021, 11, 1537. [Google Scholar] [CrossRef]
- Martins, C.S.M.; Sousa, H.B.A.; Prior, J.A.V. From Impure to Purified Silver Nanoparticles: Advances and Timeline in Separation Methods. Nanomaterials 2021, 11, 3407. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M. Silver Nanoparticles: Synthesis, Detection, Characterization and Assessment in Environment. Nanomaterials 2022, 12, 167. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Hu, T.; Chen, H.; Wang, C.; Huang, M.; Zhao, F. Study of electroless Sn-coated Cu microparticles and their application as a high temperature thermal interface material. Surf. Coat. Technol. 2017, 319, 230–240. [Google Scholar] [CrossRef]
- Chen, H.; Hu, T.; Li, M.; Zhao, Z. Cu@Sn Core–Shell Structure Powder Preform for High-Temperature Applications Based on Transient Liquid Phase Bonding. IEEE Trans. Power Electron. 2017, 32, 441–451. [Google Scholar] [CrossRef]
- Hu, T.; Chen, H.; Li, M. Die attach materials with high remelting temperatures created by bonding Cu@Sn microparticles at lower temperatures. Mater. Des. 2016, 108, 383–390. [Google Scholar] [CrossRef]
- Hu, B.; Yang, F.; Peng, Y.; Ji, H.; Yang, S.; Yang, M.; Li, M. Rapid formation of Cu–Cu joints with high shear strength using multiple-flocculated Ag nanoparticle paste. J. Mater. Sci. Mater. Electron. 2019, 30, 8071–8079. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, L.; Mei, Y.-H.; Lu, G.-Q. A novel multiscale silver paste for die bonding on bare copper by low-temperature pressure-free sintering in air. Mater. Des. 2018, 140, 64–72. [Google Scholar] [CrossRef]
- Gadaud, P.; Caccuri, V.; Bertheau, D.; Carr, J.; Milhet, X. Ageing sintered silver: Relationship between tensile behavior, mechanical properties and the nanoporous structure evolution. Mater. Sci. Eng. A 2016, 669, 379–386. [Google Scholar] [CrossRef]
- Suganuma, K.; Sakamoto, S.; Kagami, N.; Wakuda, D.; Kim, K.S.; Nogi, M. Low-temperature low-pressure die attach with hybrid silver particle paste. Microelectron. Reliab. 2012, 52, 375–380. [Google Scholar] [CrossRef]



| No. | Chemicals | Formula | Manufacturers |
|---|---|---|---|
| 1 | Disodium EDTA (AR) | C10H14N2Na2O8 | Aladdin, China |
| 2 | Thiourea (AR) | CH4N2S | Aladdin, China |
| 3 | Sodium hypophosphite (AR) | NaH2PO2·H2O | Aladdin, China |
| 4 | Stannous chloride (AR) Ascorbic acid (AR) | SnCl2·2H2O | Aladdin, China |
| 5 | C6H5O6 | Aladdin, China | |
| 6 | Hydroquinone (AR) | C6H4(OH)2 | Aladdin, China |
| 7 | Hydrochloric acid (AR) | HCl | Aladdin, China |
| 8 | Pine Oil Alcohol (AR) | C10H18O | Aladdin, China |
| 9 | Ethyl cellulose (AR) | (C12H22O5)n | Aladdin, China |
| 10 | Sulfosalicylic acid (AR) | C7H6O6S·2H2O | Aladdin, China |
| 11 | Anhydrous ethanol (AR) | C2H6O | Aladdin, China |
| 12 | Span™ 85 (AR) | C60H108O8 | Aladdin, China |
| 13 | Dibutyl phthalate (AR) | C16H22O4 | Aladdin, China |
| 14 | Deionized water (AR) | H2O | Watsons, China |
| 15 | Polyethylene glycol (AR) | HO(CH2CH2O) H | Aladdin, China |
| 16 | Micron copper powder (3.0–4.5 μm) | Cu | Tianjiu, China |
| 17 | Polyvinyl pyrrolidone (AR) | (C6H9NO)n | Aladdin, China |
| 18 | Silver nitrate (AR) | AgNO3 | Aladdin, China |
| 19 | Ferrous sulfate (AR) | FeSO4 | Aladdin, China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, X.; Zhang, L.; Zhang, L.; Duan, F.; Wang, F.; Zhang, W.; Wang, J.; Zhang, Z.; Hang, C.; et al. A Multilayer Paste Based on Ag Nanoparticles with Cu@Sn for Die Attachment in Power Device Packaging. Materials 2022, 15, 914. https://doi.org/10.3390/ma15030914
Wang J, Wang X, Zhang L, Zhang L, Duan F, Wang F, Zhang W, Wang J, Zhang Z, Hang C, et al. A Multilayer Paste Based on Ag Nanoparticles with Cu@Sn for Die Attachment in Power Device Packaging. Materials. 2022; 15(3):914. https://doi.org/10.3390/ma15030914
Chicago/Turabian StyleWang, Jintao, Xinjie Wang, Lin Zhang, Luobin Zhang, Fangcheng Duan, Fengyi Wang, Weiwei Zhang, Jianqiang Wang, Zheng Zhang, Chunjin Hang, and et al. 2022. "A Multilayer Paste Based on Ag Nanoparticles with Cu@Sn for Die Attachment in Power Device Packaging" Materials 15, no. 3: 914. https://doi.org/10.3390/ma15030914





