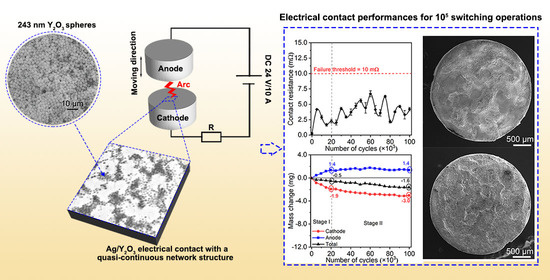
Quasi-Continuous Network Structure Greatly Improved the Anti-Arc-Erosion Capability of Ag/Y2O3 Electrical Contacts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of Spherical Y2O3 Powders with Different Sizes
2.3. Fabrication of Ag/Y2O3 Sintered Compacts
2.4. Characterization
3. Results and Discussion
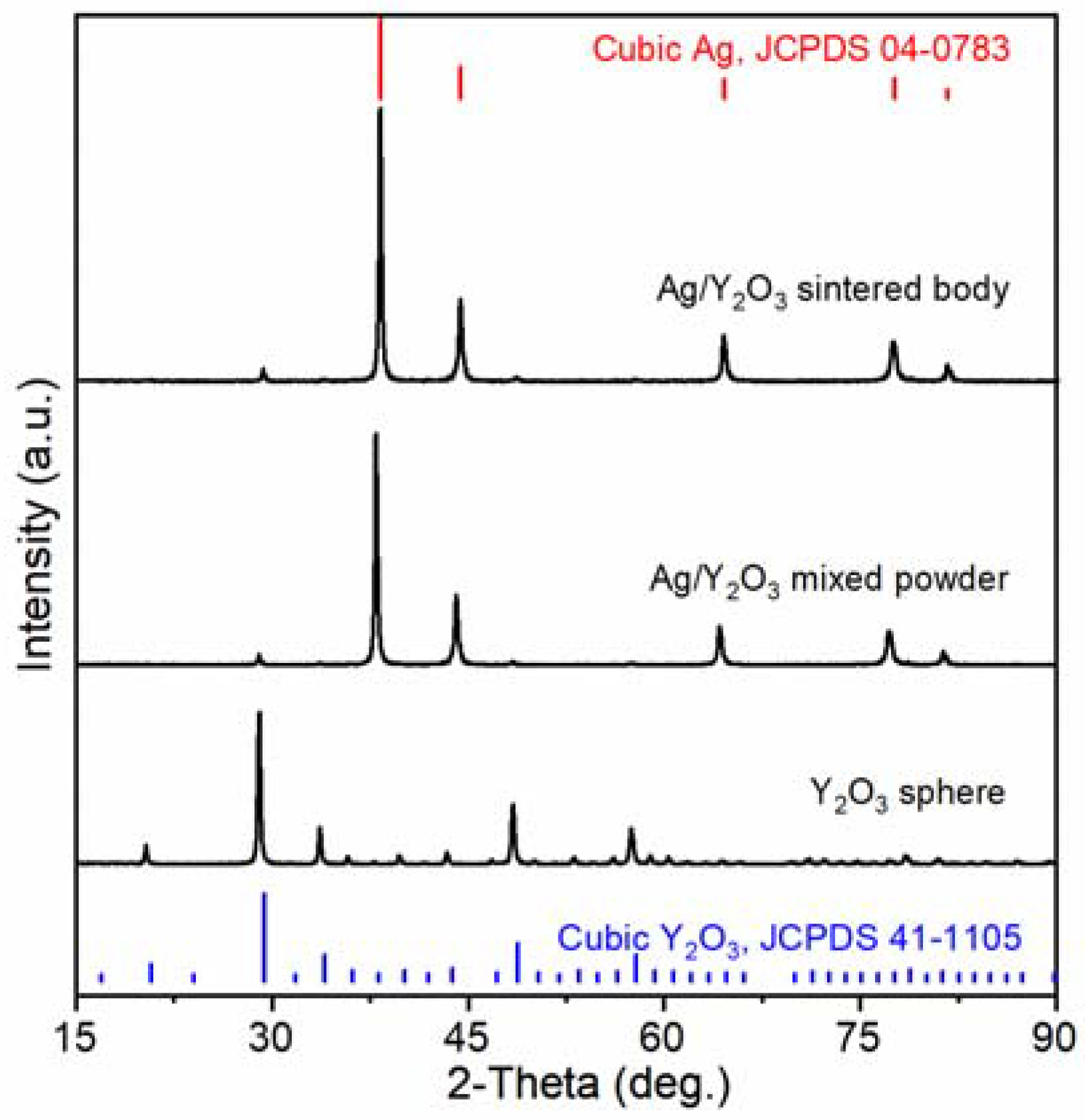
3.1. Phase, Microstructure, and Physical Properties
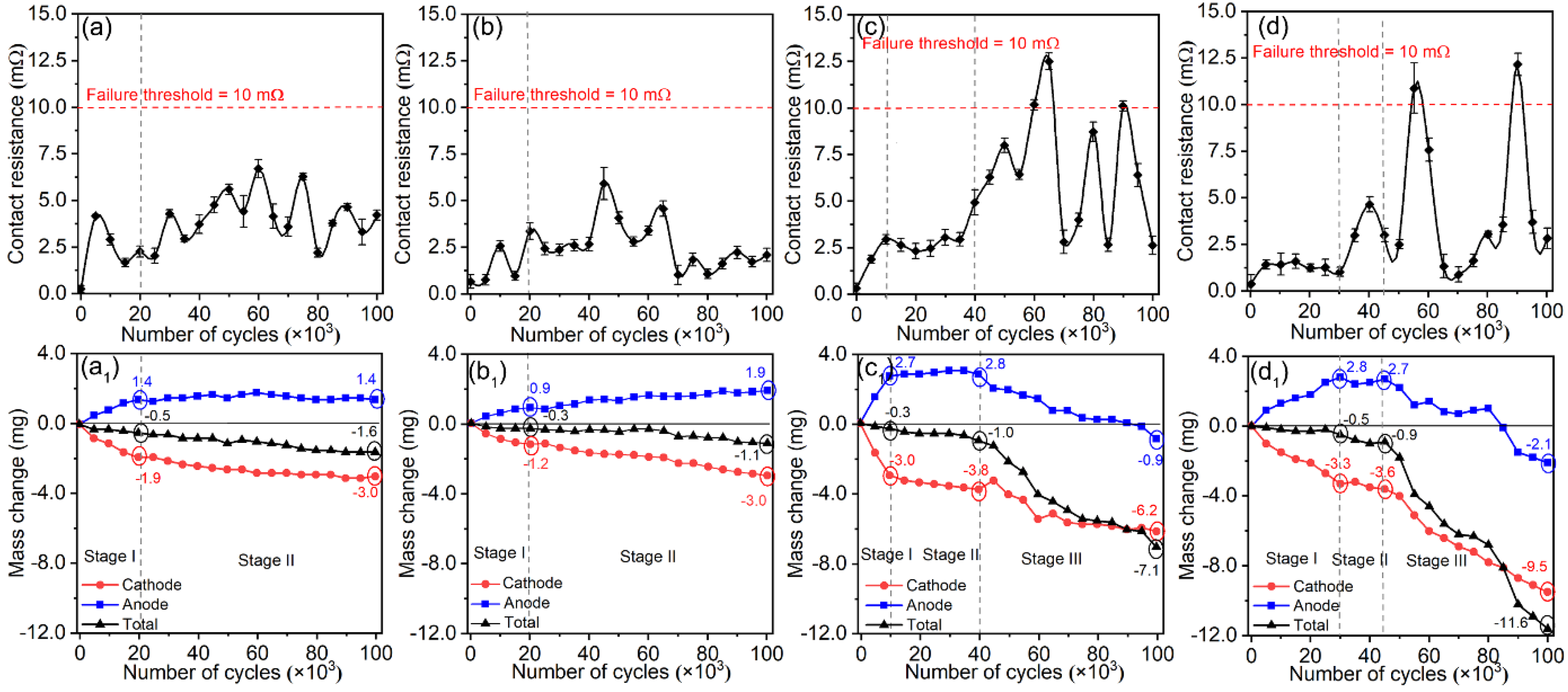
3.2. Electrical Contact Performances of Ag/Y2O3 Contacts
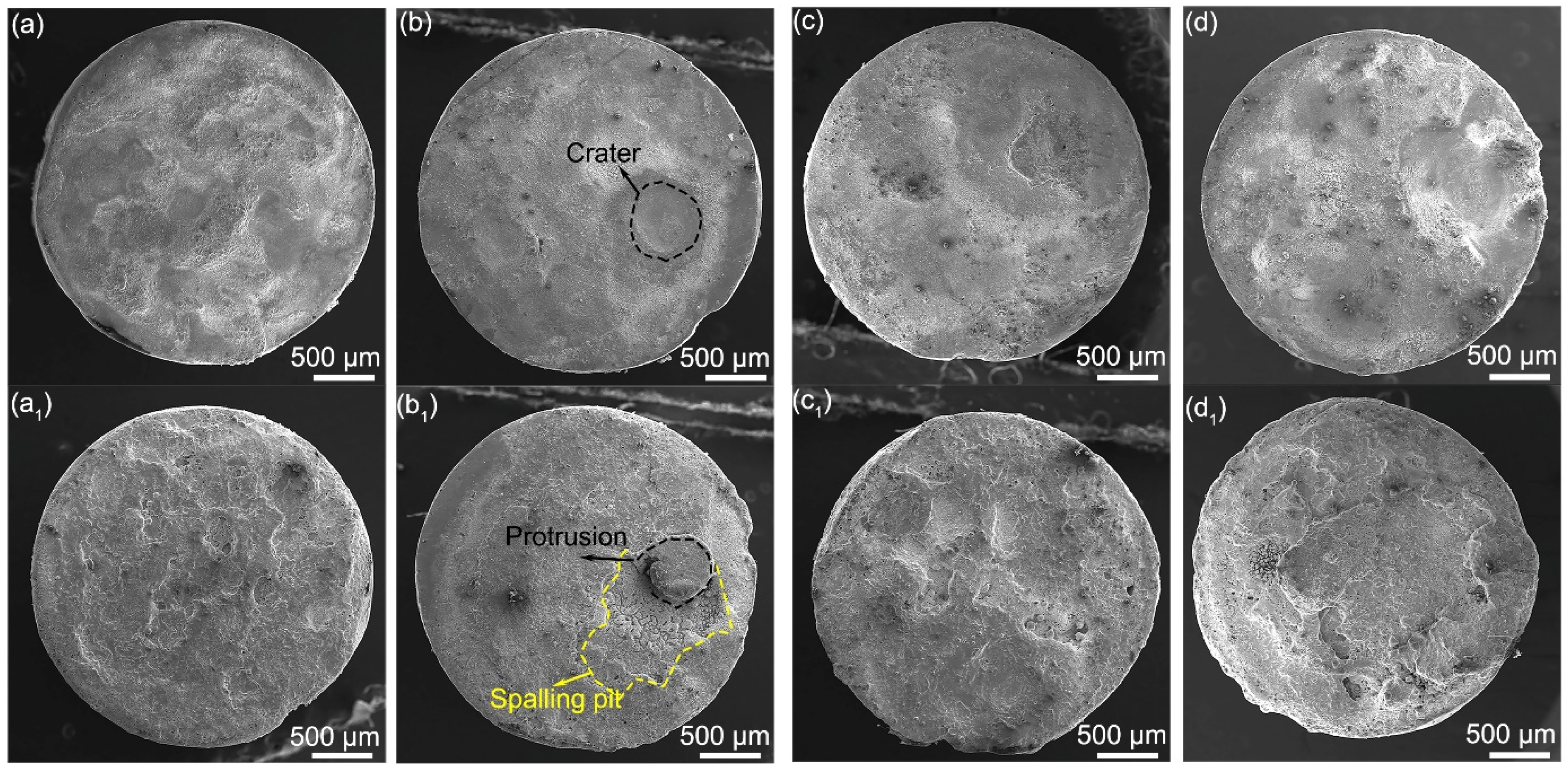
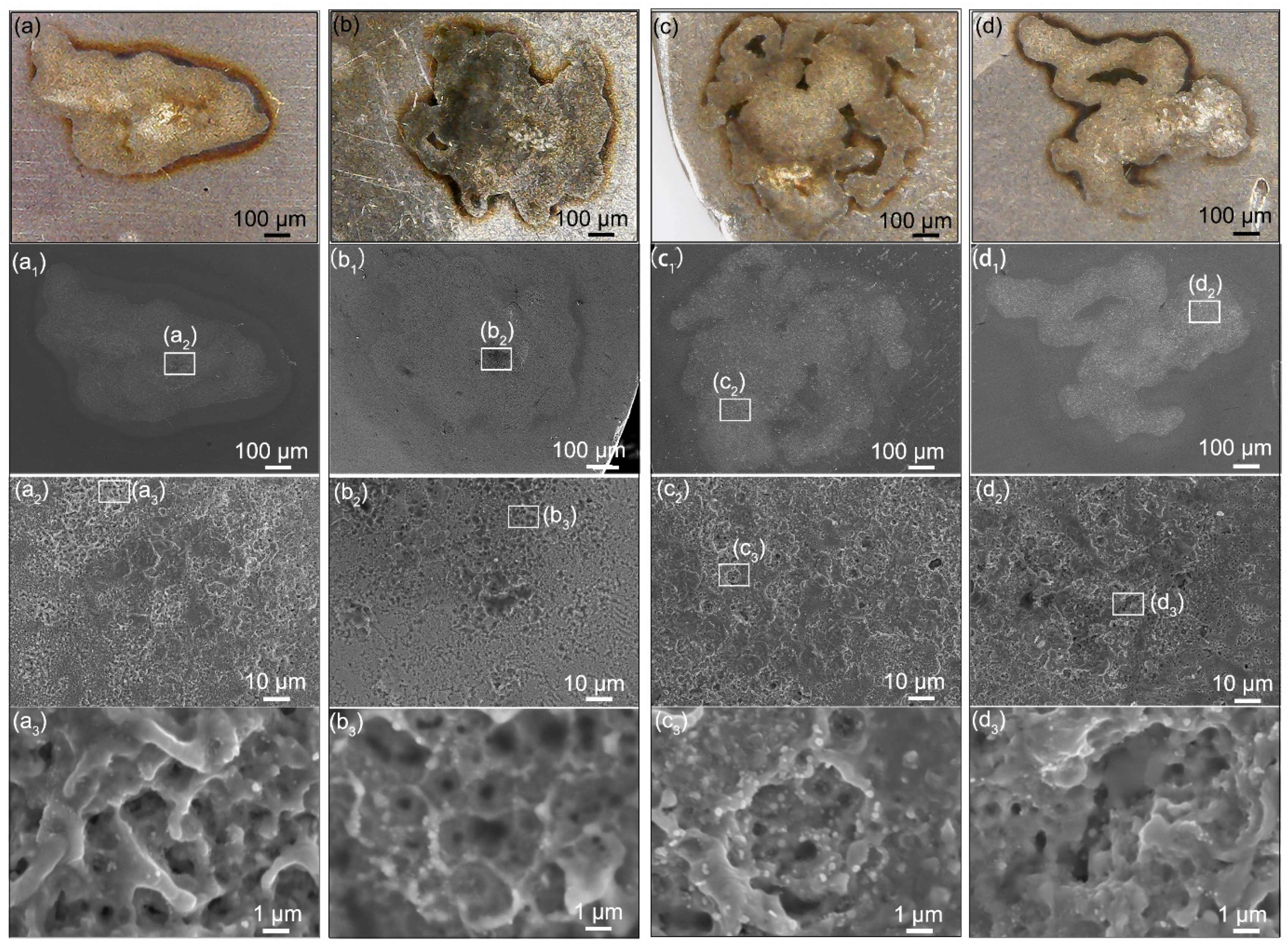
3.3. Microstructure Change after One-Time Arcing Erosion
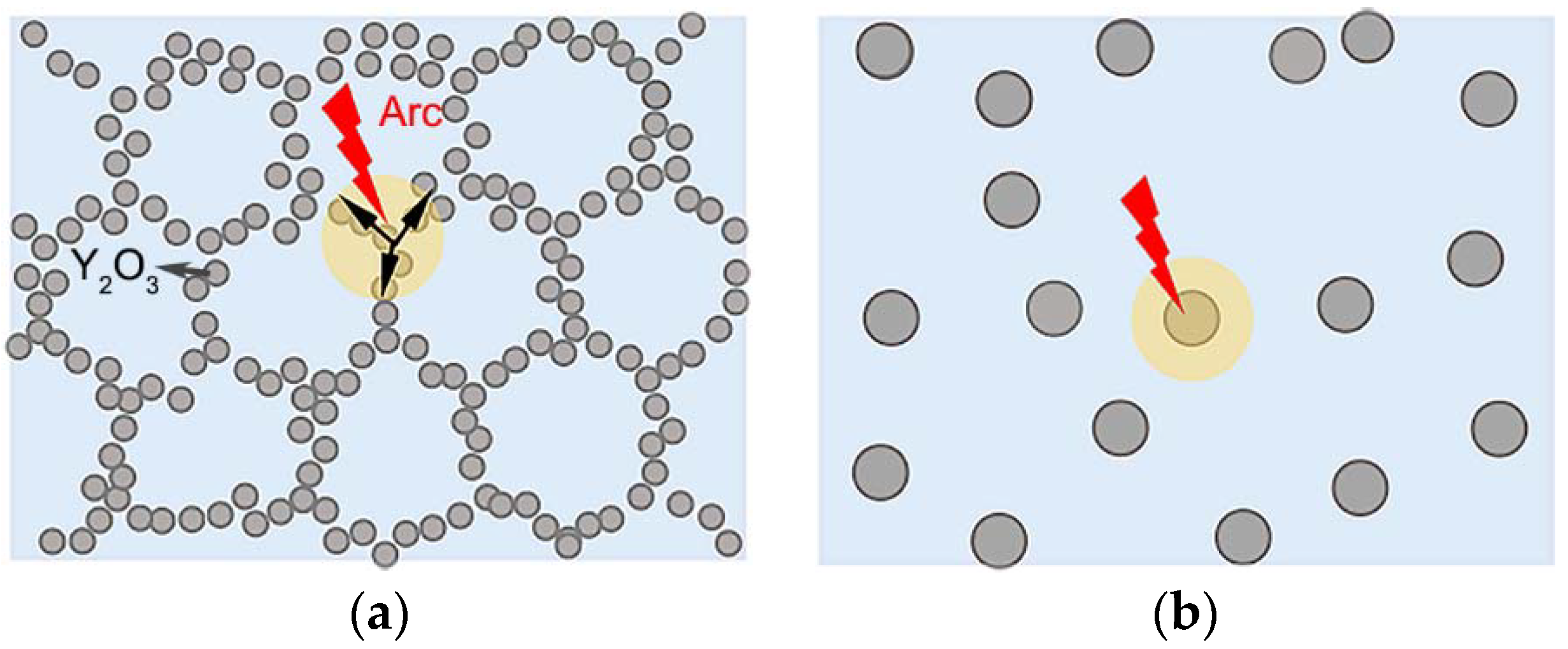
3.4. Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slade, P.G. Electrical Contacts: Principles and Applications, 2nd ed.; CRC Press: New York, NY, USA, 2013; pp. 3–943. [Google Scholar]
- Zhou, L.; Zhu, S.; Zheng, W.; Li, T.; Wu, L.; Zhang, Z.; Lei, Z. Constant current induction brazing process optimization of AgCdO15-Cu electrical contact. J. Manuf. Process. 2020, 51, 122–129. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, G.; Yuan, M.; Wu, C. Influence of operation numbers on arc erosion of Ag/CdO electrical contact materials. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 845–857. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liang, Y.; Zeng, Y.; Chen, X.; Liu, M.; Dai, P.; Chen, J.; Sun, X. Morphology-tunable synthesis and formation mechanism of SnO2 particles and their application in Ag–SnO2 electrical contact materials. Ceram. Int. 2021, 48, 6052–6061. [Google Scholar] [CrossRef]
- Li, G.; Ma, Y.; Zhang, X.; Fang, X.; Feng, W. Interface strengthening and fracture characteristics of the Ag-based contact materials reinforced with nanoporous SnO2(Cu, CuO) phases. Appl. Surf. Sci. 2021, 543, 148812. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Yuan, Z.; Wang, Z.; Du, D. Microstructure and arc erosion behaviors of Ag-CuO contact material prepared by selective laser melting. J. Alloys Compd. 2021, 860, 158494. [Google Scholar] [CrossRef]
- Wang, J.; Kang, Y.; Wang, C.; Wang, J.; Fu, C. Resistance to arc erosion characteristics of CuO skeleton-reinforced Ag-CuO contact materials. J. Alloys Compd. 2018, 756, 202–207. [Google Scholar] [CrossRef]
- Guzmán, D.; Aguilar, C.; Rojas, P.; Criado, J.M.; Diánez, M.J.; Espinoza, R.; Guzmán, A.; Martínez, C. Production of Ag−ZnO powders by hot mechanochemical processing. Trans. Nonferrous Met. Soc. China 2019, 29, 365–373. [Google Scholar] [CrossRef]
- Wang, D.D.; Tian, W.B.; Ding, J.X.; Ma, A.B.; Zhu, Y.F.; Zhang, P.G.; He, W.; Sun, Z.M. Anisotropic arc erosion resistance of Ag/Ti3AlC2 composites induced by the alignment of Ti3AlC2. Corros. Sci. 2020, 171, 108633. [Google Scholar] [CrossRef]
- Ding, J.; Tian, W.; Wang, D.; Zhang, P.; Chen, J.; Zhang, Y.; Sun, Z. Corrosion and degradation mechanism of Ag/Ti3AlC2 composites under dynamic electric arc discharge. Corros. Sci. 2019, 156, 147–160. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liu, J.; Zhang, H.; Fei, Y. Effect of electric load characteristics on the arc-erosion behavior of Ag-4 wt.%TiB2-4 wt.%Ni electrical contact material. Appl. Phys. A Mater. Sci. Process. 2021, 127, 433. [Google Scholar] [CrossRef]
- Shang, S.; Wang, Z.; Li, W.; Han, C.; Wang, Z. The performance degradation comparison test and failure mechanism of silver metal oxide contact materials. In Proceedings of the 2019 IEEE Holm Conference on Electrical Contacts, Milwaukee, WI, USA, 14–18 September 2019. [Google Scholar]
- Hu, B.-L.; Han, J.-Y.; Ge, S.-W.; Hua, X.-J.; Li, S.-L.; Xing, H.-R.; Wang, K.-S.; Hu, P.; Fu, J.-B.; Zhang, W.; et al. Secondary phases strengthening-toughening effects in the Mo–TiC–La2O3 alloys. Mater. Sci. Eng. A 2022, 831, 142271. [Google Scholar] [CrossRef]
- Kołacz, D.; Księżarek, S.; Borkowski, P.; Karwan-Baczewska, J.; Lis, M.; Kamińska, M.; Juszczyk, B.; Kulasa, J.; Kowalski, A.; Wierzbicki, Ł.; et al. The Influence of mechanical alloying and plastic consolidation on the resistance to arc erosion of the Ag–Re composite contact material. Materials 2021, 14, 3297. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.S.; Babaei, F.; Ansarian, A.; Ahadzadeh, I. Incorporation of Y2O3 nanoparticles and glycerol as an appropriate approach for corrosion resistance improvement of Ni-Fe alloy coatings. Ceram. Int. 2019, 45, 10951–10960. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Yu, H.; Pan, W. High performance of La-doped Y2O3 transparent ceramics. J. Adv. Ceram. 2020, 9, 493–502. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Ma, Y. Sintering behavior and thermal conductivity of Y2O3 fully stabilized HfO2 ceramics. Rare Met. 2021, 40, 1255–1266. [Google Scholar] [CrossRef]
- Fu, S.; Xie, M.; Chen, L.; Yang, Y.; Li, Y.; Liu, Y. Ag-SnO2-Y2O3 electrical contact materials prepared by alloy powder pre-oxidation method. Rare Met. 2005, 29, 448–451. [Google Scholar]
- Mu, Z.; Geng, H.-R.; Li, M.-M.; Nie, G.-L.; Leng, J.-F. Effects of Y2O3 on the property of copper based contact materials. Compos. Part B Eng. 2013, 52, 51–55. [Google Scholar] [CrossRef]
- Yang, R.; Liu, S.; Chen, J.; Cui, H.; Liu, M.; Zhu, F.; Yang, Y.; Xie, M.; Sun, X.; Li, X. Porous Y2O3 fiber-reinforced silver composite exhibiting enhanced mechanical and electrical properties. Ceram. Int. 2019, 45 Pt A, 1881–1886. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Wang, M.; Liu, E.; Zhao, N.; Shi, C.; Lin, D.; Zhu, F.; He, C. A powder-metallurgy-based strategy toward three-dimensional graphene-like network for reinforcing copper matrix composites. Nat. Commun. 2020, 11, 2775. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.-J.; Wang, J.-M.; Sun, S.-H.; Zhang, S.-F.; Yuan, S.; Qiao, Z.-J.; Yu, Z.-Y.; Kang, J.-L.; Li, W.-J. Tuning dual three-dimensional porous copper/graphite composite to achieve diversified utilization of copper current collector for lithium storage. Rare Met. 2021, 40, 2802–2809. [Google Scholar] [CrossRef]
- Lin, Z.; Fan, S.; Liu, M.; Liu, S.; Li, J.-G.; Li, J.; Xie, M.; Chen, J.; Sun, X. Excellent anti-arc erosion performance and corresponding mechanisms of a nickel-belt-reinforced silver-based electrical contact material. J. Alloys Compd. 2019, 788, 163–171. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Hu, Z.; Guo, X. Investigation on arc behavior of AgNi electrical contact material with three-dimensional network structure. Vacuum 2020, 175, 109290. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Jiang, S.; Qu, Y.; Ou, D.; Wang, J. Arc erosion dynamic of island- and skeleton-restricted microstructure evolution modes in Ag–CuO contact materials. J. Alloys Compd. 2020, 828, 154412. [Google Scholar] [CrossRef]
- Salur, E.; Aslan, A.; Kuntoğlu, M.; Acarer, M. Effect of ball milling time on the structural characteristics and mechanical properties of nano-sized Y2O3 particle reinforced aluminum matrix composites produced by powder metallurgy route. Adv. Powder Technol. 2021, 32, 3826–3844. [Google Scholar] [CrossRef]
- Toozandehjani, M.; Matori, K.A.; Ostovan, F.; Abdul Aziz, S.; Mamat, M.S. Effect of milling time on the microstructure, physical and mechanical properties of Al-Al2O3 nanocomposite synthesized by ball milling and powder metallurgy. Materials 2017, 10, 1232. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Geng, H.; Zeng, W.; Zheng, D.; Pan, H.; Kong, C.; Munroe, P.; Sha, G.; Suryanarayana, C.; Zhang, D. High temperature stabilization of a nanostructured Cu-Y2O3 composite through microalloying with Ti. Mater. Sci. Eng. A 2018, 712, 80–87. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Pareek, V.; Panwar, J.; Gupta, S. Synthesis and characterization of ecofriendly silver nanoparticles combined with yttrium oxide (Ag-Y2O3) nanocomposite with assorted adsorption capacity for Cu(II) and Cr(VI) removal: A mechanism perspective. J. Water Process. Eng. 2019, 32, 100917. [Google Scholar] [CrossRef]
- Laporte, J.; Fouvry, S.; Alquier, O. Prediction of electrical contact resistance failure of Ag/Ag plated contact subjected to complex fretting-reciprocating sliding. Wear 2017, 376–377, 656–669. [Google Scholar] [CrossRef]
- Yang, D.; Liao, G.; Huang, S. Invisible photonic prints shown by UV illumination: Combining photoluminescent and noniridescent structural colors. J. Mater. Chem. C 2019, 7, 11776–11782. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, G.; Zhao, C.; Huang, R.; Yuan, M.; Wu, C. Influence of preparation technology on the microstructure and properties of Ag/SnO2Bi2O3CuO composite materials. Mater. Charact. 2022, 183, 111537. [Google Scholar] [CrossRef]
- Pfeffer, R.; Dave, R.N.; Wei, D.; Ramlakhan, M. Synthesis of engineered particulates with tailored properties using dry particle coating. Powder Technol. 2001, 117, 40–67. [Google Scholar] [CrossRef]
- Kim, W.J.; Lee, T.J.; Han, S.H. Multi-layer graphene/copper composites: Preparation using high-ratio differential speed rolling, microstructure and mechanical properties. Carbon 2014, 69, 55–65. [Google Scholar] [CrossRef]
- Huang, X.; Feng, Y.; Li, L.; Li, Z. Erosion behavior of a Cu-Ti3AlC2 cathode by multi-electric arc. Materials 2019, 12, 2947. [Google Scholar] [CrossRef] [Green Version]
- Yeheskel, O.; Tevet, O. Elastic Moduli of Transparent Yttria. J. Am. Ceram. Soc. 1999, 82, 136–144. [Google Scholar] [CrossRef]
- Greenwood, J.A. Constriction resistance and the real area of contact. Br. J. Appl. Phys. 1966, 17, 1621–1632. [Google Scholar] [CrossRef]
- Echeverrigaray, F.G.; de Mello, S.R.S.; Leidens, L.M.; Boeira, C.D.; Michels, A.F.; Braceras, I.; Figueroa, C.A. Electrical contact resistance and tribological behaviors of self-lubricated dielectric coating under different conditions. Tribol. Int. 2020, 143, 106086. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Yi, P.; Lai, X. A micro contact model for electrical contact resistance prediction between roughness surface and carbon fiber paper. Int. J. Mech. Sci. 2017, 124–125, 37–47. [Google Scholar] [CrossRef]
- Liu, X.-L.; Cai, Z.-B.; Liu, S.-B.; Peng, J.-F.; Zhu, M.-H. Effect of roughness on electrical contact performance of electronic components. Microelectron. Reliab. 2017, 74, 100–109. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Niu, C.; Rong, M.; Sun, H.; Niu, L.; Xiong, Q.; Liu, W. Evolution of anodic erosion components and heat transfer efficiency for W and W80Ag20 in atmospheric-pressure arcs. J. Phys. D Appl. Phys. 2020, 53, 475203. [Google Scholar] [CrossRef]
- Guan, W.; Yuan, J.; Lv, H.; Zhu, T.; Fang, Y.; Liu, J.; Wang, H.; Li, Z.; Tang, Z.; Yang, W. Homogeneous arc ablation behaviors of CuCr cathodes improved by chromic oxide. J. Mater. Sci. Technol. 2021, 81, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Wang, J.; Fu, C.; Chang, Y. Effect of CuO additives on the formation of SnO2-rich layers in Ag-SnO2 materials. J. Alloys Compd. 2019, 770, 920–925. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Y.; Zhu, T.; Long, Y.; Yang, J.; Yao, F.; Chen, D.; Tang, Z. Arc spot formation conditions and influencing factors of a micro multi-electrode technology. J. Phys. D Appl. Phys. 2021, 55, 025203. [Google Scholar] [CrossRef]
- Guan, W.; Gao, M.; Lv, H.; Yuan, J.; Chen, D.; Zhu, T.; Fang, Y.; Liu, J.; Wang, H.; Tang, Z.; et al. Laser cladding of layered Zr/Cu composite cathode with excellent arc discharge homogeneity. Surf. Coat. Technol. 2021, 421, 127454. [Google Scholar] [CrossRef]
- Guo, X.; Song, K.; Xu, W.; Li, G.; Zhang, Z. Effect of TiB2 particle size on the material transfer behaviour of Cu–TiB2 composites. Mater. Sci. Technol. 2020, 36, 1685–1694. [Google Scholar] [CrossRef]




| Y2O3 Mean Size (nm) | Relative Density (%) | Hardness (Hv0.1) | Conductivity (IACS%) |
|---|---|---|---|
| 243 | 96.8 | 107.2 ± 3.9 | 67.6 |
| 387 | 98.1 | 109.2 ± 1.8 | 73.0 |
| 792 | 96.1 | 105.8 ± 3.4 | 69.9 |
| 980 | 94.9 | 103.7 ± 2.1 | 66.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Liu, S.; Cui, H.; Yang, H.; Zeng, Y.; Liu, M.; Chen, J.; Wen, M.; Wang, W.; Luo, Z.; et al. Quasi-Continuous Network Structure Greatly Improved the Anti-Arc-Erosion Capability of Ag/Y2O3 Electrical Contacts. Materials 2022, 15, 2450. https://doi.org/10.3390/ma15072450
Yang R, Liu S, Cui H, Yang H, Zeng Y, Liu M, Chen J, Wen M, Wang W, Luo Z, et al. Quasi-Continuous Network Structure Greatly Improved the Anti-Arc-Erosion Capability of Ag/Y2O3 Electrical Contacts. Materials. 2022; 15(7):2450. https://doi.org/10.3390/ma15072450
Chicago/Turabian StyleYang, Rui, Shaohong Liu, Hao Cui, Hongwei Yang, Yiming Zeng, Manmen Liu, Jialin Chen, Ming Wen, Wei Wang, Zhengtang Luo, and et al. 2022. "Quasi-Continuous Network Structure Greatly Improved the Anti-Arc-Erosion Capability of Ag/Y2O3 Electrical Contacts" Materials 15, no. 7: 2450. https://doi.org/10.3390/ma15072450