Effects of Surfactants on Zein Cast Films for Simultaneous Delivery of Two Hydrophilic Active Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Procedure of Zein Film
2.3. Characterization Methods
2.3.1. Scanning Electron Microscope
2.3.2. Fourier Transform Infrared Spectra
2.3.3. Mechanical Properties
2.3.4. Differential Scanning Calorimetry
2.4. LY Release Profiles and Anti-Microbial Potential of Films
2.5. LY Activities in Different Surfactant-Containing Solutions
2.6. AA Release Profiles and Anti-Oxidant Capacity of Films
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microstructures
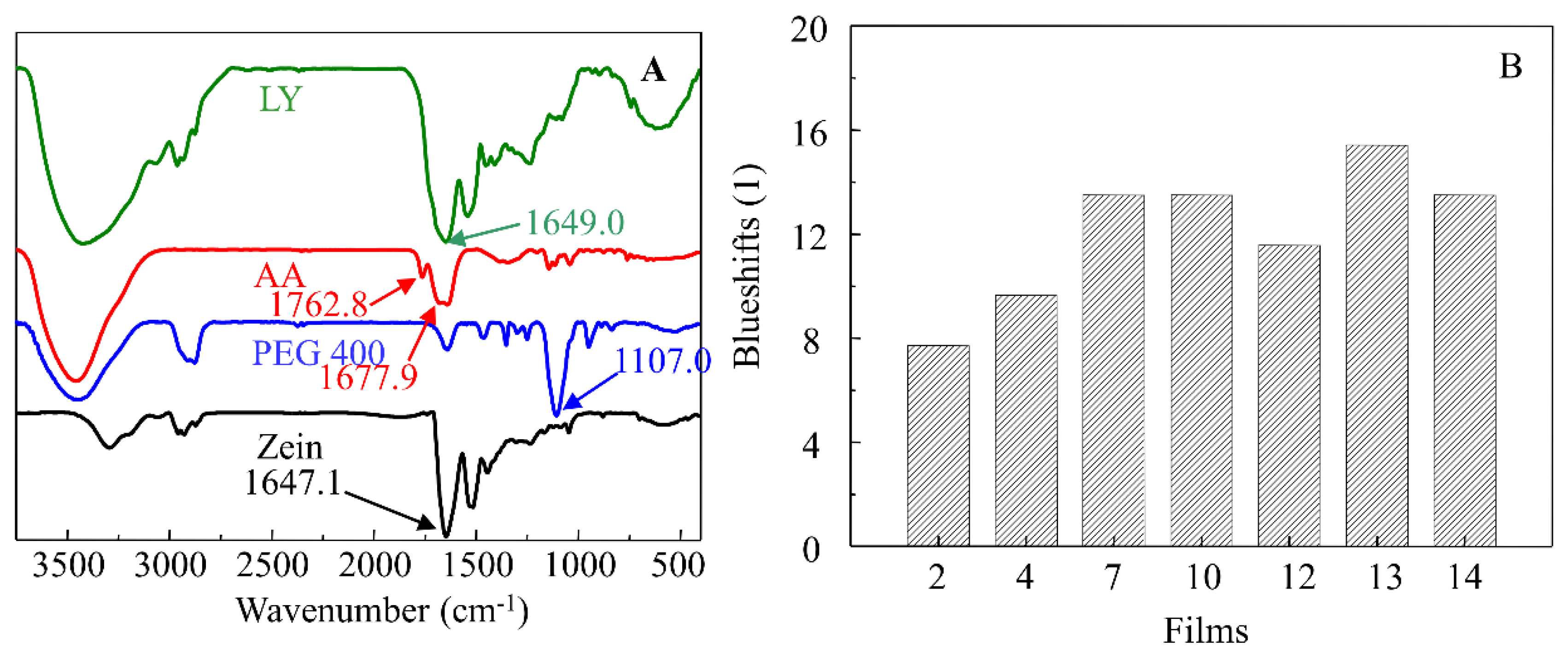
3.2. FT-IR Characterization
3.3. Mechanical Properties of Films
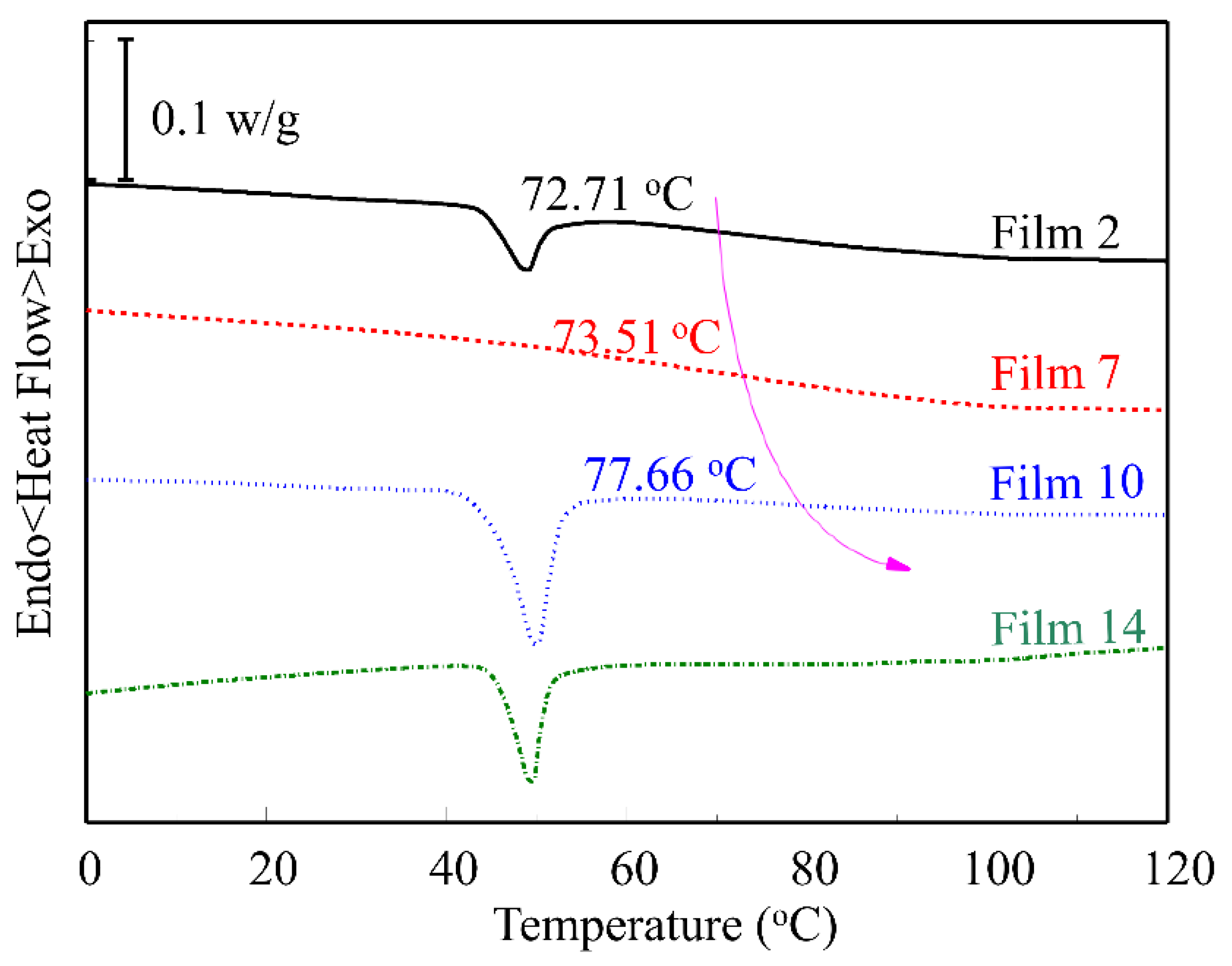
3.4. DSC Characterization
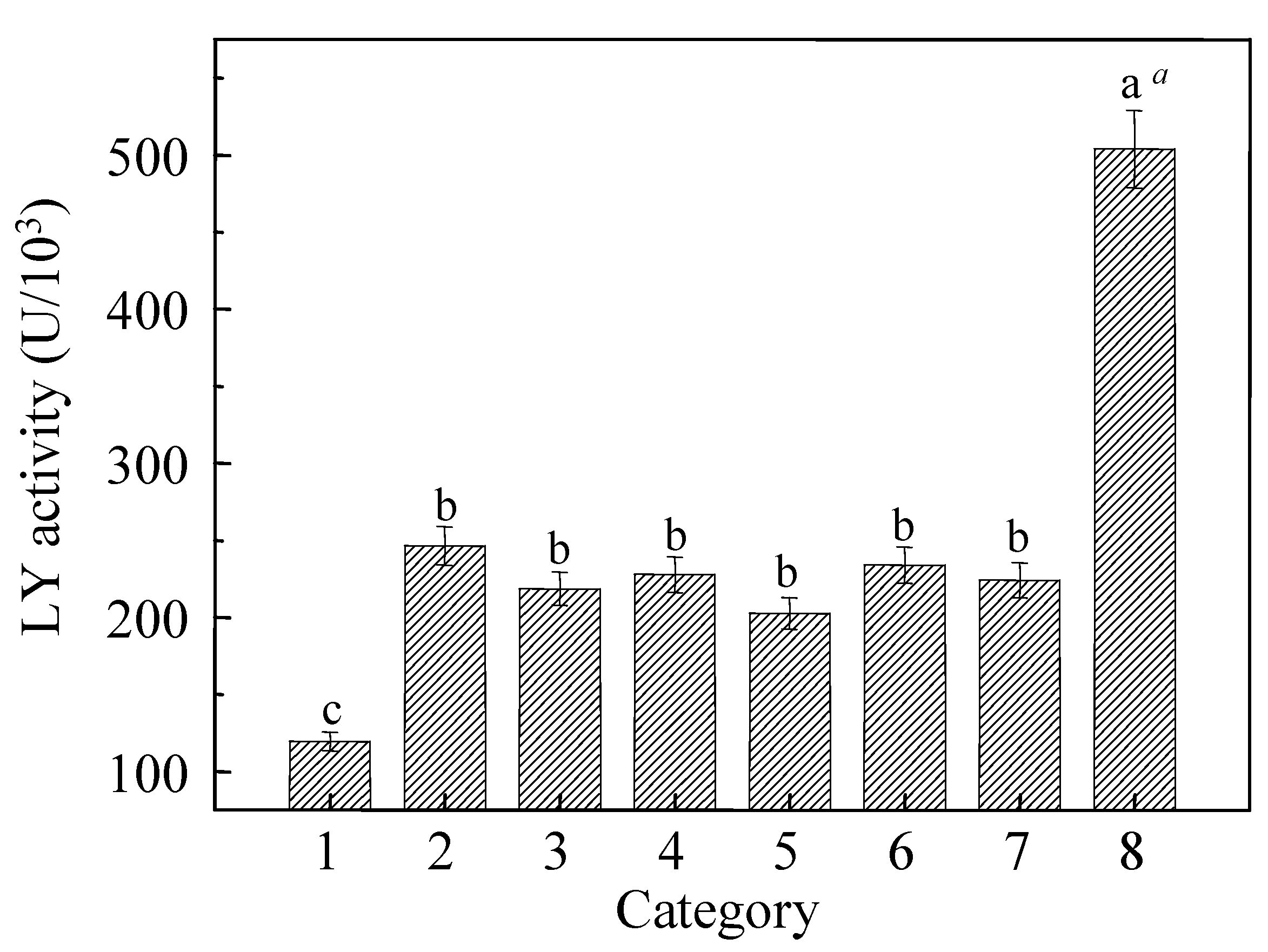
3.5. LY Activities in Different Surfactants Contained Aqueous Ethanol
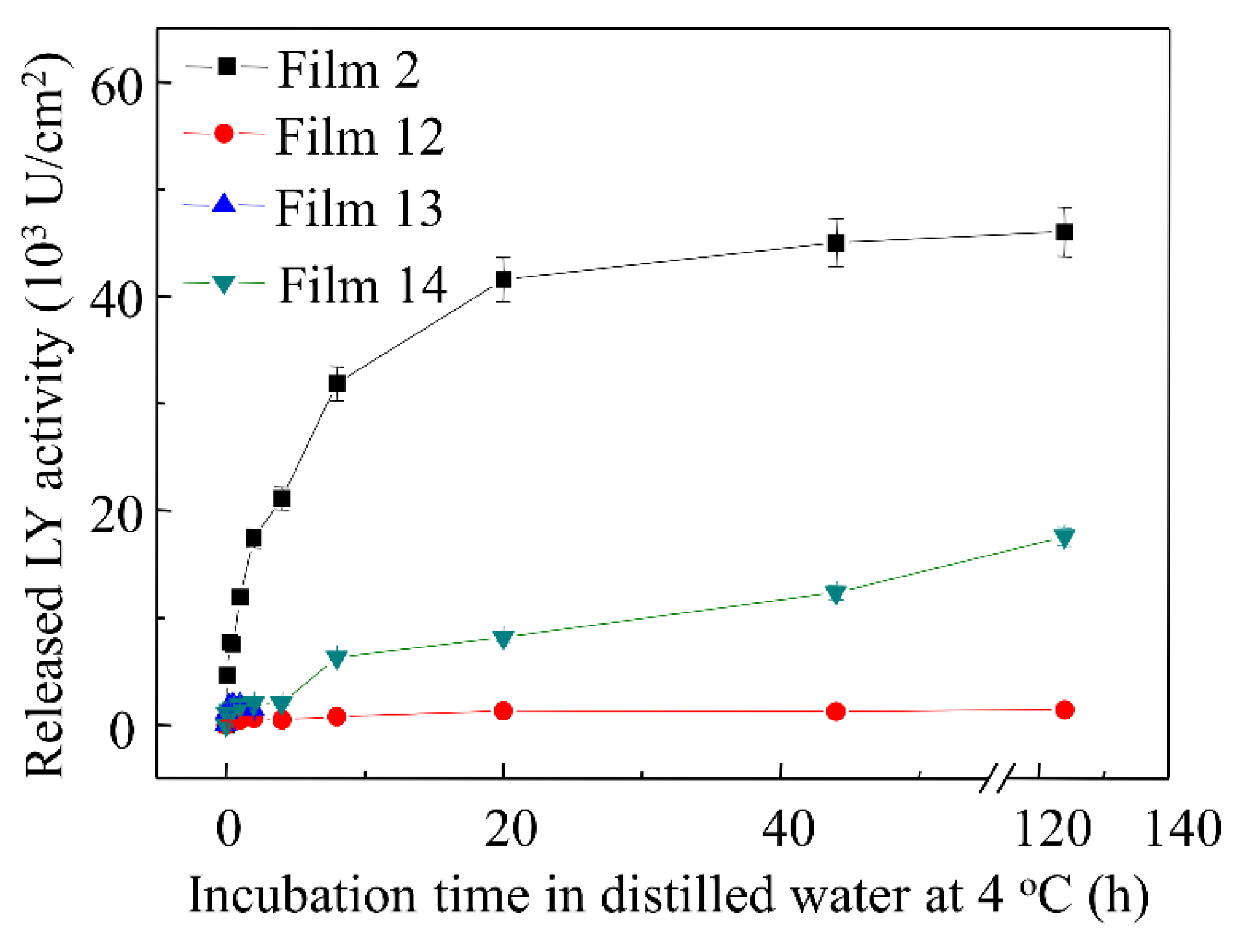
3.6. Effect of Single Surfactant on LY Release Profiles
3.7. Effects of Multiple Emulsifiers on LY Release Profiles
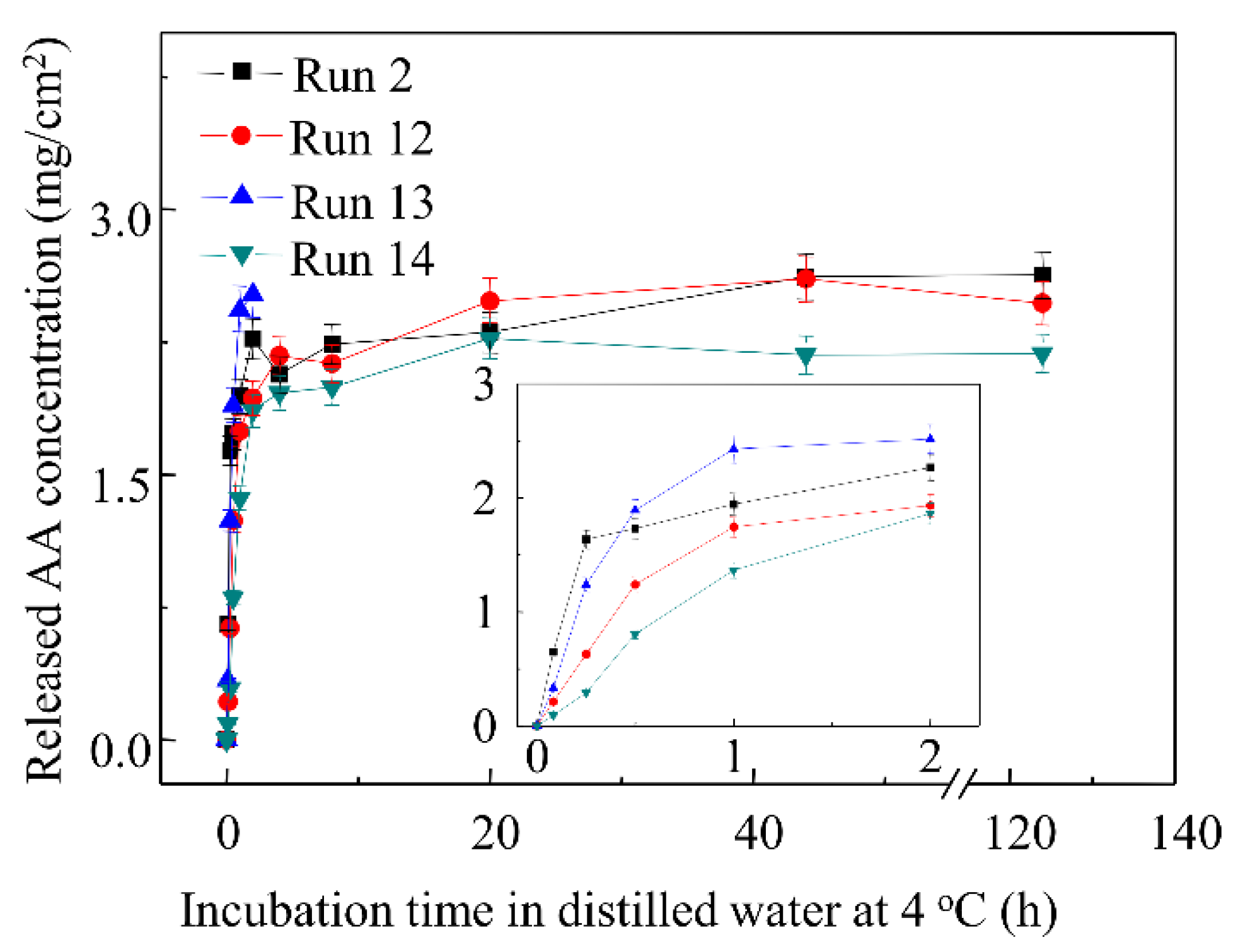
3.8. Effects of Surfactants on AA Released Profiles
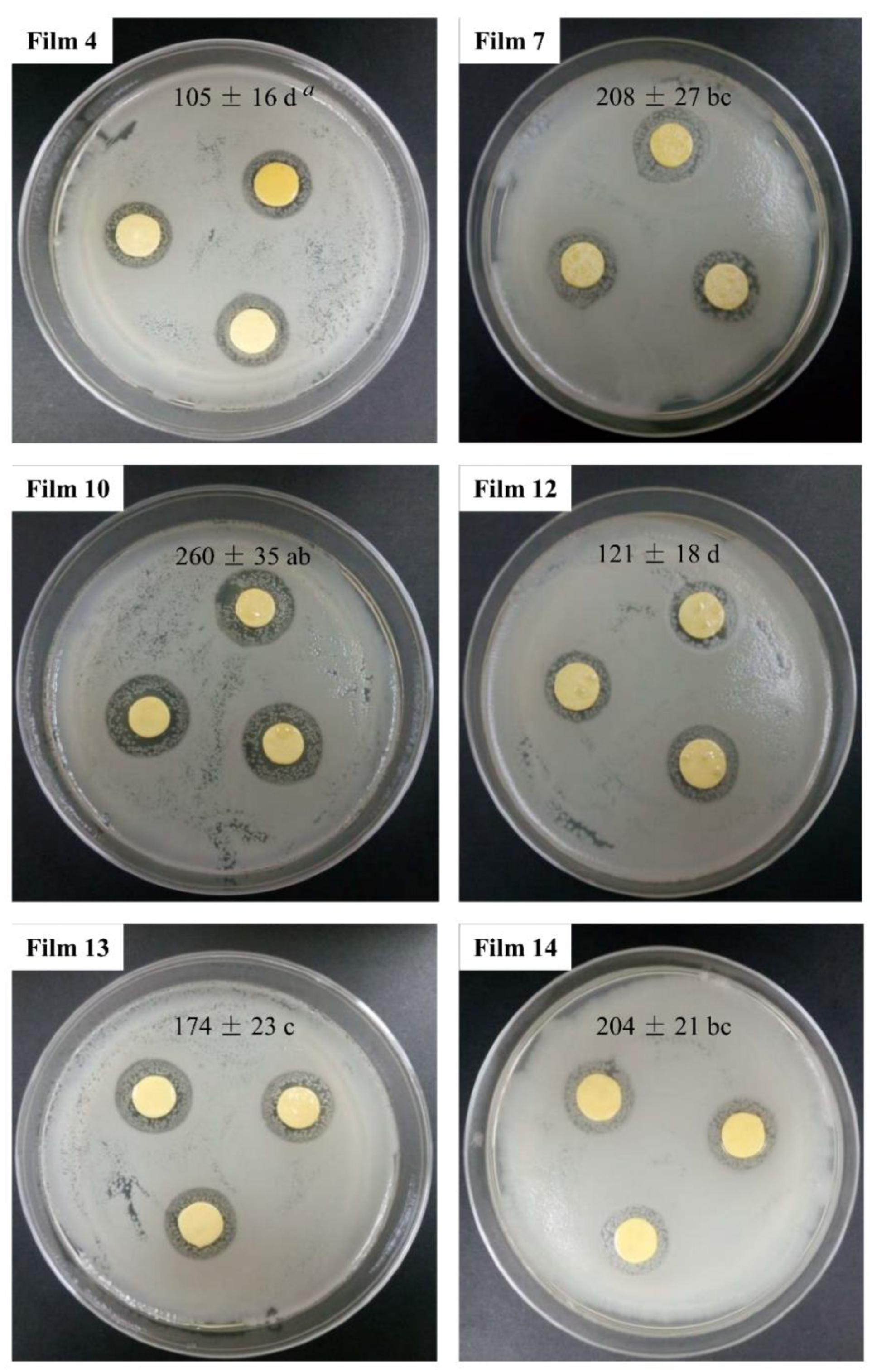
3.9. Anti-Microbial and Anti-Oxidant Potentials of Films
3.10. Emulsified Mechanisms of Surfactants in Zein Matrix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehdizadeh, A.; Shahidi, S.A.; Shariatifar, N.; Shiran, M.; Ghorbani-Hasansaraei, A. Physicochemical characteristics and antioxidant activity of the chitosan/zein films incorporated withpulicaria gnaphalodesl. extract-loaded nanoliposomes. J. Food Meas. Charact. 2022, 16, 1252–1262. [Google Scholar] [CrossRef]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Exploration of the antioxidant and antimicrobial capacity of two sunflower protein concentrate films with naturally present phenolic compounds. Food Hydrocoll. 2012, 29, 374–381. [Google Scholar] [CrossRef]
- Coroli, A.; Romano, R.; Saccani, A.; Raddadi, N.; Mascia, L. An in-vitro evaluation of the characteristics of zein-based films for the release of lactobionic acid and the effects of oleic acid. Polymers 2021, 13, 1826. [Google Scholar] [CrossRef]
- Federici, E.; Selling, G.W.; Campanella, O.H.; Jones, O.G. Thermal treatment of dry zein to improve rheological properties in gluten-free dough. Food Hydrocoll. 2021, 115, 106629. [Google Scholar] [CrossRef]
- Li, J.; Feng, H.; He, J.; Li, C.; Mao, X.; Xie, D.; Chu, B. Coaxial electrospun zein nanofibrous membrane for sustained release. J. Biomat. Sci.-Polym. Ed. 2013, 24, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, W.; Xiao, J.; Chen, Y.; Cao, Y. Interfacial engineering of pickering emulsion co-stabilized by zein nanoparticles and tween 20: Effects of the particle size on the interfacial concentration of gallic acid and the oxidative stability. Nanomaterials 2020, 10, 1068. [Google Scholar] [CrossRef]
- Lza, B.; Kl, A.; Dya, B.; Jmrb, C.; Jda, B.; Wc, A. Chitosan/zein bilayer films with one-way water barrier characteristic: Physical, structural and thermal properties. Int. J. Biol. Macromol. 2022, 200, 378–387. [Google Scholar]
- Liu, G.; Wei, D.; Wang, H.; Hu, Y.; Jiang, Y. Self-assembly of zein microspheres with controllable particle size and narrow distribution using a novel built-in ultrasonic dialysis process. Chem. Eng. J. 2016, 284, 1094–1105. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Huang, Y.; Wang, H.; Jiang, Y. Incorporation of 10-hydroxycamptothecin nanocrystals into zein microspheres. Chem. Eng. Sci. 2016, 155, 405–414. [Google Scholar] [CrossRef]
- Paramawati, R.; Yoshino, T.; Isobe, S. Properties of plasticized-zein film as affected by plasticizer treatments. Food Sci. Technol. Res. 2001, 7, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Bromberg, L. Blends and Semiinterpenetrating Networks of Zein and Poly (N,N-dimethylacrylamide). J. Phys. Chem. 1996, 100, 13811–13814. [Google Scholar] [CrossRef]
- Wongsasulak, S.; Puttipaiboon, N.; Yoovidhya, T. Fabrication, gastromuco- adhesivity, swelling, and degradation of zein–chitosan composite ultrafine fibers. J. Food Sci. 2013, 78, N926–N935. [Google Scholar] [CrossRef] [PubMed]
- Gontard, N.; Duchez, C.; Cuq, J.L. Edible composite films of wheat gluten and lipids: Water vapour permeability and other physical properties. Int. J. Food Sci. Tech. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT-Food Sci. Tech. 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- Huang, W.; Zou, T.; Li, S. Drug-loaded zein nanofibers prepared using a modified coaxial electrospinning process. AAPS PharmSciTech 2013, 14, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Coke, M.; Wilde, P.J.; Russell, E.J.; Clark, D.C. The influence of surface composition and molecular diffusion on the stability of foams formed from protein/surfactant mixtures. J. Colloid Interf. Sci. 1990, 138, 489–504. [Google Scholar] [CrossRef]
- La Mesa, C. Polymer–surfactant and protein–surfactant interactions. J. Colloid Interf. Sci. 2005, 286, 148–157. [Google Scholar] [CrossRef]
- Shaheen, A.; Kaur, I.; Mahajan, R.K. Potentiometric studies of micellization behavior of cationic surfactants in the presence of glycol additives and triblock polymer (Pluronic F68), using surfactant-selective sensors based on neutral ion-pair complexes. Ind. Eng. Chem. Res. 2007, 46, 4706–4709. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Lee, T.C.; Huang, Q. Structure and physical properties of zein/pluronic F127 composite films. J. Agric. Food Chem. 2013, 61, 1309–1318. [Google Scholar] [CrossRef]
- Xiao, D.; Zhong, Q. In vitro release kinetics of nisin as affected by Tween 20 and glycerol co-encapsulated in spray-dried zein capsules. J. Food Eng. 2011, 106, 65–73. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Yoshii, F.; Nagasawa, N.; Wach, R.A.; Mitomo, H. Degradation of chitosan and sodium alginate by gamma radiation, sonochemical and ultraviolet methods. Radiat. Phys. Chem. 2005, 73, 287–295. [Google Scholar] [CrossRef]
- Nagasawa, N.; Mitomo, H.; Yoshii, F.; Kume, T. Radiation-induced degradation of sodium alginate. Polym. Degrad. Stabil. 2000, 69, 279–285. [Google Scholar] [CrossRef]
- Gacesa, P. Alginates. Carbohyd. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Rossi, M. Use of lecithin and lecithin fractions. Bioact. Egg Compound. 2007, 27, 229–239. [Google Scholar]
- Murhammer, D.W.; Goochee, C.F. Structural features of nonionic polyglycol polymer molecules responsible for the protective effect in sparged animal cell bioreactors. Biotechnol. Progr. 1990, 6, 142–148. [Google Scholar] [CrossRef]
- Ghebeh, H.; Gillis, J.; Butler, M. Measurement of hydrophobic interactions of mammalian cells grown in culture. J. Biotechnol. 2002, 95, 39–48. [Google Scholar] [CrossRef]
- Nakamae, K.; Nizuka, T.; Miyata, T. Lysozyme loading and release from hydrogels carrying pendant phosphate groups. J. Biomat. Sci.-Polym. Ed. 1998, 9, 43–53. [Google Scholar] [CrossRef]
- Subbaraman, L.N.; Jones, L. Kinetics of lysozyme activity recovered from conventional and silicone hydrogel contact lens materials. J. Biomat. Sci.-Polym. Ed. 2010, 21, 343–358. [Google Scholar] [CrossRef]
- Lin, Y.T.; Liang, C.; Yu, C.W. Trichloroethylene degradation by various forms of iron activated persulfate oxidation with or without the assistance of ascorbic acid. Ind. Eng. Chem. Res. 2016, 55, 2302–2308. [Google Scholar] [CrossRef]
- Pénicaud, C.; Bohuon, P.; Peyron, S.; Gontard, N.; Guillard, V. Influence of the experimental errors and their propagation on the accuracy of identified kinetics parameters: Oxygen and temperature effects on ascorbic acid oxidation during storage. Ind. Eng. Chem. Res. 2012, 51, 1131–1142. [Google Scholar] [CrossRef]
- Soliman, E.A.; Mohy Eldin, M.S.; Furuta, M. Biodegradable zein-based films: Influence of γ-irradiation on structural and functional properties. J. Agric. Food Chem. 2009, 57, 2529–2535. [Google Scholar] [CrossRef]
- Mizutani, Y.; Matsumura, Y.; Imamura, K.; Nakanishi, K.; Mori, T. Effects of water activity and lipid addition on secondary structure of zein in powder systems. J. Agric. Food Chem. 2003, 51, 229–235. [Google Scholar] [CrossRef] [PubMed]
- ASTM. Standard test method for tensile properties of thin plastic sheeting D882-02. In Annual Book of American Standard Testing Methods; ASTM: Philadelphia, PA, USA, 2002. [Google Scholar]
- Arcan, I.; Yemenicioğlu, A. Controlled release properties of zein–fatty acid blend films for multiple bioactive compounds. J. Agric. Food Chem. 2014, 62, 8238–8246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, M.; Tajik, H.; Rohani, S.M.R.; Mahmoudian, A. Antioxidant and antimicrobial effects of zein edible film impregnated with Zataria multiflora Boiss. essential oil and monolaurin. LWT-Food Sci. Tech. 2016, 72, 37–43. [Google Scholar] [CrossRef]
- Cupello, A.; Rapallino, M.V.; Tabaton, M.; Lunardi, G.L. A simple, inexpensive, and precise spectrophotometric method for evaluating the concentration of ascorbic acid in CSF samples: Data from different neurological pathologies. Int. J. Neurosci. 2002, 112, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloid Surf. A 2016, 503, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Fischer, H.; Polikarpov, I.; Craievich, A.F. Average protein density is a molecular-weight-dependent function. Protein Sci. 2004, 13, 2825–2828. [Google Scholar] [CrossRef]
- Johnson, W.C.; Wang, J.; Chen, Z. Surface structures and properties of polystyrene/poly (methyl methacrylate) blends and copolymers. J. Phys. Chem. B 2005, 109, 6280–6286. [Google Scholar] [CrossRef]
- Qi, X.L.; Carl, H.O.L.T.; Mcnulty, D.; Clarke, D.T.; Brownlow, S.; Jones, G.R. Effect of temperature on the secondary structure of β-lactoglobulin at pH 6.7, as determined by CD and IR spectroscopy: A test of the molten globule hypothesis. Biochem. J. 1997, 324, 341–346. [Google Scholar] [CrossRef]
- Duodu, K.G.; Taylor, J.R.N.; Belton, P.S.; Hamaker, B.R. Factors affecting sorghum protein digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wang, T. Oxidative stability of egg and soy lecithin as affected by transition metal ions and pH in emulsion. J. Agric. Food Chem. 2008, 56, 11424–11431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghimi, S.M.; Hunter, A.C.; Dadswell, C.M.; Savay, S.; Alving, C.R.; Szebeni, J. Causative factors behind poloxamer 188 (Pluronic F68, Flocor™)-induced complement activation in human sera: A protective role against poloxamer-mediated complement activation by elevated serum lipoprotein levels. BBA-Mol. Basis Dis. 2004, 1689, 103–113. [Google Scholar] [CrossRef]
- Deo, N.; Jockusch, S.; Turro, N.J.; Somasundaran, P. Surfactant interactions with zein protein. Langmuir 2003, 19, 5083–5088. [Google Scholar] [CrossRef]
- Moore, P.N.; Puvvada, S.; Blankschtein, D. Role of the Surfactant Polar Head Structure in Protein-Surfactant Complexation: Zein Protein Solubilization by SDS and by SDS/C12E n Surfactant Solutions. Langmuir 2003, 19, 1009–1016. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Tanford, C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc. Natl. Acad. Sci. USA 1970, 66, 1002–1007. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Stühn, B. Crystallization and melting behavior of low molar weight PEO-PPO-PEO triblock copolymers. Colloid Polym. Sci. 2007, 285, 371–379. [Google Scholar] [CrossRef]

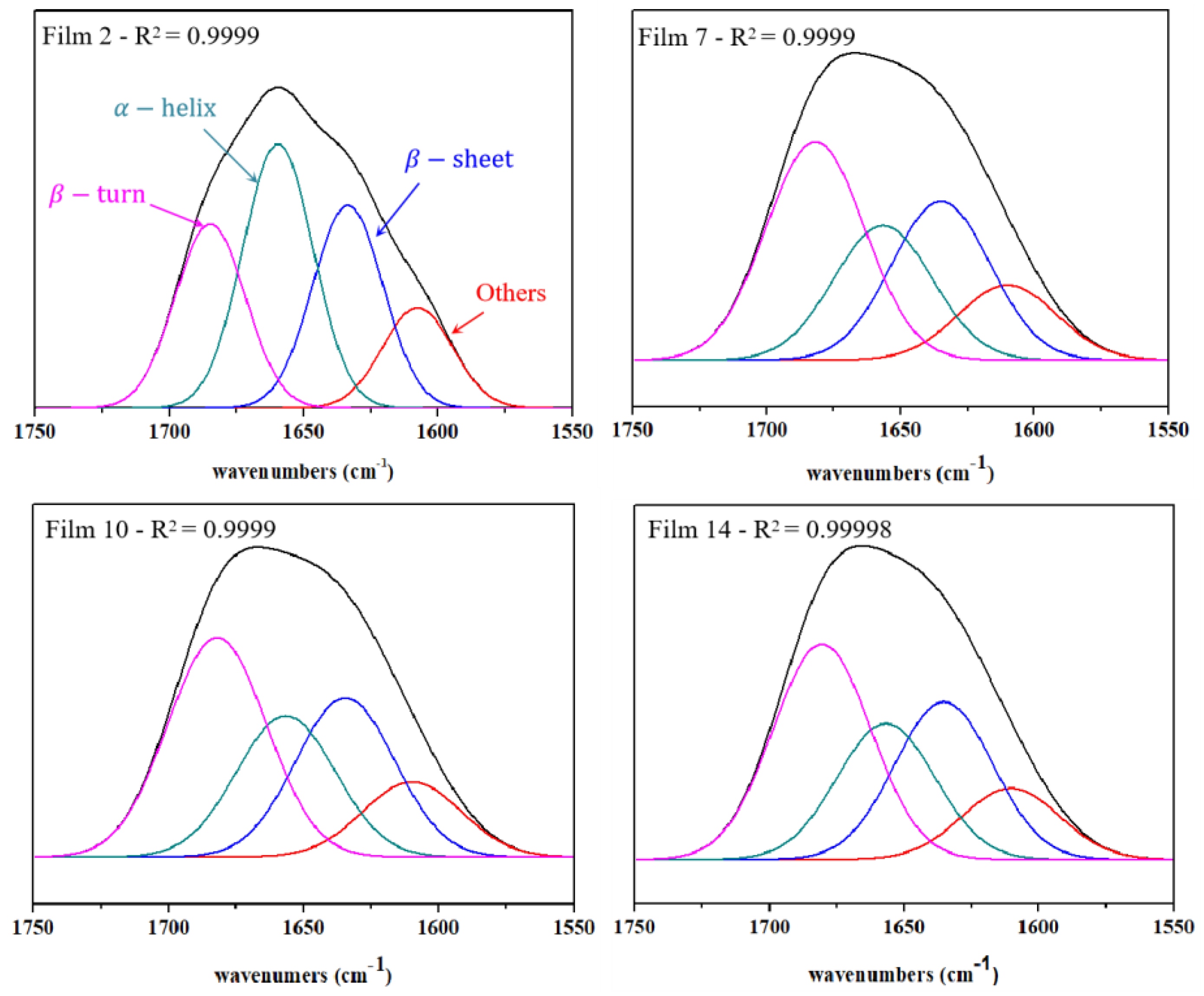

| Films | Others | |||||||
|---|---|---|---|---|---|---|---|---|
| (cm−1) | A (%) | (cm−1) | A (%) | (cm−1) | A (%) | (cm−1) | A (%) | |
| 2 | 1659.6 | 35.1 | 1633.6 | 27.0 | 1684.9 | 24.5 | 1607.6 | 13.4 |
| 7 | 1656.6 | 22.9 | 1634.9 | 27.0 | 1681.9 | 37.2 | 1610.0 | 12.9 |
| 10 | 1656.6 | 23.7 | 1634.6 | 26.8 | 1682.1 | 36.9 | 1609.9 | 12.6 |
| 14 | 1656.8 | 23.4 | 1635.3 | 27.2 | 1680.6 | 37.1 | 1610.5 | 12.3 |
| Film Composition a | TS (MPa) | EB (%) | Young’s Modulus (MPa) | Film Thickness (μm) | |||
|---|---|---|---|---|---|---|---|
| Films | SA (%) | SL (%) | PF-68 (%) | ||||
| 1 | - | - | - | 1.65 ± 0.62 a b | 30 ± 9 i | 43.00 ± 21 a | 167 ± 9 |
| AA (3.1 mg/cm2) and LY (4.1 mg/cm2) | |||||||
| 2 | - | - | - | 1.02 ± 0.17 de | 88 ± 8 efg | 13.02 ± 0.15 fg | 281 ± 20 |
| 3 | 5 | - | - | 0.96 ± 0.05 de | 121 ± 19 bcd | 6.84 ± 0.53 ij | 354 ± 13 |
| 4 | 10 | - | - | 1.52 ± 0.05 ab | 88 ± 21 efg | 17.16 ± 2.76 e | 295 ± 14 |
| 5 | 15 | - | - | 1.28 ± 0.08 bcd | 141 ± 8 ab | 11.04 ± 0.42 gh | 312 ± 20 |
| 6 | - | 5 | - | 1.63 ± 0.10 a | 59 ± 7 gh | 26.51 ± 2.21 c | 346 ± 30 |
| 7 | - | 10 | - | 1.39 ± 0.01 abc | 71 ± 7.3 fg | 20.33 ± 2.92 d | 298 ± 9 |
| 8 | - | 15 | - | 1.57 ± 0.20 ab | 32 ± 6 h | 30.24 ± 2.44 b | 340 ± 33 |
| 9 | - | - | 5 | 1.24 ± 0.06 bcd | 132 ± 20 abc | 7.46 ± 0.16 ij | 317 ± 4 |
| 10 | - | - | 10 | 0.94 ± 0.07 de | 129 ± 17 abc | 9.20 ± 1.73 i | 321 ± 16 |
| 11 | - | - | 15 | 0.78 ± 0.07 e | 157 ± 25 a | 5.02 ± 0.15 j | 319 ± 5 |
| 12 | 5 | 5 | - | 1.28 ± 0.20 bcd | 81 ± 17 efg | 15.03 ± 3.46 ef | 333 ± 12 |
| 13 | 5 | - | 5 | 1.01 ± 0.06 de | 106 ± 28 cde | 13.75 ± 1.75 efg | 355 ± 23 |
| 14 | - | 5 | 5 | 1.07 ± 0.04 cde | 99 ± 16 def | 15.15 ± 0.47 ef | 356 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Zhou, F.; Wang, H.; Liu, G.; Fang, J.; Jiang, Y. Effects of Surfactants on Zein Cast Films for Simultaneous Delivery of Two Hydrophilic Active Components. Materials 2022, 15, 2795. https://doi.org/10.3390/ma15082795
Wei D, Zhou F, Wang H, Liu G, Fang J, Jiang Y. Effects of Surfactants on Zein Cast Films for Simultaneous Delivery of Two Hydrophilic Active Components. Materials. 2022; 15(8):2795. https://doi.org/10.3390/ma15082795
Chicago/Turabian StyleWei, Dongwei, Fanhui Zhou, Hongdi Wang, Guijin Liu, Jun Fang, and Yanbin Jiang. 2022. "Effects of Surfactants on Zein Cast Films for Simultaneous Delivery of Two Hydrophilic Active Components" Materials 15, no. 8: 2795. https://doi.org/10.3390/ma15082795







