Softened Microstructure and Properties of 12 μm Thick Rolled Copper Foil
Abstract
:1. Introduction
2. Experimental Materials and Methods
3. Results and Discussion
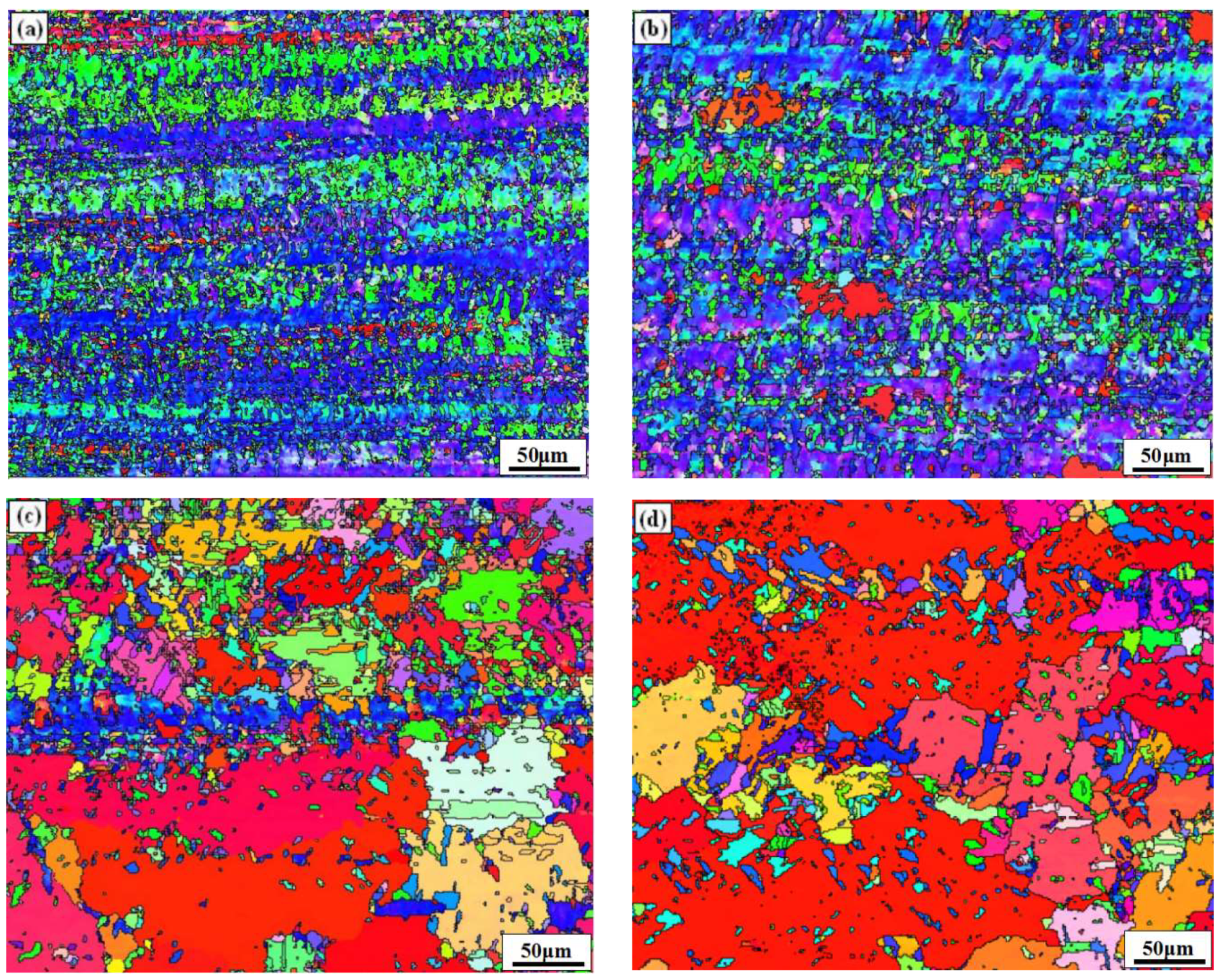
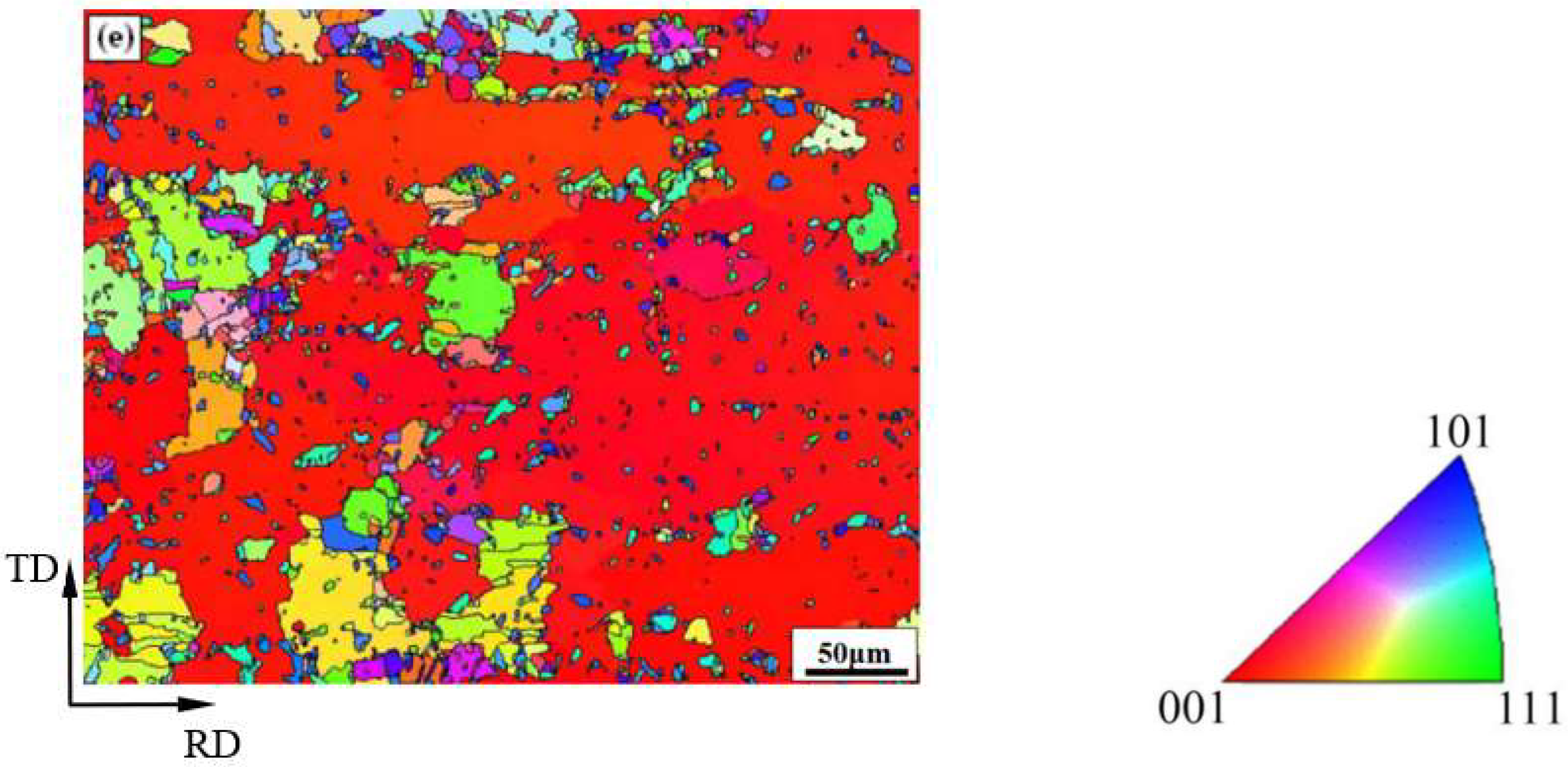
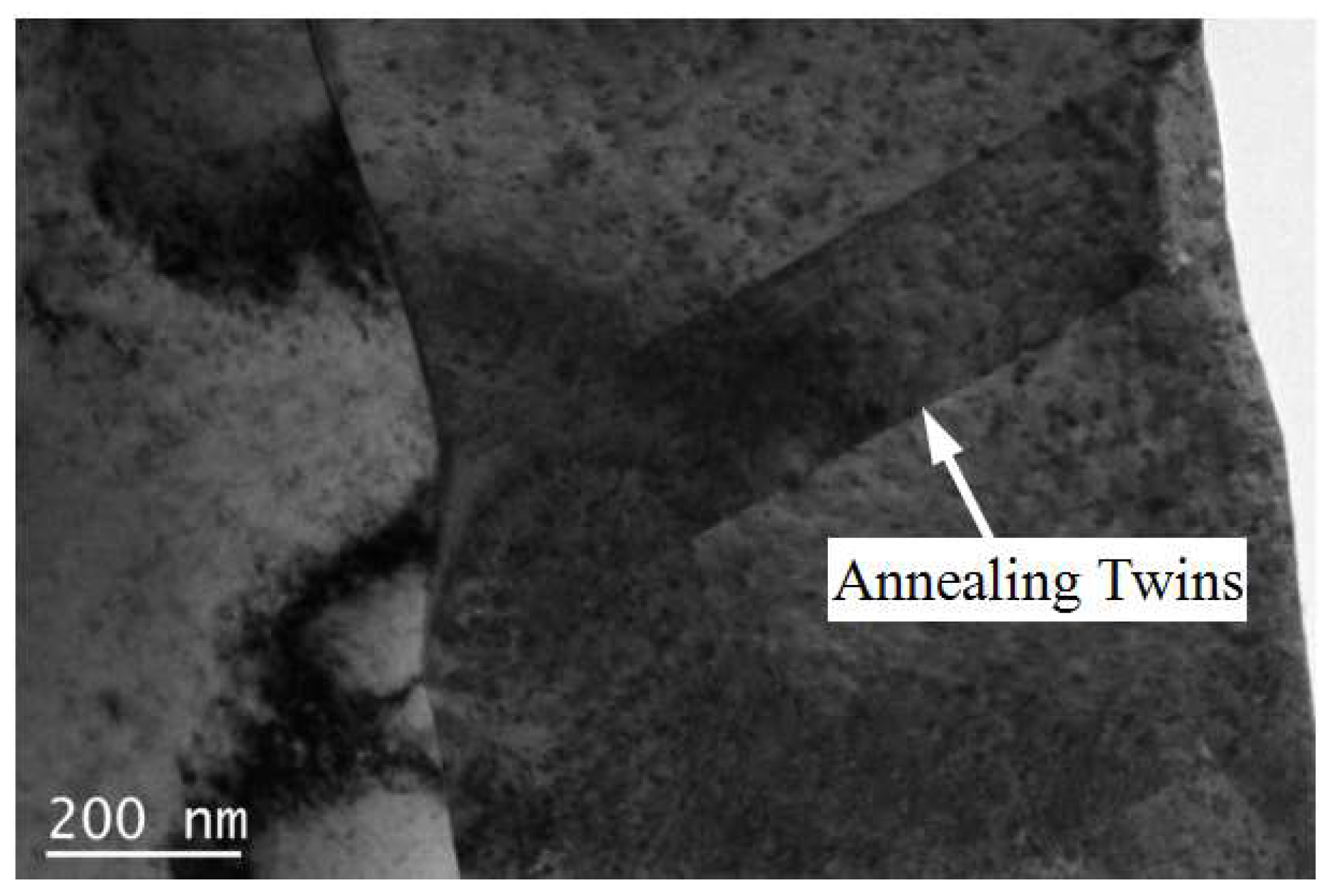
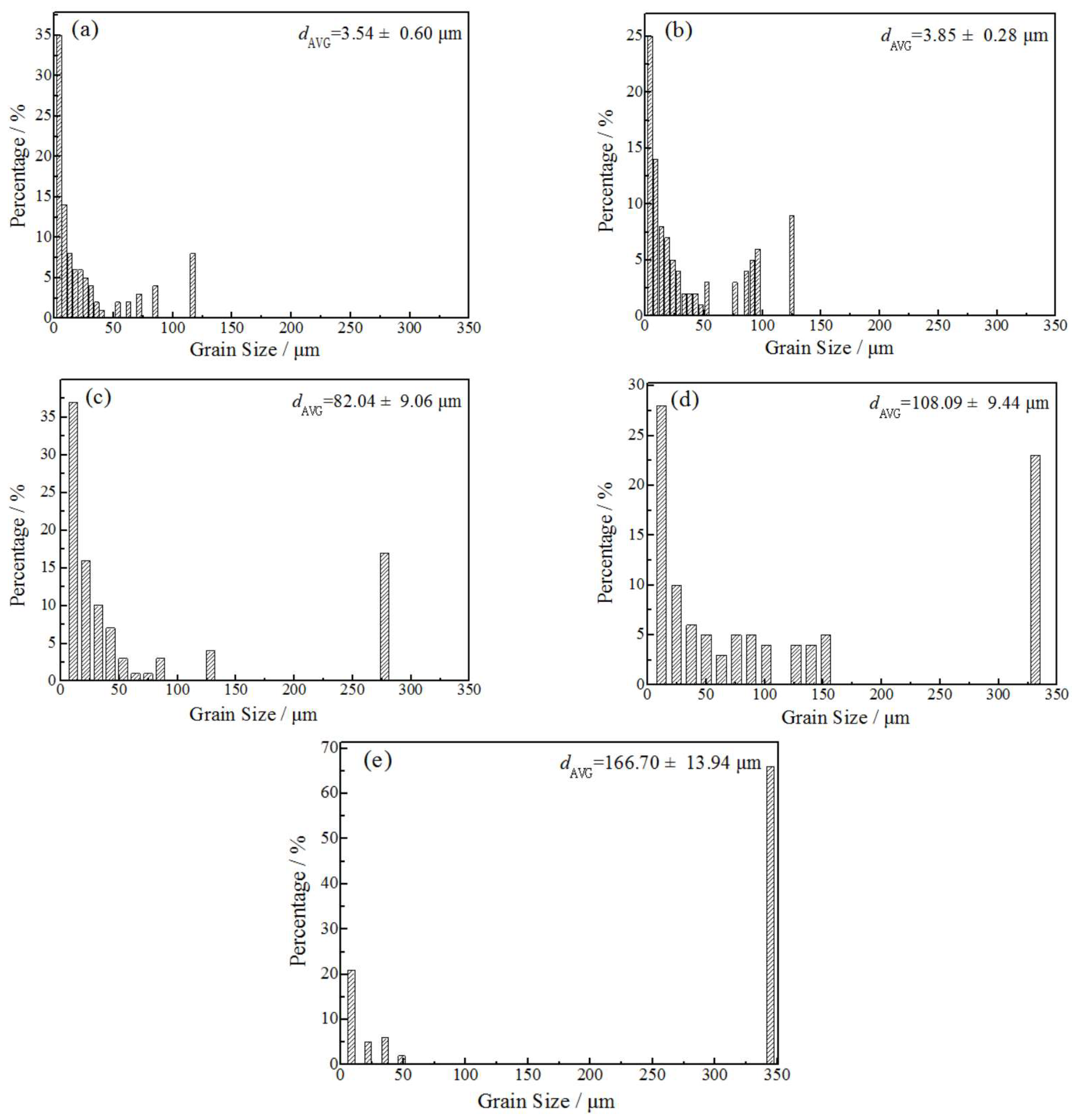
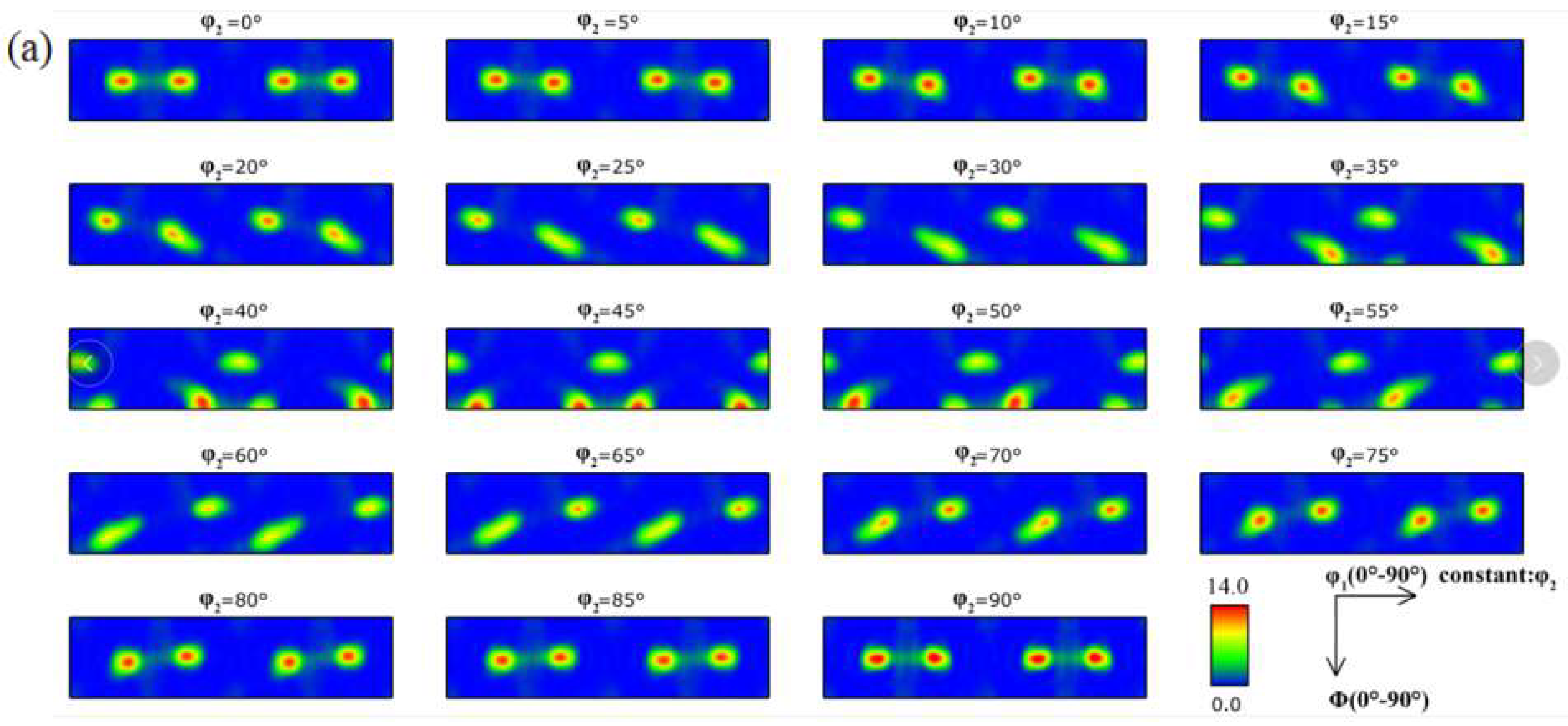
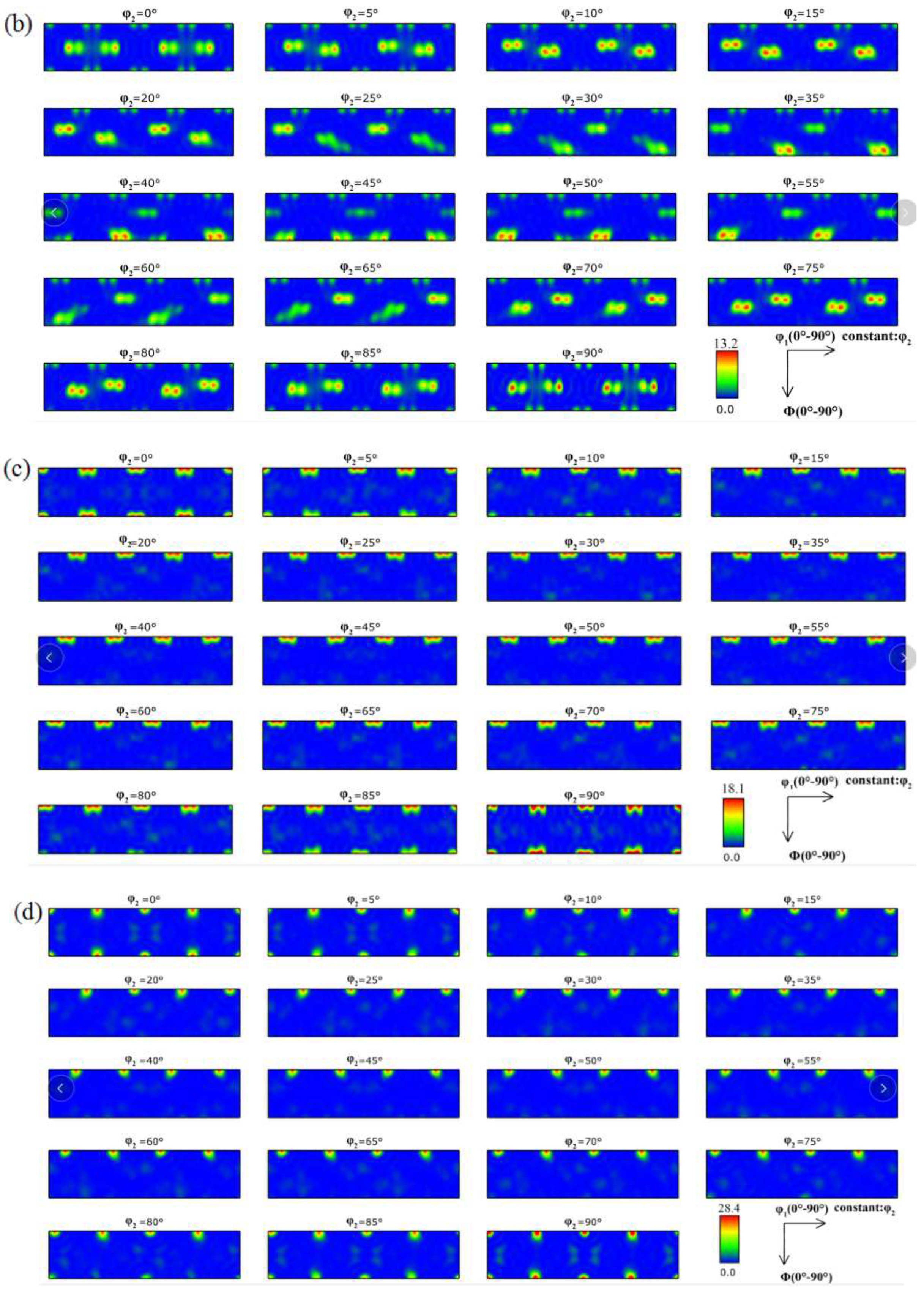
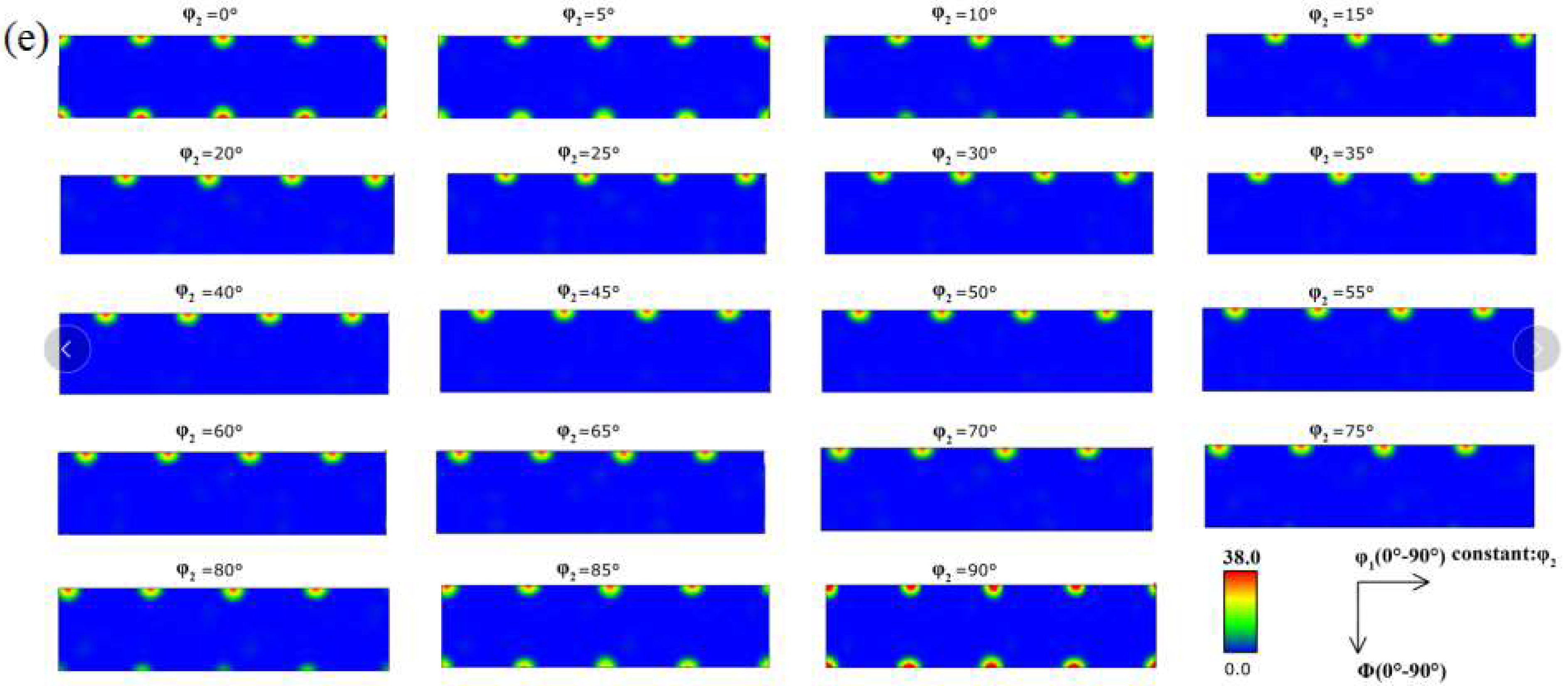
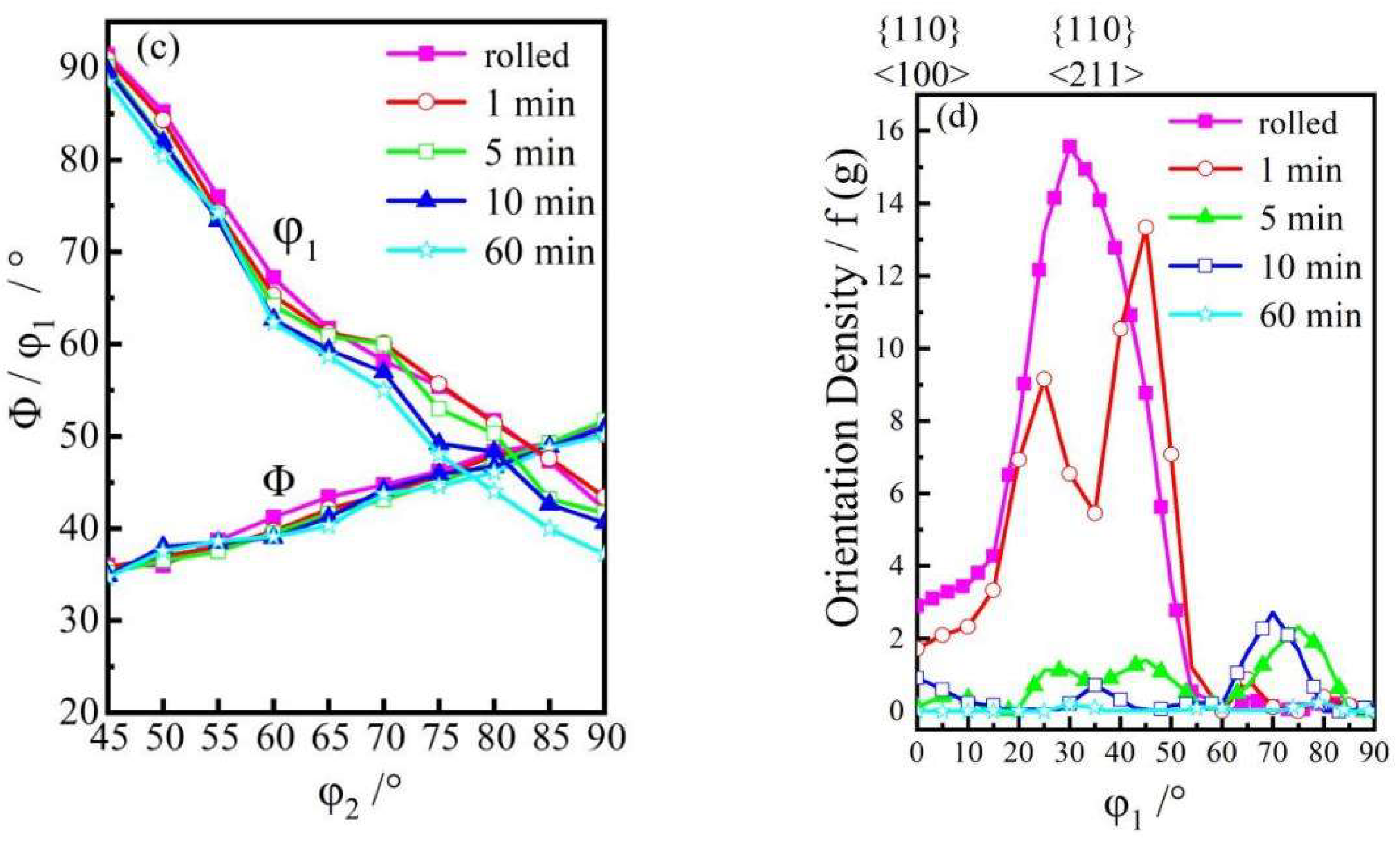
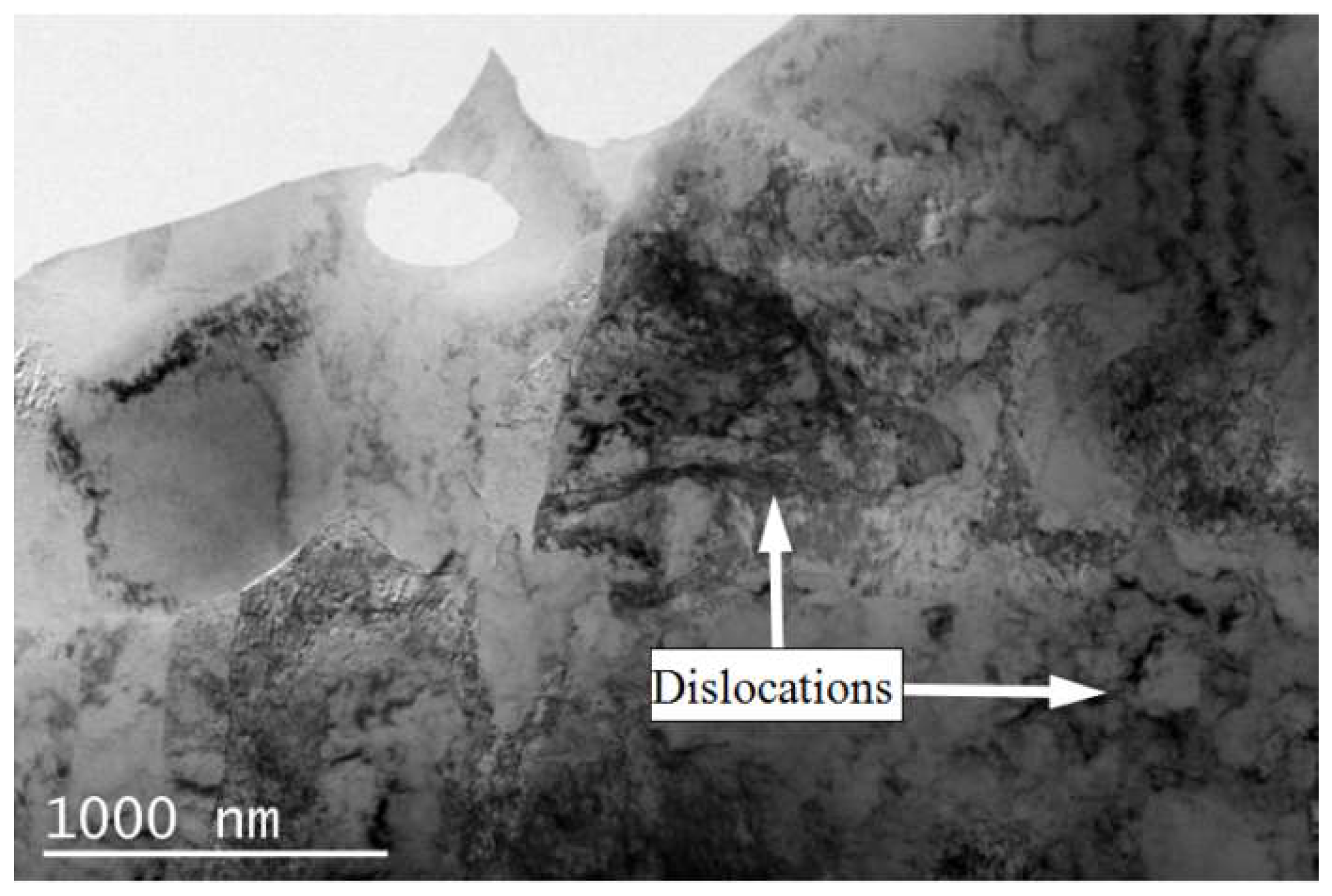
3.1. Microstructure
3.2. Mechanical Properties
3.2.1. Tensile Properties
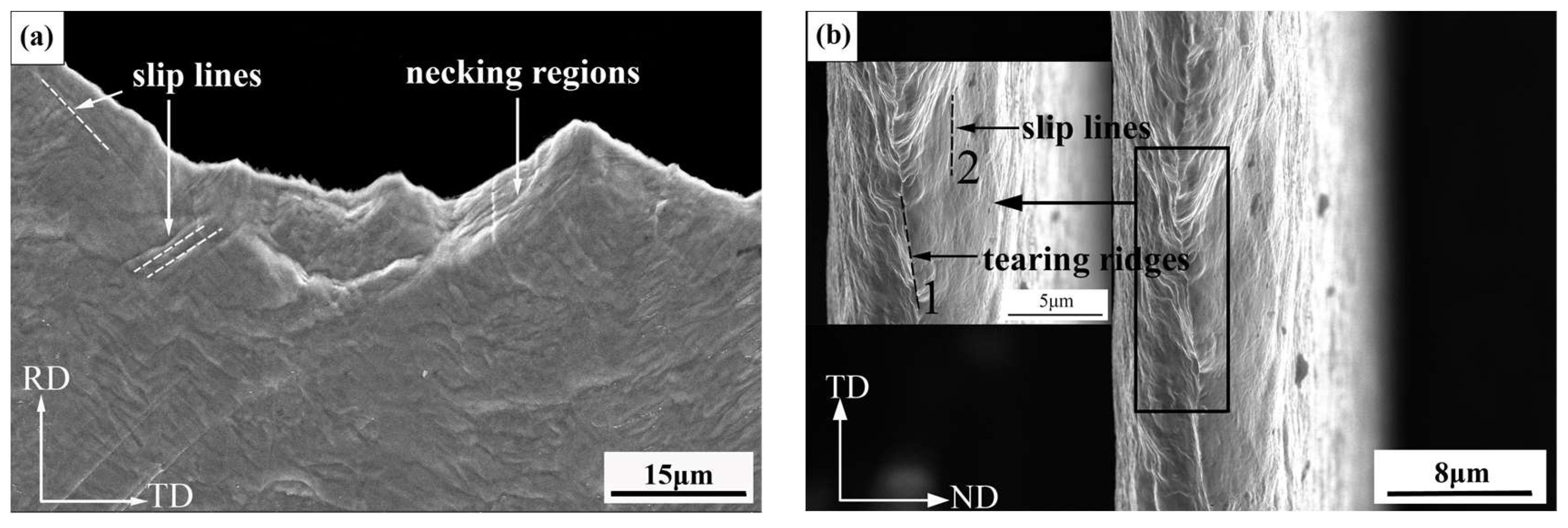
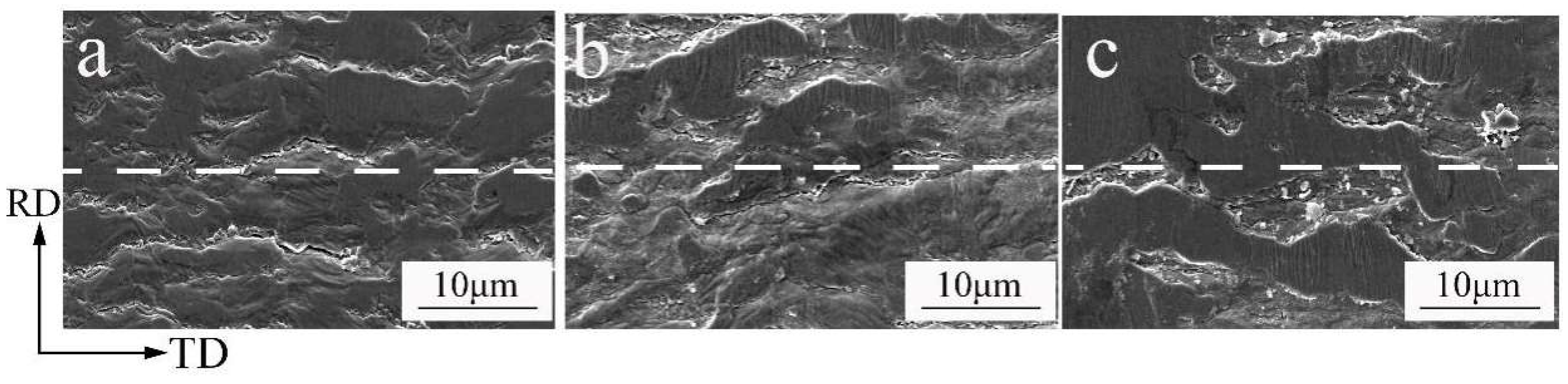
3.2.2. Bending Resistance Properties
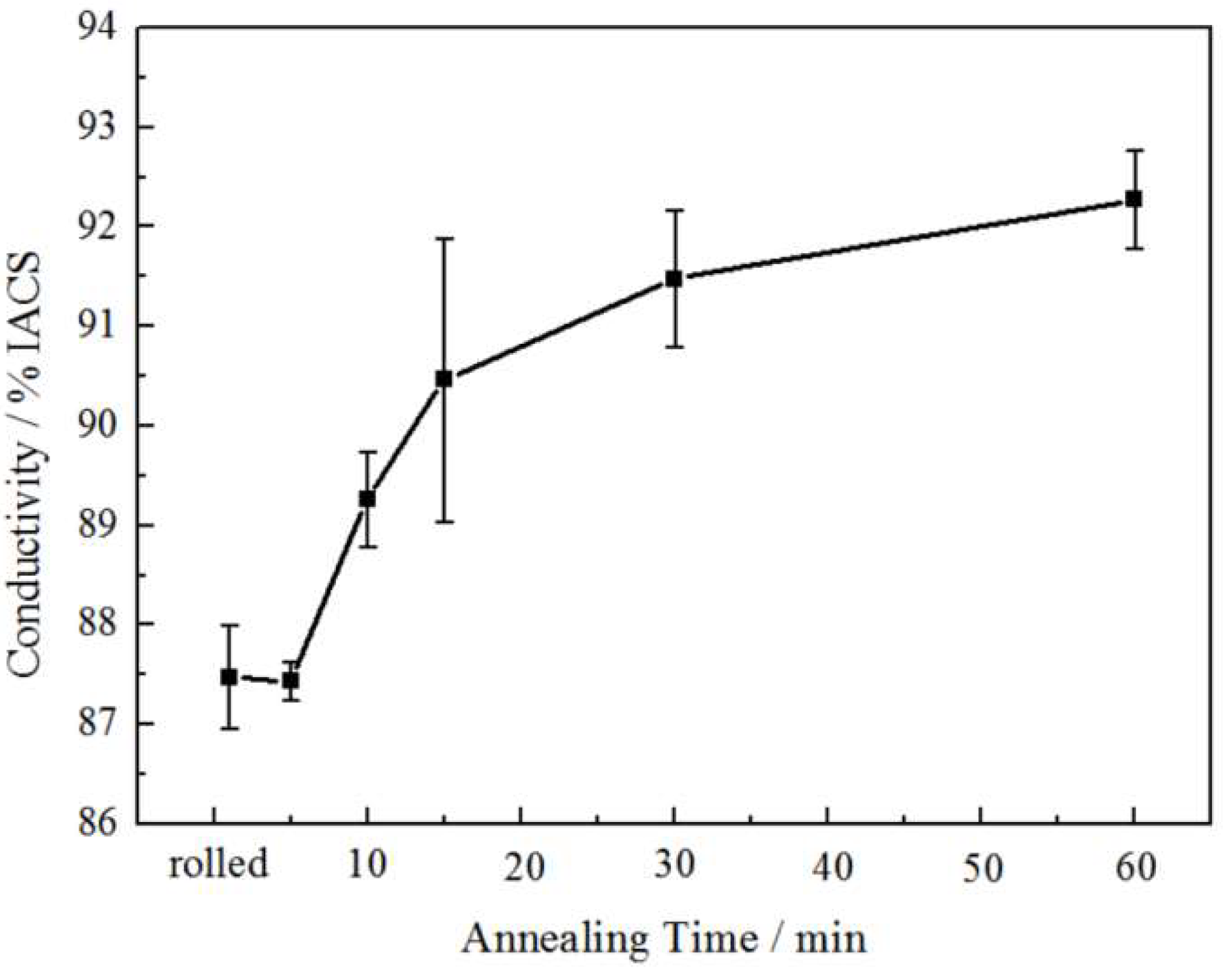
3.3. Electrical Performance
4. Discussion
4.1. Relationship between the Bending Resistance Properties and Microstructure
4.2. Relationship between the Electrical Conductivity and Microstructure
5. Conclusions
- (1)
- When the rolled copper foil is annealed at 180 °C, the microstructure changes regularly with different annealing times. After annealing for 1–10 min, the recrystallization of copper foil occurs. After annealing for 10–60 min, the grains grow, and the annealing texture is mainly cubic texture. With the increase in annealing time, the deformation texture fraction decreases, while the annealing texture fraction increases. When annealed for 10 min, the Σ3 grain boundaries fraction is the highest. The HAGBs fraction increases with the increase in annealing time.
- (2)
- When the rolled copper foil is annealed at 180 °C, the mechanical properties change regularly with different annealing times. After annealing for 1–5 min, the tensile strength decreases sharply because the LAGBs decrease sharply during the annealing time. After annealing for 10–60 min, the tensile strength tends to be stable. The elongation increases with the increase in annealing time. After annealing for 1–5 min, the bending resistance performance decreases significantly. After annealing for 5–60 min, the bending resistance performance improves slightly.
- (3)
- When the rolled copper foil is annealed at 180 °C, the electrical conductivity changes regularly with different annealing times. The electrical conductivity increases gradually with the increase in annealing time. The electrical conductivity of the copper foil reaches 92% IACS after annealing for 60 min. The electrical conductivity can be used as a fast and effective method to analyze the microstructure of metals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, L.; Li, Y.; Zhao, S.; Xiong, S.; Jiang, Z. Corrosion characteristics of rolling oil on the rolled copper foil. Materials 2020, 13, 4933. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Xie, J. Double-coating and porous treatments and evaluation of rolled copper foil surface. Surf. Coat. Technol. 2014, 254, 284–290. [Google Scholar] [CrossRef]
- Li, J.K.; Ren, X.P.; Zhang, Y.L.; Hou, H.L.; Yan, Q. Microstructural response of copper foil to a novel double-cross rolling process. J. Mater. Res. Technol. 2020, 9, 15153–15163. [Google Scholar] [CrossRef]
- Hatano, T.; Kurosawa, Y.; Miyake, J. Effect of material processing on fatigue of FPC rolled copper foil. J. Electron. Mater. 2000, 29, 611–616. [Google Scholar] [CrossRef]
- Sharma, K.P.; Mahyavanshi, R.D.; Kalita, G.; Tanemura, M. Influence of copper foil polycrystalline structure on graphene anisotropic etching. Appl. Surf. Sci. 2017, 393, 428–433. [Google Scholar] [CrossRef]
- Nolden, R.; Zöll, K.; Schwarz-Pfeiffer, A. Development of flexible and Functional sequins using subtractive technology and 3D printing for embroidered wearable textile application. Materials 2021, 14, 2633. [Google Scholar] [CrossRef] [PubMed]
- Yung, K.C.; Liem, H.; Choy, H.S.; Yue, T.M. Impact of plasma etching on fabrication technology of liquid crystal polyer printed circuit board. J. Mater. Sci.-Mater. Electron. 2010, 21, 954–962. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Ou, B.L.; Lee, Y.L. Effect of thermo-process on anodic capacitor foil manufacturing for AC etching. J. Mater. Sci.-Mater. Electron. 2017, 18, 1239–1245. [Google Scholar] [CrossRef]
- Yoshihara, N.; Noda, M. Chemical etching of copper foils for single-layer graphene growth by chemical vapor deposition. Chem. Phys. Lett. 2017, 685, 40–46. [Google Scholar] [CrossRef]
- Xue, S.; Wang, C.; Chen, P.; Xu, Z.; Cheng, L.; Guo, B.; Shan, D. Investigation of electrically-assisted rolling process of corrugated surface microstructure with T2 copper foil. Materials 2019, 12, 4144. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Sun, J.; Xu, Y.; Yan, X. Effect of lubricants and annealing treatment on the electrical conductivity and microstructure of rolled copper foil. J. Electron. Mater. 2015, 44, 2432–2439. [Google Scholar] [CrossRef]
- Li, J.; Ren, X.; Ling, Z.; Wang, H. Improving bending property of copper foil by the combination of double-rolling and cross rolling. J. Mater. Res. Technol. 2020, 9, 6922–6927. [Google Scholar] [CrossRef]
- Anand, G.; Barai, K.; Madhavan, R.; Chattopadhyay, P.P. Evolution of annealing texture in cryo-rolled copper. Mater. Sci. Eng. A 2015, 638, 114–120. [Google Scholar] [CrossRef]
- Tseng, I.; Hsu, Y.; Leu, J.; Tu, K.; Chen, C. Effect of thermal stress on anisotropic grain growth in nano-twinned and un-twinned copper films. Acta Mater. 2021, 206, 116637. [Google Scholar] [CrossRef]
- Chen, K.T.; Yang, Y.C.; Yi, Y.H.; Zheng, X.T.; Tuan, H.Y. A carbon ink for use in thin, conductive, non peelable, amphiphilic, antioxidant, and large-area current collector coating with enhanced lithium ion battery performance. J. Colloid Interface Sci. 2021, 598, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Moelans, N.; Zhang, Y.; Jensen, D.J. Influence of geometrical alignment of the deformation microstructure on local migration of grain boundaries during recrystallization: A phase-field study. Scr. Mater. 2021, 191, 116–119. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, H.; Qin, S.; Liu, J.; Xu, X. Effect of deformation parameters on twinning evolution during hot deformation in a typical nickel-based superalloy. Mater. Sci. Eng. A 2017, 696, 290–298. [Google Scholar] [CrossRef]
- He, Q.; Jiang, X.; Cai, P.; Zhang, L.; Sun, T.; Yang, X.; Zhou, K.; Zhang, L. Effect of annealing on microstructure and corrosion behavior of interstitial free steel. Materials 2022, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Popova, E.N.; Deryagina, I.L.; Valova-Zaharevskaya, E.G.; Ruello, M.L.; Popov, V.V. Microstructural Features in Multicore Cu–Nb Composites. Materials 2021, 14, 7033. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, X.; Huang, Y.; Liu, Y.; Zhao, L.; Zhou, W. Evolution of shear texture during the asymmetric rolling and its annealing behavior in a twin-roll casting AA6016 sheet: An ex-situ electron backscatter diffraction study. J. Mater. Res. Technol. 2020, 9, 6420–6433. [Google Scholar] [CrossRef]
- Song, M.; Liu, X.; Liu, L. Size effect on mechanical properties and texture of pure copper foil by cold rolling. Materials 2017, 10, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishin, V.V.; Glukhov, P.A.; Shishov, I.A.; Stolyarov, O.N.; Kasatkin, I.A. Structure evolution and mechanical properties of beryllium foils subjected to cold rolling and high-vacuum annealing. Mater. Sci. Eng. A 2019, 750, 60–69. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wang, X.; Xie, J. Relations of rolling reduction and microstructure, texture and bending property of rolled copper foils. Chin. J. Mater. Res. 2014, 28, 241–247. [Google Scholar]
- Chen, Q.Z.; Jones, C.N.; Knowles, D.M. The grain boundary microstructures of the base and modified RR 2072 bicrystal superalloys and their effects on the creep properties. Mater. Sci. Eng. A 2004, 385, 402–418. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P.; He, H.; Shi, B. Enhanced the thermal conductivity of flexible copper foil by introducing graphene. Mater. Des. 2020, 187, 108373. [Google Scholar] [CrossRef]
- Girard, G.; Martiny, M.; Mercier, S. Experimental characterization of rolled annealed copper film used in flexible printed circuit boards: Identification of the elastic-plastic and low-cycle fatigue behaviors. Microelectron. Reliab. 2020, 115, 113976. [Google Scholar] [CrossRef]
- Chen, J.Q.; Gao, H.T.; Hu, X.L.; Yang, L.Q.; Ke, D.W.; Liu, X.H.; Yan, S.; Lu, R.H.; Misra, R.D.K. The significant size effect on nucleation and propagation of crack during tensile deformation of copper foil: Free surface roughening and crystallography study. Mater. Sci. Eng. A 2020, 790, 139678. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Fan, B.; Shan, H.; Chen, Q.; Jiang, C.; Hou, G.; Tang, Y. Study on the relationship between crystal plane orientation and strength of electrolytic copper foil. J. Alloys Compd. 2021, 884, 161044. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Misra, R.D.K.; Chen, Z. Grain size effect on shearing performance of copper foil: A polycrystal plasticity investigation. Mech. Mater. 2022, 166, 104212. [Google Scholar] [CrossRef]
- Pucillo, G.P.; Esposito, L.; Leonetti, D. On the effects of unilateral boundary conditions on the crack growth rate under cycling bending loads. Int. J. Fatigue 2019, 124, 245–252. [Google Scholar] [CrossRef]
- Lezaack, M.; Hannard, F.; Simar, A. Understanding the ductility versus toughness and bendability decoupling of large elongated and fine grained Al 7475-T6 alloy. Mater. Sci. Eng. A 2022, 839, 142816. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Xie, J. Mechanism of surface texture evolution in pure copper strips subjected to double rolling. Prog. Nat. Sci.-Mater. Int. 2014, 24, 75–82. [Google Scholar] [CrossRef]
- Liao, W.; Liu, X.; Yang, Y.; Wang, S.; Du, M. Effect of cold rolling reduction rate on mechanical properties and electrical conductivity of Cu-Ni-Si alloy prepared by temperature controlled mold continuous casting. Mater. Sci. Eng. A 2019, 763, 138068. [Google Scholar] [CrossRef]
- Huang, H.L.; Ho, N.J. The study of fatigue in polycrystalline copper under various strain amplitude at stage I: Crack initiation and propagation. Mater. Sci. Eng. A 2000, 293, 7–14. [Google Scholar] [CrossRef]
- Paik, S.; Dutta, B.K.; Kumar, N.N.; Tewari, R. Fracture initiation in a single crystal copper edge-crack specimen for various crystallographic orientations. Theor. Appl. Fract. Mec. 2021, 114, 103019. [Google Scholar] [CrossRef]
- Chembarisova, R.G. Influence of grain boundaries on the electrical conductivity of copper alloys. Tech. Phys. 2020, 65, 593–601. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Gao, H.; Yan, S.; Chen, S.; Liu, X.; Hu, X. Rolled electrodeposited copper foil with modified surface morphology as anode current collector for high performance lithium-ion batteries. Surf. Coat. Technol. 2021, 410, 126881. [Google Scholar] [CrossRef]
- Dominguez, D.; Sevostianov, I. Cross-property connection between work-hardening coefficient and electrical resistivity of stainless steel during plastic deformation. Int. J. Fatigue 2011, 167, 281–287. [Google Scholar] [CrossRef]
- Breidi, A.; Dudarev, S.L. Dislocation dynamics simulation of thermal annealing of a dislocation loop microstructure. J. Nucl. Mater. 2022, 562, 153552. [Google Scholar] [CrossRef]
- Feng, R.; Li, S.; Li, Z.; Tian, L. Variations of microstructure and properties of 690 MPa grade low carbon bainitic steel after tempering. Mater. Sci. Eng. A 2012, 558, 205–210. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Zhan, M.; Zheng, Z.; Gao, J.; Shao, G. Electron force-induced dislocations annihilation and regeneration of a superalloy through electrical in-situ transmission electron microscopy observations. J. Mater. Sci. Technol. 2020, 36, 79–83. [Google Scholar] [CrossRef]
- Yang, F.; Dong, L.; Cai, L.; Wang, L.; Xie, Z.; Fang, F. Effect of cold drawing strain on the microstructure, mechanical properties and electrical conductivity of low-oxygen copper wires. Mater. Sci. Eng. A 2021, 818, 141348. [Google Scholar] [CrossRef]
- Ablyaz, T.R.; Shlykov, E.S.; Muratov, K.R.; Zhurin, A.V. Study of the EDM process of bimetallic materials using a composite electrode tool. Materials 2022, 15, 750. [Google Scholar] [CrossRef] [PubMed]







| Annealing Time/min | Rolled | 1 | 5 | 10 | 60 |
|---|---|---|---|---|---|
| LAGBs | 68.5% | 61.2% | 29.1% | 24.1% | 16.9% |
| HAGBs | 31.5% | 38.8% | 70.9% | 75.9% | 83.1% |
| Σ3 Grain Boundaries | 2.7% | 4.2% | 47.5% | 63.3% | 57.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, R.; Zhao, W.; Sun, Y.; Wang, X.; Gong, B.; Chang, B.; Feng, T. Softened Microstructure and Properties of 12 μm Thick Rolled Copper Foil. Materials 2022, 15, 2249. https://doi.org/10.3390/ma15062249
Feng R, Zhao W, Sun Y, Wang X, Gong B, Chang B, Feng T. Softened Microstructure and Properties of 12 μm Thick Rolled Copper Foil. Materials. 2022; 15(6):2249. https://doi.org/10.3390/ma15062249
Chicago/Turabian StyleFeng, Rui, Weichao Zhao, Yumei Sun, Xiaowen Wang, Benkui Gong, Baoping Chang, and Tianjie Feng. 2022. "Softened Microstructure and Properties of 12 μm Thick Rolled Copper Foil" Materials 15, no. 6: 2249. https://doi.org/10.3390/ma15062249






