Laser Powder-Bed Fusion of Ceramic Particulate Reinforced Aluminum Alloys: A Review
Abstract
:1. Introduction
- (i)
- (ii)
- The addition of grain refiners (stable, non-soluble solid ceramic particulates) to reduce hot-tear susceptibility, grain growth and dislocation motion by developing aluminum matrix composites (AMC) [8,33]. The latter conveys a combination of properties of two or more physically distinct phases with the aim to produce parts with far superior properties to the individual components [34].
- (iii)

Reinforcement with Ceramic Particulates
2. Non-Oxide Additives
2.1. Borides: Grain Refining and Strengthening Effect of TiB2, LaB6, CaB6
| System | Used Device, Process Parameters | Relative Density (%) | Average Grain Size (μm) | σy/σu (MPa) | ε/εc (%) | Hardness (HV) | N |
|---|---|---|---|---|---|---|---|
| AlSi10Mg/ 1 wt.% TiB2 | SLM 150 HL P = 350–450 W ν = 1800 mm/s d = 50 μm h = 50 μm Ev = 77.7–100.0 J/mm3 | 99.95 | ~6.3 | - | - | ~126 HV0.2 | [78] |
| AlSi10Mg/ 3.4 vol.%TiB2 | Prox DMP 200 SLM P = 210 W ν = 1000 mm/s d = 30 μm h = 100 μm Ev = 70 J/mm3 | 99.975 | 2.08 | σu = 522.9–529 | ε ≈ 7.5–8.6 | - | [59] |
| AlSi10Mg/ 1 wt.%TiB2 | SLM 150 P = 450 W ν = 1600–2600 mm/s d = 50 μm h = 50 μm Ev = 69.2–112.5 J/mm3 | Up to 99.09 | 6.32 ± 0.07 | σy ≈ 270 σu = 397 | ε ≈ 3.6 | ~124 HV0.2 | [73] |
| AlSi10Mg/ 2 wt.% TiB2 | Up to 99 | 2.20 ± 0.11 | σy ≈ 283 σu ≈ 444 | ε ≈ 4.2 | ~127 HV0.2 | ||
| AlSi10Mg/ 5 wt.% TiB2 | ~96–97.8 | 1.55 ± 0.14 | σy ≈ 270 σu = 422 | ε ≈ 4.1 | ~129 HV0.2 | ||
| AlSi10Mg | Prox DMP 200, 3D Systems P = 220–280 W ν = 800–2000 mm/s d = 30 μm h = 90 μm | 99.56 ± 0.16 | 4.64 | σy = 270.1 ± 4.3 σu = 430.7 ± 1.6 | ε = 4.7 ± 0.4 | 125.9 ± 1.4 HV10 | [77] |
| AlSi10Mg/ 0.5 wt.% TiB2 | 99.82 ± 0.10 | 3.45 | σy = 317.6 ± 2.1 σu = 484.1 ± 3.3 | ε = 9.5 ± 0.3 | 140.5 ± 1.3 HV10 | ||
| AlSi10Mg/ 2 wt.% TiB2 | 99.92 ± 0.04 | 2.0 | σy = 320.1 ± 3.2 σu = 500.7 ± 3.5 | ε = 12.7 ± 0.2 | 147.1 ± 1.5 HV10 | ||
| AlSi10Mg/ 5 wt.% TiB2 | 99.91 ± 0.02 | ~2.0 | σy = 323.7 ± 1.9 σu = 522.9 ± 3.6 | ε = 8.7 ± 0.5 | 151.1 ± 2.1 HV10 | ||
| AlSi10Mg/ 8 wt.% TiB2 | 99.92 ± 0.05 | ~2.0 | σy = 340.8 ± 1.7 σu = 544.4 ± 2.6 | ε = 6.2 ± 0.2 | 161.5 ± 2.5 HV10 | ||
| AlSi10Mg/ 6.5 wt.%TiB2 | BLT-S310 P = 260–350 W ν = 900–1500 mm/s d = 30 μm h = 110–170 μm | >99.5 | 1.63 μm for top | σy = 332.3 ± 6.7 σu = 536.9 ± 14.4 | ε = 16.5 ± 1.7 | - | [79] |
| 1.38 μm for side | σy = 277.9 ± 6.9 σu = 517.3 ± 9.1 | ε = 15.4 ± 1.6 | |||||
| AlSi10Mg/ 11.6 wt.% TiB2 | House-built P = 200–300 W ν = 800–2000 mm/s d = 30 μm h = 105 μm Ev = 31.7–119.0 J/mm3 | 99.5 | ~2 | σu = 530 ± 16 | ε = 15.5 ± 1.2 | 191 ± 4 HV0.3 | [80] |
| AlCu/ ~4.7 wt.% TiB2 | Renishaw AM400 P = 250–300 W ν = 1125–4500 mm/s d = 30 μm h = 90 μm | Up to 99.5 | 0.5–2 | σu = 391 ± 7.3 σy = 317.8 ± 9.3 | ε = 12.5 ± 0.8 | - | [50] |
| Al-Cu-Mg-Si/ 5 vol.% TiB2 | SLM 250 HL P = 190 W ν = 165 mm/s d = 40 μm h = 80 μm Ev = 359.8 J/mm3 | >99.0 | 2.5 ± 0.1 | σyc = 191 ± 12 | εc ≈ 60 | - | [81] |
| Al-Cu/ ~4 wt.% TiB2 | Aconity LAB P = 200 W ν = 1000 mm/s d = 30 μm h = 100 μm Ev = 66.67 J/mm3 | 99.9 ± 0.1 | 0.64 ± 0.26 | σu = 401 ± 2 | ε = 17.7 ± 0.8 | 113 ± 2 HV10 | [82] |
| Al-12Si | SLM 250 HL P = 320 W ν = 1655 mm/s d = 50 µm h = 110 µm Ev = 35.1 J/mm3 | - | - | σyc = 211 ± 4 | - | 119 HV0.05 | [64,83] |
| Al-12Si/ 2 wt.% TiB2 | ≈99.1 | ~5.1 | σyc = 225 ± 4 | εc ≈ 30 | 142 ± 6 HV0.05 | ||
| AlSi10Mg | SLM125HL P = 300 W ν = 1650 mm/s d = 30 μm h = 130 μm Ev = 46.6 J/mm3 T = 200 °C | 99.08 ± 0.1 | 6.1 | σy = 243 ± 9 σu = 420 ± 9 | εtr ≈ 5.5 εlong ≈ 3.7 | - | [84] |
| AlSi10Mg/ 0.05 wt.% LaB6 | 99.03 ± 0.08 | 4.0 | σy ≈ 242 σu ≈ 430 | εtr ≈ 6.4 εlong ≈ 4.8 | |||
| AlSi10Mg/ 0.2 wt.% LaB6 | 99.17 ± 0.05 | 2.5 | σy ≈ 245 σu ≈ 435 | εtr ≈ 7 εlong ≈ 6.5 | |||
| AlSi10Mg/ 0.5 wt.% LaB6 | 99.46 ± 0.18 | 2.2 | σy ≈ 240 σu ≈ 427 | εtr ≈ 6.5 εlong ≈ 6.9 | |||
| AlSi10Mg/ 1 wt.% LaB6 | 99.49 ± 0.13 | 1.8 | σy ≈ 235 σu ≈ 429 | εtr ≈ 7.1 εlong ≈ 5.8 | |||
| AlSi10Mg/ 2 wt.% LaB6 | 99.48 ± 0.22 | 1.6 | σy ≈ 238 σu ≈ 445 | εtr ≈ 7.0 εlong ≈ 5.6 | |||
| 2024 Al alloy | Aconity LAB machine P = 200–300 W ν = 600–1200 mm/s d = 30 µm h = 100 µm Ev = 56–167 J/mm3 | 98.3 | - | - | - | 66 ± 6 HV5 | [28] |
| 2024 Al alloy/ 2 wt.% CaB6 | >99.5 | 0.91 ± 0.32 | σy = 348 ± 16 σu = 391 ± 22 | ε = 12.6 ± 0.6 | 132 ± 4 HV5 |

2.2. Carbides: Grain Refining and Strengthening Effect of TiC, SiC, B4C
2.2.1. Titanium Carbide: TiC

2.2.2. Silicon Carbide: SiC
2.3. Nitrides: Grain Refinement and Strengthening Effect
2.3.1. Titanium Nitride: TiN
2.3.2. Aluminum Nitride: AlN
2.3.3. Boron Nitride: BN
2.3.4. Silicon Nitride: Si3N4
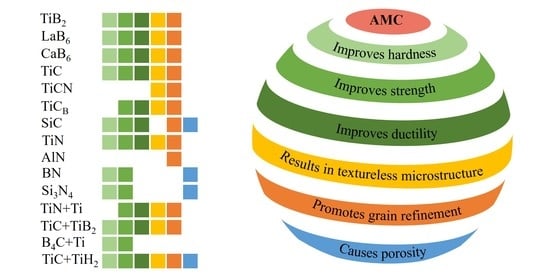
3. Comparison of Ceramic Reinforcements’ Influence on LPBF Process and the Properties of the AMCs
4. Summary and Outlook
- Generally, an incorporation of the ceramic particles into Al alloys results in a significant improvement in strength, ductility and hardness of the fabricated parts accompanied by a refined microstructure and with randomization of crystallographic orientation of reinforced AMCs.
- Most of the AMCs can be densified to over 99% relative density; moreover, non-oxide ceramic additives significantly improve laser absorptivity of a powder feedstock.
- The addition of ceramic particulates shifts the process window to a higher energy regime; however, an applied excess energy may result in the evaporation or decomposition of ceramics particles (mainly SiC).
- The application of a laser re-melting strategy can further increase the densification degree and the surface quality of AMCs; however, it also can cause the evaporation and loss of ceramic particles.
- Hybrid reinforcements are proven to be the effective additives, providing the formation of a wide variety of reinforcing phases with a coherent interface with matrices.
- The use of ceramics with a fine-particle size results in an increased degree of densification, microstructural and compositional uniformity, as well as an apparent grain refinement.
- The addition of TiB2, CaB6, TiC, TiN to Al alloys leads to a considerable grain refinement, down to the submicron level, due to the intensive heterogeneous nucleation and grain growth inhibition.
- An addition of matching ceramics prevents the hot tearing and gives the prospect to consolidate crack-susceptible Al alloys by a laser powder-bed fusion technique.
- The highest elongation of 17.7% is demonstrated by the AlSi10Mg/TiB2 composite; however, the highest strength of 613 MPa is recorded for the hybrid TiN-Ti reinforced AMCs.
- The highest hardness of 316 HV is estimated for SiC reinforced AMCs, which possess a relatively high strength and moderate ductility.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spierings, A.B.; Dawson, K.; Uggowitzer, P.J.; Wegener, K. Influence of SLM scan-speed on microstructure, precipitation of Al3Sc particles and mechanical properties in Sc- and Zr-modified Al-Mg alloys. Mater. Des. 2018, 140, 134–143. [Google Scholar] [CrossRef]
- Otani, Y.; Sasaki, S. Effects of the addition of silicon to 7075 aluminum alloy on microstructure, mechanical properties, and selective laser melting processability. Mater. Sci. Eng. A 2020, 777, 139079. [Google Scholar] [CrossRef]
- Muhammad, M.; Nezhadfar, P.; Thompson, S.; Saharan, A.; Phan, N.; Shamsaei, N. A comparative investigation on the microstructure and mechanical properties of additively manufactured aluminum alloys. Int. J. Fatigue 2021, 146, 106165. [Google Scholar] [CrossRef]
- Li, P.; Li, R.; Yang, H.; Yuan, T.; Niu, P.; Wang, M.; Li, L.; Chen, C. Selective laser melting of Al-3.48Cu-2.03Si-0.48Sc-0.28Zr alloy: Microstructure evolution, properties and metallurgical defects. Intermetallics 2020, 129, 107008. [Google Scholar] [CrossRef]
- Qian, W.; Zhao, Y.; Kai, X.; Yan, Y.; Gao, X.; Jin, L. Microstructure and properties of 6111Al matrix composites reinforced by the cooperation of in situ ZrB2 particles and Y. J. Alloys Compd. 2020, 829, 154624. [Google Scholar] [CrossRef]
- Qbau, N.; Nam, N.; Hien, N.; Ca, N. Development of light weight high strength aluminum alloy for selective laser melting. J. Mater. Res. Technol. 2020, 9, 14075–14081. [Google Scholar] [CrossRef]
- Totten, G.E.; Tiryakioğlu, M.; Kessler, O. (Eds.) Encyclopedia of Aluminum and Its Alloys; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Wang, M.; Song, B.; Wei, Q.; Shi, Y. Improved mechanical properties of AlSi7Mg/nano-SiCp composites fabricated by selective laser melting. J. Alloys Compd. 2019, 810, 151926. [Google Scholar] [CrossRef]
- Tan, Q.; Fan, Z.; Tang, X.; Yin, Y.; Li, G.; Huang, D.; Zhang, J.; Liu, Y.; Wang, F.; Wu, T.; et al. A novel strategy to additively manufacture 7075 aluminium alloy with selective laser melting. Mater. Sci. Eng. A 2021, 821, 141638. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2018, 35, 270–284. [Google Scholar] [CrossRef]
- Gu, D.; Chang, F.; Dai, D. Selective Laser Melting Additive Manufacturing of Novel Aluminum Based Composites With Multiple Reinforcing Phases. J. Manuf. Sci. Eng. 2015, 137, 021010. [Google Scholar] [CrossRef]
- Famodimu, O.H.; Stanford, M.; Oduoza, C.F.; Zhang, L. Effect of process parameters on the density and porosity of laser melted AlSi10Mg/SiC metal matrix composite. Front. Mech. Eng. 2018, 13, 520–527. [Google Scholar] [CrossRef]
- Chang, F.; Gu, D.; Dai, D.; Yuan, P. Selective laser melting of in-situ Al4SiC4 + SiC hybrid reinforced Al matrix composites: Influence of starting SiC particle size. Surf. Coatings Technol. 2015, 272, 15–24. [Google Scholar] [CrossRef]
- Zhou, L.; Huynh, T.; Park, S.; Hyer, H.; Mehta, A.; Song, S.; Bai, Y.; McWilliams, B.; Cho, K.; Sohn, Y. Laser powder bed fusion of Al–10 wt% Ce alloys: Microstructure and tensile property. J. Mater. Sci. 2020, 55, 14611–14625. [Google Scholar] [CrossRef]
- Wallis, C.; Buchmayr, B.; Bermejo, R.; Supancic, P. Fabrication of 3D metal-ceramic (Al-AlN) architectures using laser-powder bed fusion process. Addit. Manuf. 2020, 38, 101799. [Google Scholar] [CrossRef]
- Minasyan, T.; Aghayan, M.; Liu, L.; Aydinyan, S.; Kollo, L.; Hussainova, I.; Rodríguez, M.A. Combustion synthesis of MoSi2 based composite and selective laser sintering thereof. J. Eur. Ceram. Soc. 2018, 38, 3814–3821. [Google Scholar] [CrossRef]
- Minasyan, T.; Ivanov, R.; Toyserkani, E.; Hussainova, I. Laser powder-bed fusion of Mo(Si,Al)2—Based composite for elevated temperature applications. J. Alloys Compd. 2021, 884, 161034. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Luo, L.; Cai, X.; Wang, B.; Zhao, J.; Cheng, Z.; Wang, L.; Su, Y.; Xue, X.; et al. Selective laser melting of high-strength TiB2/AlMgScZr composites: Microstructure, tensile deformation behavior, and mechanical properties. J. Mater. Res. Technol. 2021, 16, 786–800. [Google Scholar] [CrossRef]
- Minasyan, T.; Ivanov, R.; Toyserkani, E.; Hussainova, I. Mo(Si,Al)2 by laser powder bed fusion of AlSi10Mg and combustion synthesized MoSi2. Mater. Lett. 2021, 307, 131041. [Google Scholar] [CrossRef]
- Minasyan, T.; Aydinyan, S.; Toyserkani, E.; Hussainova, I. Parametric Study on In Situ Laser Powder Bed Fusion of Mo(Si1−x,Alx)2. Materials 2020, 13, 4849. [Google Scholar] [CrossRef]
- Kuai, Z.; Li, Z.; Liu, B.; Liu, W.; Yang, S. Effects of remelting on the surface morphology, microstructure and mechanical properties of AlSi10Mg alloy fabricated by selective laser melting. Mater. Chem. Phys. 2022, 125901. [Google Scholar] [CrossRef]
- Gu, D.; Yuan, P. Thermal evolution behavior and fluid dynamics during laser additive manufacturing of Al-based nanocomposites: Underlying role of reinforcement weight fraction. J. Appl. Phys. 2015, 118, 233109. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Sing, S.; Chua, C.; Kuo, C.; Tian, X. Particle-reinforced metal matrix nanocomposites fabricated by selective laser melting: A state of the art review. Prog. Mater. Sci. 2019, 104, 330–379. [Google Scholar] [CrossRef]
- Kumar, M.B.; Sathiya, P. Methods and materials for additive manufacturing: A critical review on advancements and challenges. Thin-Walled Struct. 2020, 159, 107228. [Google Scholar] [CrossRef]
- Bayat, M.; Nadimpalli, V.K.; Pedersen, D.B.; Hattel, J.H. A fundamental investigation of thermo-capillarity in laser powder bed fusion of metals and alloys. Int. J. Heat Mass Transf. 2020, 166, 120766. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Griffiths, S.; Rossell, M.D.; Croteau, J.; Vo, N.Q.; Dunand, D.C.; Leinenbach, C. Effect of laser rescanning on the grain microstructure of a selective laser melted Al-Mg-Zr alloy. Mater. Charact. 2018, 143, 34–42. [Google Scholar] [CrossRef]
- Mair, P.; Goettgens, V.S.; Rainer, T.; Weinberger, N.; Letofsky-Papst, I.; Mitsche, S.; Leichtfried, G. Laser powder bed fusion of nano-CaB6 decorated 2024 aluminum alloy. J. Alloys Compd. 2021, 863, 158714. [Google Scholar] [CrossRef]
- Zhou, L.; Hyer, H.; Chang, J.; Mehta, A.; Huynh, T.; Yang, Y.; Sohn, Y. Microstructure, mechanical performance, and corrosion behavior of additively manufactured aluminum alloy 5083 with 0.7 and 1.0 wt% Zr addition. Mater. Sci. Eng. A 2021, 823, 141679. [Google Scholar] [CrossRef]
- Plotkowski, A.; Sisco, K.; Bahl, S.; Shyam, A.; Yang, Y.; Allard, L.; Nandwana, P.; Rossy, A.M.; Dehoff, R. Microstructure and properties of a high temperature Al–Ce–Mn alloy produced by additive manufacturing. Acta Mater. 2020, 196, 595–608. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, X.; Fu, Z.; Niu, B.; Chen, J.; Hu, Y.; Chang, C.; Yi, J. In situ formation of D022-Al3Ti during selective laser melting of nano-TiC/AlSi10Mg alloy prepared by electrostatic self-assembly. Vacuum 2021, 188, 110179. [Google Scholar] [CrossRef]
- Kaufman, J.G. Introduction to Aluminum Alloys and Tempers. Available online: https://books.google.ee/books?hl=en&lr=&id=idmZIDcwCykC&oi=fnd&pg=PR7&dq=Introduction+to+Aluminum+Alloys+and+Tempers&ots=YF2Do8uYO4&sig=lVfaG-D2QRHLKNTb8Nivh0VpmmA&redir_esc=y#v=onepage&q=Introduction%20to%20Aluminum%20Alloys%20and%20Tempers&f=false (accessed on 25 August 2021).
- Montero-Sistiaga, M.L.; Mertens, R.; Vrancken, B.; Wang, X.; Van Hooreweder, B.; Kruth, J.-P.; Van Humbeeck, J. Changing the alloy composition of Al7075 for better processability by selective laser melting. J. Mater. Process. Technol. 2016, 238, 437–445. [Google Scholar] [CrossRef]
- Astfalck, L.; Kelly, G.K.; Li, X.; Sercombe, T.B. On the Breakdown of SiC during the Selective Laser Melting of Aluminum Matrix Composites. Adv. Eng. Mater. 2017, 19, 1600835. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Saunders, M.; Suvorova, A.; Zhang, L.; Liu, Y.; Fang, M.; Huang, Z.; Sercombe, T. A selective laser melting and solution heat treatment refined Al–12Si alloy with a controllable ultrafine eutectic microstructure and 25% tensile ductility. Acta Mater. 2015, 95, 74–82. [Google Scholar] [CrossRef]
- Zhuo, L.; Wang, Z.; Zhang, H.; Yin, E.; Wang, Y.; Xu, T.; Li, C. Effect of post-process heat treatment on microstructure and properties of selective laser melted AlSi10Mg alloy. Mater. Lett. 2018, 234, 196–200. [Google Scholar] [CrossRef]
- Wang, M.; Song, B.; Wei, Q.; Zhang, Y.; Shi, Y. Effects of annealing on the microstructure and mechanical properties of selective laser melted AlSi7Mg alloy. Mater. Sci. Eng. A 2018, 739, 463–472. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, H.; Hu, Z.; Zhang, L.; Zeng, X. A comparative study on single-laser and multi-laser selective laser melting AlSi10Mg: Defects, microstructure and mechanical properties. Mater. Sci. Eng. A 2019, 746, 416–423. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Liu, J.; Zhang, A.; Zhou, Y.; Wei, Q.; Yan, C.; Shi, Y. Effect of heat treatment on AlSi10Mg alloy fabricated by selective laser melting: Microstructure evolution, mechanical properties and fracture mechanism. Mater. Sci. Eng. A 2016, 663, 116–125. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Li, X. Design materials based on simulation results of silicon induced segregation at AlSi10Mg interface fabricated by selective laser melting. J. Mater. Sci. Technol. 2020, 46, 145–155. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J. Effect of hot isostatic pressing on nanoparticles reinforced AlSi10Mg produced by selective laser melting. Mater. Sci. Eng. A 2020, 788, 139570. [Google Scholar] [CrossRef]
- Bi, J.; Lei, Z.; Chen, Y.; Chen, X.; Tian, Z.; Lu, N.; Qin, X.; Liang, J. Microstructure, tensile properties and thermal stability of AlMgSiScZr alloy printed by laser powder bed fusion. J. Mater. Sci. Technol. 2020, 69, 200–211. [Google Scholar] [CrossRef]
- Thapliyal, S.; Shukla, S.; Zhou, L.; Hyer, H.; Agrawal, P.; Agrawal, P.; Komarasamy, M.; Sohn, Y.; Mishra, R.S. Design of heterogeneous structured Al alloys with wide processing window for laser-powder bed fusion additive manufacturing. Addit. Manuf. 2021, 42, 102002. [Google Scholar] [CrossRef]
- Lu, J.; Lin, X.; Kang, N.; Cao, Y.; Wang, Q.; Huang, W. Keyhole mode induced simultaneous improvement in strength and ductility of Sc modified Al–Mn alloy manufactured by selective laser melting. Mater. Sci. Eng. A 2021, 811, 141089. [Google Scholar] [CrossRef]
- Thapliyal, S.; Komarasamy, M.; Shukla, S.; Zhou, L.; Hyer, H.; Park, S.; Sohn, Y.; Mishra, R.S. An integrated computational materials engineering-anchored closed-loop method for design of aluminum alloys for additive manufacturing. Materialia 2019, 9, 100574. [Google Scholar] [CrossRef]
- Yang, K.; Shi, Y.; Palm, F.; Wu, X.; Rometsch, P. Columnar to equiaxed transition in Al-Mg(-Sc)-Zr alloys produced by selective laser melting. Scr. Mater. 2018, 145, 113–117. [Google Scholar] [CrossRef]
- Kurnsteiner, P.; Bajaj, P.; Gupta, A.; Benjamin, W.; Weisheit, A.; Li, X.; Leinebach, C.; Gault, B.; Jagle, E.; Raabe, D. Control of thermally stable core-shell nano-precipitates in additively manufactured Al-Sc-Zr alloys. Addit. Manuf. 2020, 32, 100910. [Google Scholar] [CrossRef]
- Zhou, L.; Hyer, H.; Thapliyal, S.; Mishra, R.S.; McWilliams, B.; Cho, K.; Sohn, Y. Process-Dependent Composition, Microstructure, and Printability of Al-Zn-Mg and Al-Zn-Mg-Sc-Zr Alloys Manufactured by Laser Powder Bed Fusion. Met. Mater. Trans. A 2020, 51, 3215–3227. [Google Scholar] [CrossRef]
- Zhou, S.; Su, Y.; Wang, H.; Enz, J.; Ebel, T.; Yan, M. Selective laser melting additive manufacturing of 7xxx series Al-Zn-Mg-Cu alloy: Cracking elimination by co-incorporation of Si and TiB2. Addit. Manuf. 2020, 36, 101458. [Google Scholar] [CrossRef]
- Biffi, C.A.; Bassani, P.; Fiocchi, J.; Albu, M.; Tuissi, A. Selective laser melting of AlCu-TiB2 alloy using pulsed wave laser emission mode: Processability, microstructure and mechanical properties. Mater. Des. 2021, 204, 109628. [Google Scholar] [CrossRef]
- Jia, Q.; Rometsch, P.; Kürnsteiner, P.; Chao, Q.; Huang, A.; Weyland, M.; Bourgeois, L.; Wu, X. Selective laser melting of a high strength Al Mn Sc alloy: Alloy design and strengthening mechanisms. Acta Mater. 2019, 171, 108–118. [Google Scholar] [CrossRef]
- Kang, N.; El Mansori, M.; Lin, X.; Guittonneau, F.; Liao, H.; Huang, W.; Coddet, C. In-situ synthesis of aluminum/nano-quasicrystalline Al-Fe-Cr composite by using selective laser melting. Compos. Part B Eng. 2018, 155, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.; Fu, Y.; Coddet, P.; Guelorget, B.; Liao, H.; Coddet, C. On the microstructure, hardness and wear behavior of Al-Fe-Cr quasicrystal reinforced Al matrix composite prepared by selective laser melting. Mater. Des. 2017, 132, 105–111. [Google Scholar] [CrossRef]
- Demir, A.G.; Previtali, B. Multi-material selective laser melting of Fe/Al-12Si components. Manuf. Lett. 2017, 11, 8–11. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Simonelli, M.; Parry, L.; Ashcroft, I.; Tuck, C.; Hague, R. 3D printing of Aluminium alloys: Additive Manufacturing of Aluminium alloys using selective laser melting. Prog. Mater. Sci. 2019, 106, 100578. [Google Scholar] [CrossRef]
- Mair, P.; Braun, J.; Kaserer, L.; March, L.; Schimbäck, D.; Letofsky-Papst, I.; Leichtfried, G. Unique microstructure evolution of a novel Ti-modified Al-Cu alloy processed using laser powder bed fusion. Mater. Today Commun. 2022, 31, 103353. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Chen, X.; Qiu, C. Complete columnar-to-equiaxed transition and significant grain refinement in an aluminium alloy by adding Nb particles through laser powder bed fusion. Addit. Manuf. 2022, 51, 102615. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, J.; Mo, N.; Fan, Z.; Yin, Y.; Bermingham, M.; Liu, Y.; Huang, H.; Zhang, M.-X. A novel method to 3D-print fine-grained AlSi10Mg alloy with isotropic properties via inoculation with LaB6 nanoparticles. Addit. Manuf. 2020, 32, 101034. [Google Scholar] [CrossRef]
- Xiao, Y.; Bian, Z.; Wu, Y.; Ji, G.; Li, Y.; Li, M.; Lian, Q.; Chen, Z.; Addad, A.; Wang, H. Effect of nano-TiB2 particles on the anisotropy in an AlSi10Mg alloy processed by selective laser melting. J. Alloys Compd. 2019, 798, 644–655. [Google Scholar] [CrossRef]
- Kotadia, H.; Gibbons, G.; Das, A.; Howes, P. A review of Laser Powder Bed Fusion Additive Manufacturing of aluminium alloys: Microstructure and properties. Addit. Manuf. 2021, 46, 102155. [Google Scholar] [CrossRef]
- Wang, L.; Jue, J.; Xia, M.; Guo, L.; Yan, B.; Gu, D. Effect of the Thermodynamic Behavior of Selective Laser Melting on the Formation of In situ Oxide Dispersion-Strengthened Aluminum-Based Composites. Metals 2016, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Minasyan, T.; Aydinyan, S.; Liu, L.; Volubujeva, O.; Toyserkani, E.; Hussainova, I. Mo(Si1−x,Alx)2-based composite by reactive laser powder-bed fusion. Mater. Lett. 2020, 281, 128776. [Google Scholar] [CrossRef]
- Dadbakhsh, S.; Mertens, R.; Hao, L.; Van Humbeeck, J.; Kruth, J. Selective Laser Melting to Manufacture “In Situ” Metal Matrix Composites: A Review. Adv. Eng. Mater. 2018, 21, 1801244. [Google Scholar] [CrossRef] [Green Version]
- Xi, L.; Wang, P.; Prashanth, K.; Li, H.; Prykhodko, H.; Scudino, S.; Kaban, I. Effect of TiB2 particles on microstructure and crystallographic texture of Al-12Si fabricated by selective laser melting. J. Alloys Compd. 2019, 786, 551–556. [Google Scholar] [CrossRef]
- Macías, J.G.S.; Douillard, T.; Zhao, L.; Maire, E.; Pyka, G.; Simar, A. Influence on microstructure, strength and ductility of build platform temperature during laser powder bed fusion of AlSi10Mg. Acta Mater. 2020, 201, 231–243. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Zhang, M.-X.; Zhu, Q. Novel approach to additively manufacture high-strength Al alloys by laser powder bed fusion through addition of hybrid grain refiners. Addit. Manuf. 2021, 48, 102400. [Google Scholar] [CrossRef]
- Dai, D.; Gu, D.; Xia, M.; Ma, C.; Chen, H.; Zhao, T.; Hong, C.; Gasser, A.; Poprawe, R. Melt spreading behavior, microstructure evolution and wear resistance of selective laser melting additive manufactured AlN/AlSi10Mg nanocomposite. Surf. Coatings Technol. 2018, 349, 279–288. [Google Scholar] [CrossRef]
- Wang, P.; Eckert, J.; Prashanth, K.-G.; Wu, M.-W.; Kaban, I.; Xi, L.-X.; Scudino, S. A review of particulate-reinforced aluminum matrix composites fabricated by selective laser melting. Trans. Nonferrous Met. Soc. China 2020, 30, 2001–2034. [Google Scholar] [CrossRef]
- Tjong, S.C. Novel Nanoparticle-Reinforced Metal Matrix Composites with Enhanced Mechanical Properties. Adv. Eng. Mater. 2007, 9, 639–652. [Google Scholar] [CrossRef]
- Gu, D.; Wang, H.; Chang, F.; Dai, D.; Yuan, P.; Hagedorn, Y.-C.; Meiners, W. Selective Laser Melting Additive Manufacturing of TiC/AlSi10Mg Bulk-form Nanocomposites with Tailored Microstructures and Properties. Phys. Procedia 2014, 56, 108–116. [Google Scholar] [CrossRef]
- Gu, D.; Wang, H.; Dai, D.; Yuan, P.; Meiners, W.; Poprawe, R. Rapid fabrication of Al-based bulk-form nanocomposites with novel reinforcement and enhanced performance by selective laser melting. Scr. Mater. 2015, 96, 25–28. [Google Scholar] [CrossRef]
- Gu, D.; Wang, H.; Dai, D.; Chang, F.; Meiners, W.; Hagedorn, Y.-C.; Wissenbach, K.; Kelbassa, I.; Poprawe, R. Densification behavior, microstructure evolution, and wear property of TiC nanoparticle reinforced AlSi10Mg bulk-form nanocomposites prepared by selective laser melting. J. Laser Appl. 2015, 27, S17003. [Google Scholar] [CrossRef]
- Xi, L.; Gu, D.; Guo, S.; Wang, R.; Ding, K.; Prashanth, K.G. Grain refinement in laser manufactured Al-based composites with TiB2 ceramic. J. Mater. Res. Technol. 2020, 9, 2611–2622. [Google Scholar] [CrossRef]
- Kusoglu, I.M.; Gökce, B.; Barcikowski, S. Use of (nano-)additives in Laser Powder Bed Fusion of Al powder feedstocks: Research directions within the last decade. Procedia CIRP 2020, 94, 11–16. [Google Scholar] [CrossRef]
- Liu, L.; Minasyan, T.; Ivanov, R.; Aydinyan, S.; Hussainova, I. Selective laser melting of TiB2-Ti composite with high content of ceramic phase. Ceram. Int. 2020, 46, 21128–21135. [Google Scholar] [CrossRef]
- Gu, D.; Yang, Y.; Xi, L.; Yang, J.; Xia, M. Laser absorption behavior of randomly packed powder-bed during selective laser melting of SiC and TiB2 reinforced Al matrix composites. Opt. Laser Technol. 2019, 119, 105600. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, H.; Bian, Z.; Sun, T.; Ding, H.; Yang, Q.; Wu, Y.; Lian, Q.; Chen, Z.; Wang, H. Enhancing strength and ductility of AlSi10Mg fabricated by selective laser melting by TiB2 nanoparticles. J. Mater. Sci. Technol. 2021, 109, 254–266. [Google Scholar] [CrossRef]
- Xi, L.; Guo, S.; Gu, D.; Guo, M.; Lin, K. Microstructure development, tribological property and underlying mechanism of laser additive manufactured submicro-TiB2 reinforced Al-based composites. J. Alloys Compd. 2019, 819, 152980. [Google Scholar] [CrossRef]
- Feng, Z.; Tan, H.; Fang, Y.; Lin, X.; Huang, W. Selective laser melting of TiB2/AlSi10Mg composite: Processability, microstructure and fracture behavior. J. Mater. Process. Technol. 2021, 299, 117386. [Google Scholar] [CrossRef]
- Li, X.; Ji, G.; Chen, Z.; Addad, A.; Wu, Y.; Wang, H.; Vleugels, J.; Van Humbeeck, J.; Kruth, J. Selective laser melting of nano-TiB2 decorated AlSi10Mg alloy with high fracture strength and ductility. Acta Mater. 2017, 129, 183–193. [Google Scholar] [CrossRef]
- Wang, P.; Gammer, C.; Brenne, F.; Niendorf, T.; Eckert, J.; Scudino, S. A heat treatable TiB2/Al-3.5Cu-1.5Mg-1Si composite fabricated by selective laser melting: Microstructure, heat treatment and mechanical properties. Compos. Part B Eng. 2018, 147, 162–168. [Google Scholar] [CrossRef]
- Mair, P.; Kaserer, L.; Braun, J.; Weinberger, N.; Letofsky-Papst, I.; Leichtfried, G. Microstructure and mechanical properties of a TiB2-modified Al–Cu alloy processed by laser powder-bed fusion. Mater. Sci. Eng. A 2020, 799, 140209. [Google Scholar] [CrossRef]
- Xi, L.X.; Zhang, H.; Wang, P.; Li, H.C.; Prashanth, K.G.; Lin, K.J.; Kaban, I.; Gu, D.D. Comparative investigation of microstructure, mechanical properties and strengthening mechanisms of Al-12Si/TiB2 fabricated by selective laser melting and hot pressing. Ceram. Int. 2018, 44, 17635–17642. [Google Scholar] [CrossRef]
- Tan, Q.; Yin, Y.; Fan, Z.; Zhang, J.; Liu, Y.; Zhang, M.-X. Uncovering the roles of LaB6-nanoparticle inoculant in the AlSi10Mg alloy fabricated via selective laser melting. Mater. Sci. Eng. A 2020, 800, 140365. [Google Scholar] [CrossRef]
- Wearing, D.; Horsfield, A.P.; Xu, W.; Lee, P.D. Which wets TiB2 inoculant particles: Al or Al3Ti? J. Alloys Compd. 2016, 664, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Savalani, M.; Ng, C.; Li, Q.; Man, H. In situ formation of titanium carbide using titanium and carbon-nanotube powders by laser cladding. Appl. Surf. Sci. 2012, 258, 3173–3177. [Google Scholar] [CrossRef]
- Masanta, M.; Shariff, S.; Choudhury, A.R. Evaluation of modulus of elasticity, nano-hardness and fracture toughness of TiB2–TiC–Al2O3 composite coating developed by SHS and laser cladding. Mater. Sci. Eng. A 2011, 528, 5327–5335. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, S.; Liu, G.; Sun, Q.; Liu, J.; Sun, Q.; Sun, J.; Wang, Z.; Liu, X.; Wang, X. A high strength AlSi10Mg alloy fabricated by laser powder bed fusion with addition of Al Ti C B master alloy powders. Materialia 2021, 16, 101103. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, S.; Wang, C.; Duan, L.; Wei, Q.; Shi, Y. Effect of TiC content on the Al-15Si alloy processed by selective laser melting: Microstructure and mechanical properties. Opt. Laser Technol. 2019, 120, 105719. [Google Scholar] [CrossRef]
- Wang, H.; Gu, D. Nanometric TiC reinforced AlSi10Mg nanocomposites: Powder preparation by high-energy ball milling and consolidation by selective laser melting. J. Compos. Mater. 2014, 49, 1639–1651. [Google Scholar] [CrossRef]
- He, P.; Kong, H.; Liu, Q.; Ferry, M.; Kruzic, J.J.; Li, X. Elevated temperature mechanical properties of TiCN reinforced AlSi10Mg fabricated by laser powder bed fusion additive manufacturing. Mater. Sci. Eng. A 2021, 811, 141025. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Zhou, Z.; Wang, K.; Zhan, Q.; Xiao, X. Grain refinement and crack inhibition of selective laser melted AA2024 aluminum alloy via inoculation with TiC–TiH2. Mater. Sci. Eng. A 2021, 813, 141171. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, Y.; Xiao, X.; Huang, B.; Zhou, Z.; Liu, X. Microstructure and mechanical properties of a novel (TiB2+TiC)/AlSi10Mg composite prepared by selective laser melting. Mater. Sci. Eng. A 2021, 834, 142435. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, X.; Rao, J.H.; Xiao, J.; Jiang, Y. In-situ chemical reaction mechanism and non-equilibrium microstructural evolution of (TiB2 + TiC)/AlSi10Mg composites prepared by SLM-CS processing. J. Alloys Compd. 2020, 857, 157553. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuo, L.; Yin, E.; Zhao, Z. Microstructure evolution and properties of nanoparticulate SiC modified AlSi10Mg alloys. Mater. Sci. Eng. A 2021, 808, 140864. [Google Scholar] [CrossRef]
- Zhang, D.; Yi, D.; Wu, X.; Liu, Z.; Wang, W.; Poprawe, R.; Schleifenbaumc, J.H.; Zieglerd, S. SiC reinforced AlSi10Mg composites fabricated by selective laser melting. J. Alloys Compd. 2021, 894, 162365. [Google Scholar] [CrossRef]
- Xue, G.; Ke, L.; Zhu, H.; Liao, H.; Zhu, J.; Zeng, X. Influence of processing parameters on selective laser melted SiCp/AlSi10Mg composites: Densification, microstructure and mechanical properties. Mater. Sci. Eng. A 2019, 764, 138155. [Google Scholar] [CrossRef]
- Xue, G.; Ke, L.; Liao, H.; Chen, C.; Zhu, H. Effect of SiC particle size on densification behavior and mechanical properties of SiCp/AlSi10Mg composites fabricated by laser powder bed fusion. J. Alloys Compd. 2020, 845, 156260. [Google Scholar] [CrossRef]
- Gao, C.; Xiao, Z.; Liu, Z.; Zhu, Q.; Zhang, W. Selective laser melting of nano-TiN modified AlSi10Mg composite powder with low laser reflectivity. Mater. Lett. 2018, 236, 362–365. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Z.; Xiao, Z.; You, D.; Wong, K.; Akbarzadeh, A. Selective laser melting of TiN nanoparticle-reinforced AlSi10Mg composite: Microstructural, interfacial, and mechanical properties. J. Mater. Process. Technol. 2020, 281, 116618. [Google Scholar] [CrossRef]
- Gao, C.; Wu, W.; Shi, J.; Xiao, Z.; Akbarzadeh, A. Simultaneous enhancement of strength, ductility, and hardness of TiN/AlSi10Mg nanocomposites via selective laser melting. Addit. Manuf. 2020, 34, 101378. [Google Scholar] [CrossRef]
- Dai, D.; Gu, D.; Poprawe, R.; Xia, M. Influence of additive multilayer feature on thermodynamics, stress and microstructure development during laser 3D printing of aluminum-based material. Sci. Bull. 2017, 62, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Konopatsky, A.S.; Kvashnin, D.G.; Corthay, S.; Boyarintsev, I.; Firestein, K.L.; Orekhov, A.; Arkharova, N.; Golberg, D.V.; Shtansky, D.V. Microstructure evolution during AlSi10Mg molten alloy/BN microflake interactions in metal matrix composites obtained through 3D printing. J. Alloys Compd. 2021, 859, 157765. [Google Scholar] [CrossRef]
- Miao, K.; Zhou, H.; Gao, Y.; Deng, X.; Lu, Z.; Li, D. Laser powder-bed-fusion of Si3N4 reinforced AlSi10Mg composites: Processing, mechanical properties and strengthening mechanisms. Mater. Sci. Eng. A 2021, 825, 141874. [Google Scholar] [CrossRef]
- Rauchenecker, J.; Rabitsch, J.; Schwentenwein, M.; Konegger, T. Additive manufacturing of aluminum nitride ceramics with high thermal conductivity via digital light processing. Open Ceram. 2021, 9, 100215. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Wu, C.; Cheng, X. Microstructure and mechanical properties of aluminum nitride co-doped with cerium oxide via hot-pressing sintering. J. Alloys Compd. 2015, 640, 275–279. [Google Scholar] [CrossRef]
- Dai, D.; Gu, D. Influence of thermodynamics within molten pool on migration and distribution state of reinforcement during selective laser melting of AlN/AlSi10Mg composites. Int. J. Mach. Tools Manuf. 2016, 100, 14–24. [Google Scholar] [CrossRef]
- Gu, D.; Ma, C.; Xia, M.; Dai, D.; Shi, Q. A Multiscale Understanding of the Thermodynamic and Kinetic Mechanisms of Laser Additive Manufacturing. Engineering 2017, 3, 675–684. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, X.; Bourgeois, L.; Dai, P.; Mitome, M.; Zhang, C.; Yamaguchi, M.; Matveev, A.; Tang, C.; Bando, Y.; et al. Aluminum matrix composites reinforced with multi-walled boron nitride nanotubes fabricated by a high-pressure torsion technique. Mater. Des. 2015, 88, 451–460. [Google Scholar] [CrossRef]
- Minasyan, T.; Liu, L.; Aghayan, M.; Kollo, L.; Kamboj, N.; Aydinyan, S.; Hussainova, I. A novel approach to fabricate Si3N4 by selective laser melting. Ceram. Int. 2018, 44, 13689–13694. [Google Scholar] [CrossRef]
- Minasyan, T.; Liu, L.; Aghayan, M.; Rodriguez, M.A.; Aydinyan, S.; Hussainova, I. Mesoporous fibrous silicon nitride by catalytic nitridation of silicon. Prog. Nat. Sci. 2019, 29, 190–197. [Google Scholar] [CrossRef]
- Minasyan, T.; Liu, L.; Holovenko, Y.; Aydinyan, S.; Hussainova, I. Additively manufactured mesostructured MoSi2-Si3N4 ceramic lattice. Ceram. Int. 2019, 45, 9926–9933. [Google Scholar] [CrossRef]
- Worldwide Aluminum Consumption Forecast 2029|Statista. Available online: https://www.statista.com/statistics/863681/global-aluminum-consumption/ (accessed on 31 January 2022).
- Boeing: First of Many: 787 Dreamliner Celebrates 10 Years since First Flight. Available online: https://www.boeing.com/features/2019/12/787-1st-flight-anniversary-12-19.page (accessed on 31 January 2022).
- China Sets Carbon Reduction Plans for Steel, Aluminium|Argus Media. Available online: https://www.argusmedia.com/en/news/2266222-china-sets-carbon-reduction-plans-for-steel-aluminium (accessed on 31 January 2022).
























| System | Used Device, Process Parameters | Relative Density (%) | Average Grain Size (μm) | σy/σu (MPa) | ε/εc (%) | Hardness (HV) | N |
|---|---|---|---|---|---|---|---|
| Al-15Si | SLM125 P = 360 W ν = 600 mm/s d = 20 µm h = 60 µm | >98.5 | - | σu = 398 | ε = 2.6 | 154 HV1 | [89] |
| Al-15Si/ 1 wt.% TiC | σu = 578 | ε = 7.86 | 146 HV1 | ||||
| Al-15Si/ 2.5 wt.% TiC | σu ≈ 450 | ε ≈ 4 | 150 HV1 | ||||
| Al-15Si/ 10 wt.% TiC | σu ≈ 313 | ε = 2.24 | 177 HV1 | ||||
| AlSi10Mg/ 3 wt.% TiC | SLM system P = 80, 100, 120 and 140 W ν = 200 mm/s d = 50 µm h = 50 µm E = 160 J/mm3 | >98.5 | - | σu = 452 | ε = 9.8 | 157.4 HV0.1 | [71] |
| E = 200 J/mm3 | - | - | ≈173 HV0.1 | ||||
| E = 240 J/mm3 | σu = 486 | ε = 10.9 | 188.3 HV0.1 | ||||
| E = 280 J/mm3 | - | - | 180.6 HV0.1 | ||||
| AlSi10Mg/ 5 wt.% TiC | SLM system P = 110 W ν = 100–350 mm/s d = 50 µm h = 50 µm El = 1100, 733, 440, 314 J/m | >98 | - | - | - | 181.2 HV0.2 | [70] |
| AlSi10Mg/ 5 wt.% TiC | EOS M290 P = 320 W ν = 1100 mm/s d = 30 µm h = 130 µm | 99.75 | 0.5–1 | σu ≈ 456 σy ≈ 338 | ε = 2.97 | 131 HV0.05 | [31] |
| AlSi10Mg/ 5 wt.% TiC | SLM system P = 100 W ν = 150 mm/s d = 50 µm h = 50 µm | Full dense | - | σu = 482 | ε = 10.8 | 185 HV0.1 | [90] |
| AlSi10Mg/ 10 wt.% Al-Ti-C-B master alloy | 3D Systems ProX DMP 320 P = 300 W ν = 1400 mm/s d = 30 µm h = 100 µm | - | ~3 | σu = 488 ± 6 σy = 287 ± 3 | ε = 10.1 ± 2.2 | - | [88] |
| 2024 alloy | EOS M290 P = 200 W ν = 100 mm/s d = 40 µm h = 90 µm T = 180 °C | 98.2 | ~30 | σu = 240 ± 10 | ε = 0.3 ± 0.2 | 108 HV0.2 | [92] |
| 2024/ 1 wt.% TiC | 98.5 | - | - | - | |||
| 2024/ 1 wt.% TiH2 | 95.7 | - | - | - | - | ||
| 2024/ (1 wt.% TiC +1 wt.% TiH2) | 97.1 | ~2 | σu = 390 ± 15 | ε = 12.0 ± 0.5 | 120 HV0.2 | ||
| AlSi10Mg | EOS M280 P = 270 W ν = 1600 mm/s d = 30 µm h = 110 µm | 98.22 | 12.1 | σu = 393.8 ± 14.5 σy = 224.2 ± 7.2 | ε = 4.5 ± 0.9 | 127.8 ± 2.4 HV.1 | [93] |
| ASi10Mg/ 1.5 wt.% TiC +1.5 wt.% TiB2 | 99.02 | 1.5 | σu = 552.4 ± 12.1 σy = 325 ± 10.2 | ε = 12 ± 0.6 | 142 ± 2.9 HV0.1 | ||
| ASi10Mg/ 3 wt.% TiB2 | 97.12 | 7.7 | σu = 360.6 ± 8.5 σy = 200 ± 8.8 | ε = 3.8 ± 0.2 | 134.4 ± 1.4 HV0.1 | ||
| ASi10Mg/ 3 wt.% TiC | 98.23 | 1.7 | σu = 453 ± 10 σy = 267.5 ± 7.8 | ε = 4.8 ± 1.1 | 138.3 ± 1.7 HV0.1 | ||
| AlSi10Mg | SLM-125HL P = 150 W ν = 1200 mm/s d = 30 µm h = 105 µm T = 200 °C | At RT full dense | - | RT σu = 356 ± 10 σy = 220 ± 4 | ε = 4.5 ± 0.5 | - | [91] |
| 100 °C σu = 327 ± 2 σy = 230 ± 3 | ε = 5 ± 1 | ||||||
| 150 °C σu = 282 ± 3 σy = 213 ± 3 | ε = 11.5 ± 2.5 | ||||||
| 200 °C σu = 245 ± 8 σy = 194 ± 7 | ε = 11 ± 1.2 | ||||||
| AlSi10Mg/ 2 vol.% TiCN | At RT full dense | RT <1.5 | RT σu = 333 ± 2 σy = 227 ± 7 | ε = 2.8 ± 0. | - | ||
| - | 100 °C σu = 344 ± 2 σy = 245 ± 2 | ε = 3.5 ± 0.2 | |||||
| - | 150 °C σu = 308 ± 9 σy = 235 ± 4 | ε = 4.2 ± 0.2 | |||||
| - | 200 °C σu = 270 ± 1 σy = 209 ± 10 | ε = 4.9 ± 0.4 | |||||
| AlSi10Mg | SLM-120 P = 200 W ν = 1200 mm/s d = 30 µm h = 70 µm T = 200 °C | Almost full dense | - | σu = 366 σy = 193 | ε = 6.8 | ~141 HV0.2 | [94] |
| AlSi10Mg/ 0.7 wt.% (B4C+Ti) | σu = 417 σy = 234 | ε = 5.2 | ~139 HV0.2 | ||||
| AlSi10Mg/ 5.7 wt.% (B4C+Ti) | σu = 307 σy = 126 | ε = 3.6 | ~170 HV0.2 | ||||
| AlSi10Mg/ 11.5 wt.% (B4C+Ti) | σu = 218 σy = 117 | ε = 3.4 | ~175 HV0.2 | ||||
| AlSi10Mg/ 17.2 wt.% (B4C+Ti) | σu = 165 σy = 72 | ε = 1.7 | ~222 HV0.2 | ||||
| AlSi7Mg | EOSINT M280 P = 350 W ν = 1200 mm/s d = 40 µm h = 190 µm T = 80 °C | Porosity ≈0.59% | ~4.55 | σu = 388.3 ± 49.6 | ε = 7.03 ± 1.25 | ≈1.85 GPa nano-hardness | [8] |
| AlSi7Mg/ 2 wt.% SiC | Porosity ≈0.25% | ~3.14 | σu = 502.94 | ε = 10.64 ± 1.06 | ≈2.11 GPa nano-hardness | ||
| AlSi10Mg/ 2 vol.% SiC (~2.4 wt.%) | SLM280HL P = 120 W ν = 250 mm/s d = 30 µm h = 60 µm T = 150 °C Ev = 267 J/mm3 | ~92.04 | - | - | - | - | [95] |
| P = 150 W Ev = 333 J/mm3 | 98.7 | 4.44 | σu = 343 ± 59 | ε = 3.3 ± 1.7 | 134.4 ± 3.2 HV0.1 | ||
| P = 180 W Ev = 400 J/mm3 | 97.69 | 4.96 | σu = 377 ± 28 | ε = 2.9 ± 0.95 | 135.6 ± 3.5 HV0.1 | ||
| P = 210 W Ev = 467 J/mm3 | 97.36 | 6.73 | σu = 440 ± 17 | ε ≈ 7.4 | 131.7 ± 2.6 HV0.1 | ||
| P = 240 W Ev = 533 J/mm3 | 97.40 | - | σu = 450 ± 30 | ε = 4.9 | 129.7 ± 6.9 HV0.1 | ||
| Al–12Si/ 10 vol.% SiC (~11.8 wt.%) | ReaLizer SLM-100 P = 200 W ν = 375–1500 mm/s d = 50 µm h = 100 µm Ev ≈ 20–80 J/mm3 | 97.4 (by X-ray micro tomography (XMT)) | - | - | - | - | [34] |
| AlSi10Mg/ 10 wt.% SiC | EOSINT M280 P = 240–320 W ν = 500–1800 mm/s d = 30 µm h = 80–160 µm | - | 2.35 | σu ≈ 450 σy ≈ 410 | - | 208.5 HV0.1 | [96] |
| AlSi10Mg/ 15 wt.% SiC | Self-developed NRD-SLM-III P = 340–490 W ν = 600–2100 mm/s d = 40 µm h = 60–180 µm T = 200 °C | 97.7 | - | σu = 341.9 | ε ≈ 3 | 217.4 HV0.2 | [97] |
| AlSi10Mg/ 15 wt.% SiCp (300 mesh) | Self-developed NRD-SLM-III P = 500 W ν = 1200 mm/s d = 40 µm h = 120 µm T = 200 °C | ≈97.8 | - | σuc = 545.4 | εc ≈ 4.7% | ≈210 HV0.2 | [98] |
| AlSi10Mg/ 15 wt.% SiCp (600 mesh) | ≈98.5 | σuc = 642.4 | εc ≈ 6.1% | ≈240 HV0.2 | |||
| AlSi10Mg/ 15 wt% SiCp (1200 mesh) | 98.9 | σuc = 764.1 | εc ≈ 7.0% | 316.1 HV0.2 | |||
| AlSi10Mg/ 20 wt.% SiC | Self-developed P = 80–110 W Ν = 100 mm/s d = 50 µm h = 50 µm El = 800–1100 J/m | ~89.2–96.1 | - | - | - | 214 HV0.1 | [11] |
| AlSi10Mg/ 20 wt.% SiC D50SiC = 50 μm | SLM apparatus with Yb laser P = 100 W ν = 100 mm/s d = 30 µm h = 50 µm | 86.4 | - | - | - | ~127 HV0.1 | [13] |
| AlSi10Mg/ 20 wt.% SiC D50SiC = 15 μm | 93.7 | 188 HV0.1 | |||||
| AlSi10Mg/ 20 wt.% SiC D50SiC = 5 μm | ~97.2 | 218.5 HV0.1 |
| System | Used Device, Process Parameters | Relative Density (%) | Average Grain Size (μm) | σy/σu (MPa) | ε/εc (%) | Hardness (HV) | N |
|---|---|---|---|---|---|---|---|
| AlSi10Mg/ 2 wt.% TiN (D50TiN = 80 nm) | Dimetal-80 SLM system P = 100 W ν = 200–600 mm/s d = 30 µm h = 80 µm | 97.6 | 0.284 | - | - | 145 ± 4.9 HV0.1 | [99,100] |
| AlSi10Mg | SLM-280 HL P = 100 W ν = 1200 mm/s d = 30 µm h = 90 µm | Porosity =0.9% | 3.86 | σu = 359.4 ± 8.5 σy = 264 ± 10.5 | ε = 3.9 ± 0.3 | 134.6 ± 4.4 HV0.1 | [101] |
| AlSi10Mg/ 2 wt.% TiN | Porosity =0.2% | 1.37 | σu = 386.1 ± 12.6 σy = 295.9 ± 4.6 | ε = 4.4 ± 0.27 | 148.5 ± 4.1 HV0.1 | ||
| AlSi10Mg/ 4 wt.% TiN | Porosity =0.01% | 1.24 | σu = 491.8 ± 5.5 σy = 315.4 ± 5.2 | ε = 7.5 ± 0.29 | 156.9 ± 4.9 HV0.1 | ||
| AlSi10Mg/ 6 wt.% TiN | Porosity =3.7% | 1.19 | σu = 325.1 ± 14.2 σy = 261.6 ± 3.5 | ε = 2.9 ± 0.32 | 150.4 ± 3.1 HV0.1 | ||
| 7050 Al alloy | SLM-280 HL P = 210 W ν = 115 mm/s d = 30 µm h = 50 µm | 98.5 | 91.8 | σu = 75 ± 25 | ε ≈ 0.6 | - | [66] |
| 7050/0.18 wt.% TiN | 98.9 | 88 | σu = 111 ± 3 | ε = 1.1 ± 0.2 | |||
| 7050/0.36 wt.% TiN | - | - | σu ≈140 | ε ≈ 1 | |||
| 7050/0.54 wt.% TiN | - | - | σu ≈ 60 | ε ≈ 0.9 | |||
| 7050/1.82 wt.% Ti | 99.6 | 2.3 | σu = 427 ± 12 | ε = 3.9 ± 1.1 | |||
| 7050/3.64 wt.% Ti | - | - | σu ≈ 480 | ε ≈ 6.1 | |||
| 7050/5.46 wt.% Ti | - | - | σu ≈ 350 | ε ≈ 2.5 | |||
| 7050/2 wt.% (TiN+Ti) | 99.7 | 0.775 | σu ≈ 550 | ε ≈ 8.6 | |||
| 7050/4 wt.% (TiN+Ti) | - | - | σu = 613±15 | ε = 8.8 ± 0.8 | |||
| 7050/6 wt.% (TiN+Ti) | - | - | σu ≈ 408 | ε ≈ 13.2 | |||
| AlSi10Mg/ 1 wt.% AlN (50 nm) | SLM apparatus P = 200 W ν = 100–300 mm/s d = 30 µm h = 60–100 µm Ev = 1100 J/mm3 | 97 | 4.5 | - | - | - | [67] |
| Ev = 660 J/mm3 | 60 | 2 | |||||
| Ev = 420 J/mm3 | Full dense | 1.4 | |||||
| Ev = 220 J/mm3 | Full dense | 2 | |||||
| AlSi10Mg/ 2 wt.% AlN | Self-made P = 200 W ν = 100 mm/s d = 30 µm h = 80 µm | - | - | - | - | 77–85.3 HV0.05 | [102] |
| AlSi10Mg | EOSINT M290 P = 380 W ν = 1300 mm/s d = 30 µm h = 200 µm | Porosity =0.15% | - | σu ≈ 180 | ε ≈ 5.6 | 103 HV0.2 | [103] |
| AlSi10Mg/ 1 wt.% BN | Porosity =0.81% | σu = 230 | ε ≈ 2.3 | 136 HV0.2 | |||
| AlSi10Mg | EOSINT M290 P = 180–300 W ν = 300–800 mm/s d = 30 µm h = 30–70 µm T = 150 °C | - | - | σu = 432 ± 15 σy = 275 ± 13 | ε = 5.12 ± 0.29 | 128 ± 3 HV0.2 | [104] |
| AlSi10Mg/ 5 vol.% Si3N4 (~5.8 wt.%) | 99.49 ± 0.17 | σu = 447 ± 18 σy = 308 ± 12 | ε = 3.58 ± 0.15 | 140 ± 7 HV0.2 | |||
| AlSi10Mg/ 10 vol.% Si3N4 (~11.5 wt.%) | 99.18 ± 0.16 | σu = 485 ± 12 σy = 362 ± 18 | ε = 2.47 ± 0.23 | 153 ± 3 HV0.2 | |||
| AlSi10Mg/ 15 vol.% Si3N4 (~17.1 wt.%) | 98.41 ± 0.22 | σu = 399 ± 21 | ε = 0.66 ± 0.31 | 187 ± 13 HV0.2 |
| Reinforcing Compound | Influence on the LPBF Process and the Properties of the Al Alloys | Minimum Optimal Limit |
|---|---|---|
| TiB2 | Exhibits good wettability, interfacial compatibility with Al. Increases densification level, serves as grain refiner along with in situ formed Al3Ti, stabilizes grain boundaries, leads to randomized crystallographic orientation, dramatically improves strength, hardness and ductility. | 2–6.5 wt.% |
| LaB6 | Forms highly coherent interface with Al, leads to significant grain refinement, microstructural homogeneity, isotropic mechanical properties, does not have huge effect on strength enhancement, but improves ductility. | Up to 0.5 wt.% |
| CaB6 | Serves as excellent grain refiner, microstructure stabilizer at the grain boundaries, forms highly coherent interface with Al, improves hardness, tensile strength, without sacrificing ductility. | Up to 2 wt.% |
| TiC | Using fine TiC particles leads to fully dense part fabrication with improved strength, ductility and hardness. The in situ formed D022-Al3Ti inoculants provide heterogeneous nucleation of α-Al, leading to grain refinement, and remove the preferred orientation of the α-Al (200) phase. Depending on the TiC content and process parameters, novel circular (ring) structures are formed within the matrix, enhancing the mechanical performance of AMCs. | Up to 5 wt.% |
| TiCB | The gas-atomized powders release enormous TiCB particles during LPBF process, largely promoting the nucleation of Al grains, grain refinement and resulting in weak crystallographic texture of AMCs. TiCB particles along with precipitated Si enhance the yield strength, tensile strength and elongation. | ~0.5 wt.% |
| TiCN | The addition of TiCN significantly reduces the average grain size, improves yield strength and ductility over native LPBF AlSi10Mg and rarely induces the formation of brittle Al4C3. | 2 wt.% |
| TiC+TiH2 | Due to decomposition of TiH2 and reaction of Al with Ti, a well-bonded interface between L12-Al3Ti and α-Al was observed acting as substrate for α-Al heterogeneous nucleation. Meanwhile, the presence of Ti creates “Ti transition zone” between TiC and matrix, creating potent nucleation sites for α-Al as well. Owing to restriction of columnar grain growth, the joint effect of refinement strengthening, the reinforced AMCs exhibit enhanced mechanical performance, tensile strength and ductility. | 1 wt.%TiC 1 wt.%TiH2 |
| TiC+TiB2 | Dual TiB2+TiC particles induce heterogeneous nucleation of Al and significantly refine the grains of the Al matrix. Double reinforcement results in simultaneous enhancement in strength, ductility and hardness, acting more efficiently than single species. | 1.5 wt.%TiC 1.5 wt.%TiH2 |
| SiC | Use of fine (nanosized or few-micron-sized) SiC results in grain refinement, decrease in porosity, enhancement of hardness, tensile strength and ductility but, depending on the process parameters, can cause in situ formation of Al4C3 or Al4SiC4 phases. | Up to 2 wt.% |
| Ti+B4C | In situ formed TiC, TiB2 and Ti3SiC2 serve as nucleants and reinforcements. The Ti+B4C content increase results in improvement in hardness, however much lower elongation and tensile strength. The released heat during the combustion reaction allows for fabricating the materials at low applied laser energy. | 0.7 wt.% |
| Al4C3 | Al4C3 itself is a brittle and unstable phase and is best avoided. However, small amounts of formed nanosized Al4C3 can enhance the mechanical properties of AMCs. | - |
| Al4SiC4 | Al4SiC4 along with intermetallic Mg2Si increase reinforcement/matrix wettability and the resultant interfacial bonding coherence. Al4SiC4 serves as the transition zone, which hinders the direct contact of SiC and aluminum crystals. Ultrafine Al4SiC4 has a reinforcing effect, improving the mechanical properties of SiC reinforced AMCs. | - |
| TiN | TiN particles refine the α-Al grains due to intensive heterogeneous nucleation and increase the fraction of low-energy high-angle grain boundaries, enhancing the hardness and strength. Due to the Al+TiN reaction, Al3.21Si0.47 and a (Ti,Al)N graded layer is formed, which significantly enhances the hardness due to improving interface bonding strength. The coherent interfaces between the matrix, Mg2Si and TiN particles lead to precipitation strengthening, which contributes to the overall strength increase. | 4 wt.% |
| TiN+Ti | Provides crack-free microstructure and significant grain refinement due to formation of Al3Ti phase and different precipitates, improves the hardness and tensile strength. | 4 wt.% |
| AlN | The AlN particles show high chemical stability and good compatibility with Al alloy. They promote densification, refine the α-Al grains, create strain-hardened tribo-layer, enhancing the wear resistance and stabilizing the coefficient of friction. | 1 wt.% |
| BN | The formation of AlN and AlB2 phases during the solid-state reaction of Al+BN results in increased tensile strength and hardness, though at the expense of porosity increase. However, increase in BN content and particle size decreases wettability and prevents uniform metal spreading. | 1 wt.% |
| Si3N4 | Si3N4 particles increase the melt pool’s viscosity and disturb the stability, suggesting a much narrower window for LPBF process parameters. Owing to hindered dislocation motion during deformation (because of difference of Al and Si3N4) and the load-bearing effect of Si3N4 particles, the AMCs possess improved strength and elastic modulus. | 10 vol.% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minasyan, T.; Hussainova, I. Laser Powder-Bed Fusion of Ceramic Particulate Reinforced Aluminum Alloys: A Review. Materials 2022, 15, 2467. https://doi.org/10.3390/ma15072467
Minasyan T, Hussainova I. Laser Powder-Bed Fusion of Ceramic Particulate Reinforced Aluminum Alloys: A Review. Materials. 2022; 15(7):2467. https://doi.org/10.3390/ma15072467
Chicago/Turabian StyleMinasyan, Tatevik, and Irina Hussainova. 2022. "Laser Powder-Bed Fusion of Ceramic Particulate Reinforced Aluminum Alloys: A Review" Materials 15, no. 7: 2467. https://doi.org/10.3390/ma15072467