A DFT/TD-DFT Study on the ESIPT-Type Flavonoid Derivatives with High Emission Intensity
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
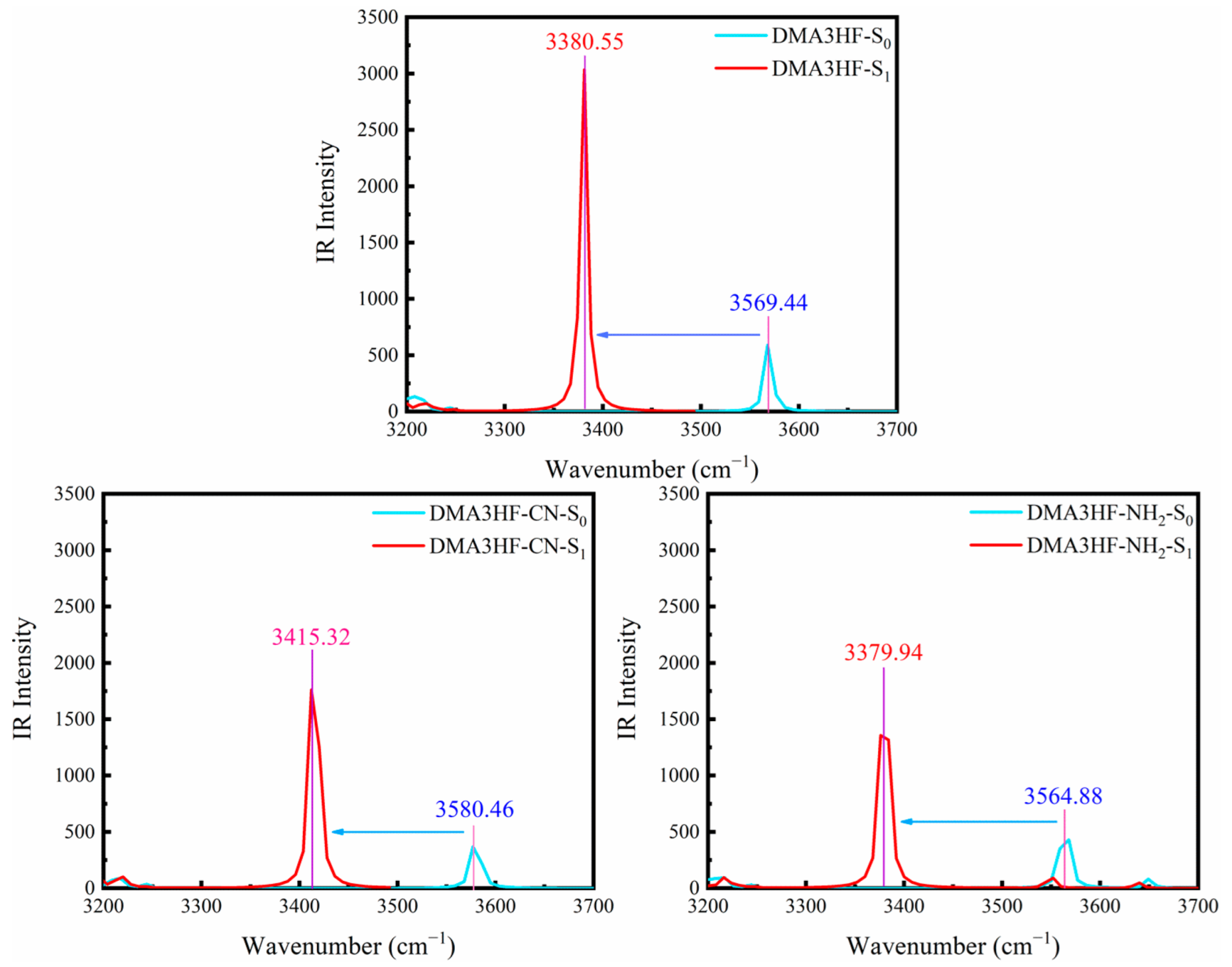
3.1. Optimized Geometric Structures and Infrared (IR) Vibrational Spectra Analysis
3.2. Natural Bond Orbital (NBO) and Fuzzy Bond Order (FBO) Analysis
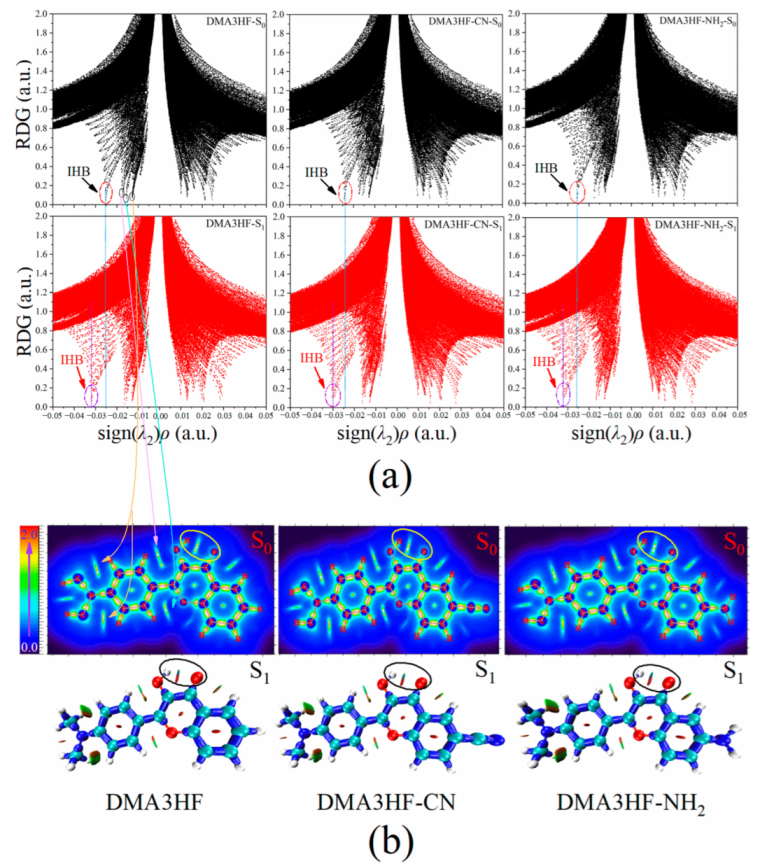
3.3. Reduced Density Gradient (RDG) Scatterplot and Topology Analysis
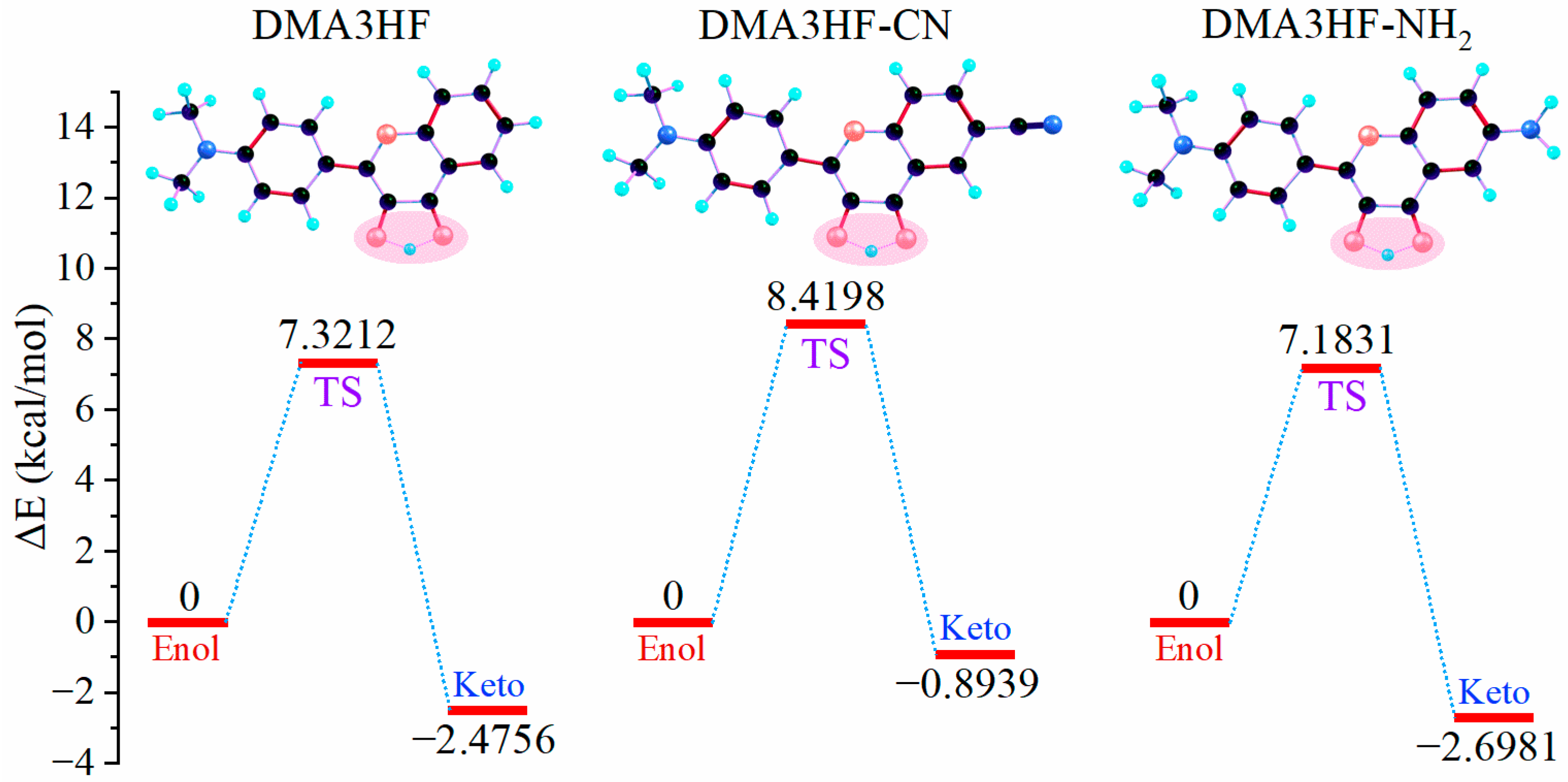
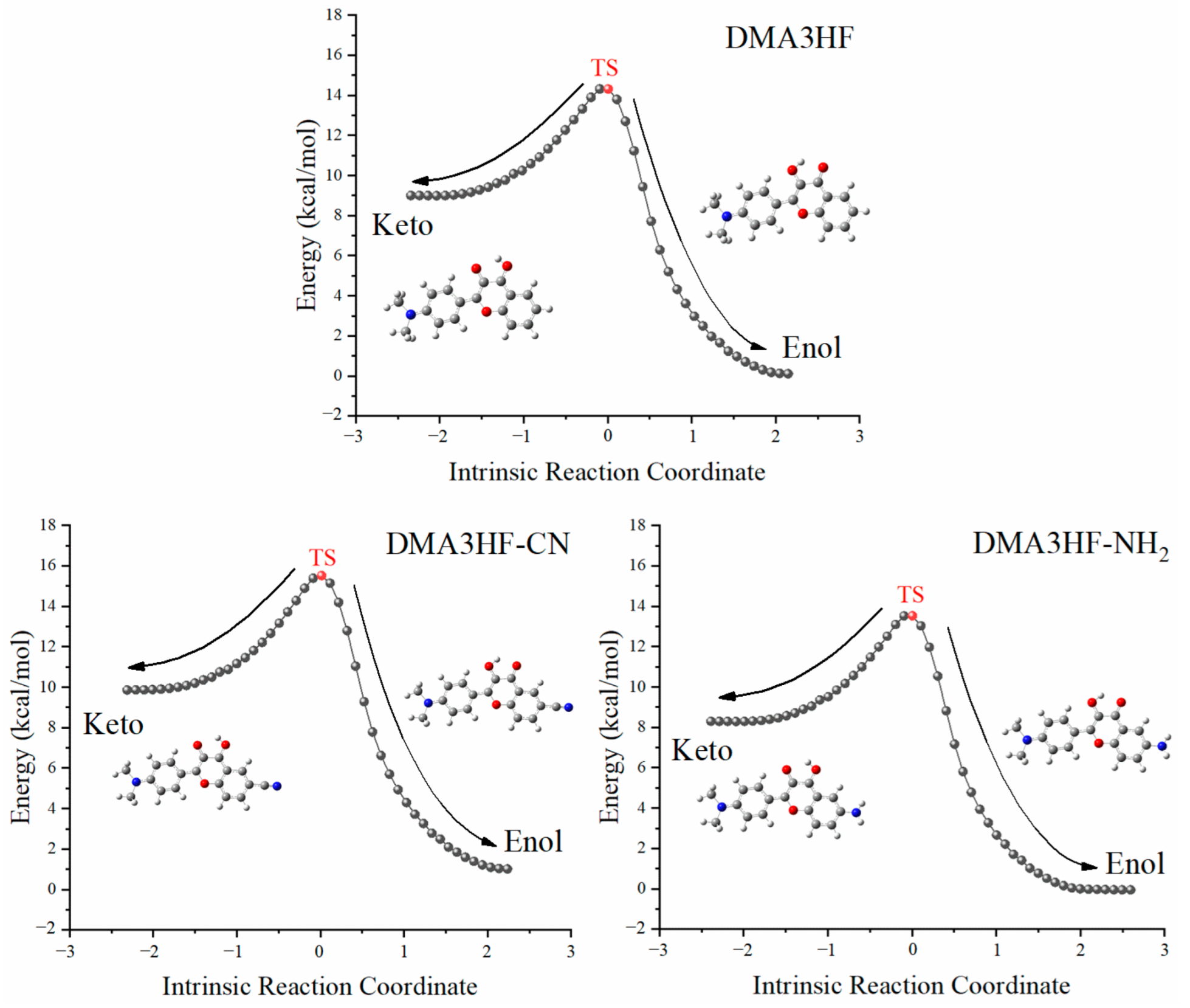
3.4. Potential Energy Curves (PECs)
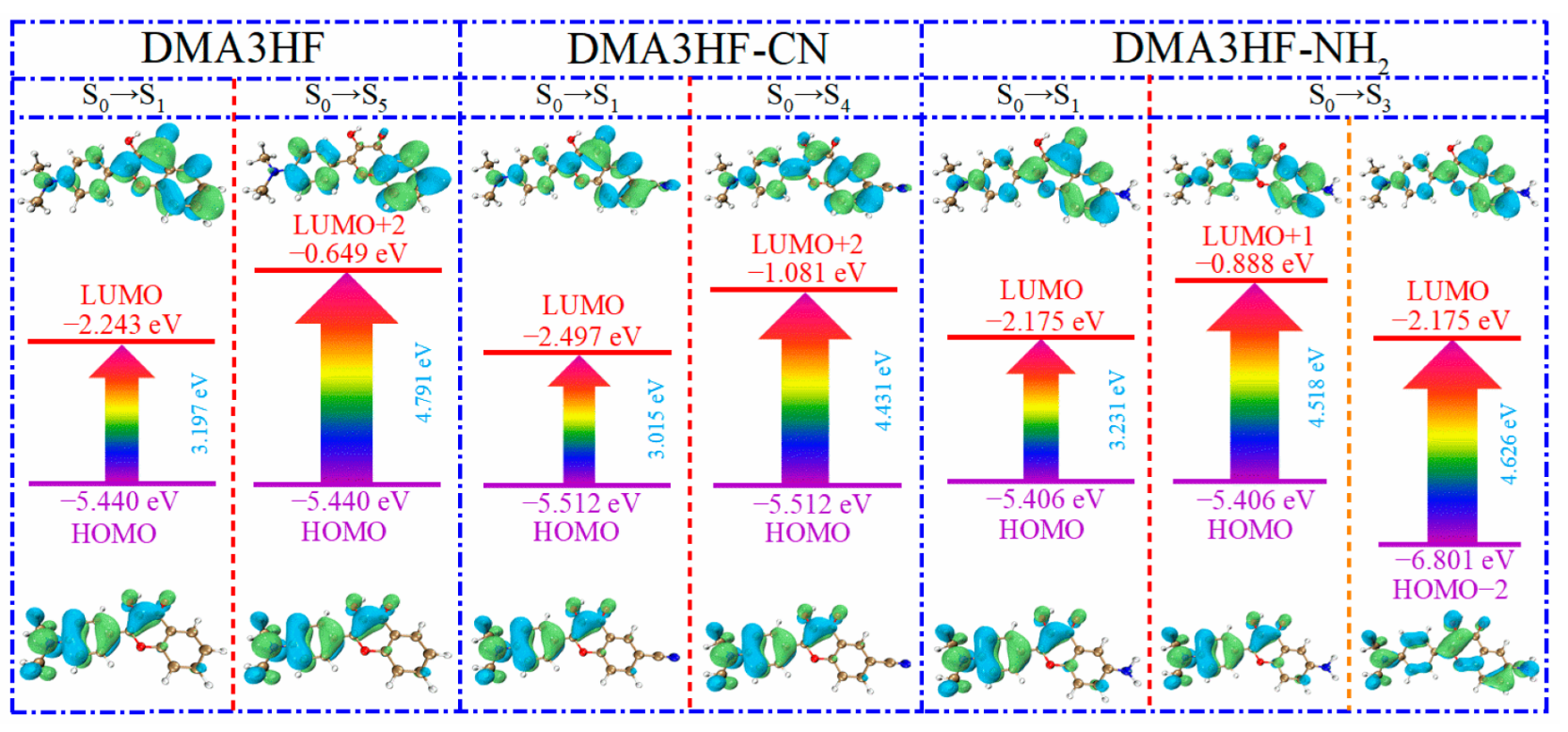
3.5. Electronic Spectra and Frontier Molecular Orbitals (FMOs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Park, S.Y. Advanced Organic Optoelectronic Materials: Harnessing Excited-State Intramolecular Proton Transfer (ESIPT) Process. Adv. Mater. 2011, 23, 3615–3642. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Chen, Y.; Guo, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT): From principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, V.S.; Seki, S. Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Wu, L.L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, A. Über die Fluoreszenz der Salizylsäure und verwandter Verbindungen. Naturwissenschaften 1955, 42, 175–176. [Google Scholar] [CrossRef]
- Chen, L.; Ye, J.W.; Wang, H.P.; Pan, M.; Yin, S.Y.; Wei, Z.W.; Zhang, L.Y.; Wu, K.; Fan, Y.N.; Su, C.Y. Ultrafast water sensing and thermal imaging by a metal-organic framework with switchable luminescence. Nat. Commun. 2017, 8, 15985. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cao, B.F.; Zhou, Q.; Zhang, X.; Li, B.; Su, X.; Shi, Y. Enhancing fluorescence of benzimidazole derivative via solvent-regulated ESIPT and TICT process: A TDDFT study. Spectrochim. Acta Part A 2021, 258, 119862. [Google Scholar] [CrossRef]
- Cao, B.F.; Han, J.H.; Zhou, Q.; Sun, C.F.; Li, Y.; Li, B.; Yin, H.; Shi, Y. Skillfully tuning 1-hydroxy-9H-fluoren-9-one forward-backward ESIPT processes by introducing electron-withdrawing groups: A theoretical exploration. J. Mol. Liq. 2020, 303, 112627. [Google Scholar] [CrossRef]
- Luo, X.; Shi, W.; Yang, Y.F.; Song, Y.Z.; Li, Y.Q. Systematic theoretical investigation of two novel molecules BtyC-1 and BtyC-2 based on ESIPT mechanism. Spectrochim. Acta Part A 2021, 258, 119810. [Google Scholar] [CrossRef]
- Zhao, G.J.; Shi, W.; Yang, Y.F.; Ding, Y.; Li, Y.Q. Substituent Effects on Excited-State Intramolecular Proton Transfer Reaction of 2-Aryloxazoline Derivatives. J. Phys. Chem. A 2021, 125, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.P.; Jia, M.; Zhang, Q.L.; Wang, Y.S. Modulating O-H-based excited-state intramolecular proton transfer by alkyl-substitutions at various positions of 1-hydroxy-11H-benzo b fluoren-11-one. J. Lumin. 2020, 219, 116913. [Google Scholar] [CrossRef]
- Yang, D.P.; Zhang, T.J.; Song, X.Y.; Gao, H.Y. Is excited state intramolecular proton transfer frustrated in 10-hydroxy-11H-benzo b fluoren-11-one? Spectrochim. Acta Part A Spectrosc. 2020, 228, 117734. [Google Scholar] [CrossRef] [PubMed]
- Daengngern, R.; Kungwan, N. Electronic and photophysical properties of 2-(2′-hydroxyphenyl)benzoxazole and its derivatives enhancing in the excited-state intramolecular proton transfer processes: A TD-DFT study on substitution effect. J. Lumin. 2015, 167, 132–139. [Google Scholar] [CrossRef]
- Daengngern, R.; Prommin, C.; Rungrotmongkol, T.; Promarak, V.; Wolschann, P.; Kungwan, N. Theoretical investigation of 2-(iminomethyl) phenol in the gas phase as a prototype of ultrafast excited-state intramolecular proton transfer. Chem. Phys. Lett. 2016, 657, 113–118. [Google Scholar] [CrossRef]
- Kanlayakan, N.; Kerdpol, K.; Prommin, C.; Salaeh, R.; Chansen, W.; Sattayanon, C.; Kungwan, N. Effects of different proton donor and acceptor groups on excited-state intramolecular proton transfers of amino-type and hydroxy-type hydrogen-bonding molecules: Theoretical insights. New J. Chem. 2017, 41, 8761–8771. [Google Scholar] [CrossRef]
- Jia, L.F.; Wang, F.; Liu, Y.F. Solvent effects on excited state intramolecular proton transfer mechanism in 4-(N,N-dimethylamino)-3-hydroxyflavone. Org. Electron. 2018, 57, 292–297. [Google Scholar] [CrossRef]
- Li, C.Z.; Li, D.L.; Ma, C.; Liu, Y.F. DFT-TDDFT investigation of excited-state intramolecular proton transfer in 2-(2′-hydroxyphenyl)benzimidazole derivatives: Effects of electron acceptor and donor groups. J. Mol. Liq. 2016, 224, 83–88. [Google Scholar] [CrossRef]
- Yang, L.; Yang, N.; Gu, P.; Wang, C.; Li, B.; Zhang, Y.; Ji, L.; He, G. A novel flavone-based ESIPT ratiometric fluorescent probe for selective sensing and imaging of hydrogen polysulfides. Spectrochim. Acta Part A 2022, 271, 120962. [Google Scholar] [CrossRef] [PubMed]
- Holt, E.L.; Krokidi, K.M.; Turner, M.A.P.; Mishra, P.; Zwier, T.S.; Rodrigues, N.D.N.; Stavros, V.G. Insights into the photoprotection mechanism of the UV filter homosalate. Phys. Chem. Chem. Phys. 2020, 22, 15509–15519. [Google Scholar] [CrossRef]
- Feng, W.X.; Fu, G.R.; Huang, Y.J.; Zhao, Y.; Yan, H.X.; Lu, X.Q. ESIPT-capable Eu3+-metallopolymer with colour-tunable emission for selective visual sensing of Zn2+ ion. J. Mater. Chem. C 2022, 10, 1090–1096. [Google Scholar] [CrossRef]
- Cao, Y.J.; Yu, X.R.; Sun, C.F.; Cui, J.A. Theoretical Investigation on the ESIPT Process and Detection Mechanism for Dual-Proton Type Fluorescent Probe. Int. J. Mol. Sci. 2022, 23, 2132. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, D. A Selective Fluorescence Turn-On Probe for the Detection of DCNP (Nerve Agent Tabun Simulant). Materials 2019, 12, 2943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.L.; Liu, Y.F. A theoretical investigation on ESIPT process of a red-emitting ratiometric fluorescent probe and its fluorescent detection mechanism for cyanide anion. J. Ind. Eng. Chem. 2021, 99, 126–133. [Google Scholar] [CrossRef]
- Kenfack, C.A.; Klymchenko, A.S.; Duportail, G.; Burger, A.; Mely, Y. Ab initio study of the solvent H-bonding effect on ESIPT reaction and electronic transitions of 3-hydroxychromone derivatives. Phys. Chem. Chem. Phys. 2012, 14, 8910–8918. [Google Scholar] [CrossRef]
- Zhu, A.; Wang, B.; White, J.O.; Drickamer, H.G. The effects of pressure on the intramolecular proton transfer and charge transfer of 4′-N-dimethylamino-3-hydroxyflavone. J. Phys. Chem. B 2004, 108, 891–894. [Google Scholar] [CrossRef]
- Karmakar, A.; Mallick, T.; Fouzder, C.; Mukhuty, A.; Mondal, S.; Pramanik, A.; Kundu, R.; Mandal, D.; Begum, N.A. Unfolding the Role of a Flavone-Based Fluorescent Antioxidant towards the Misfolding of Amyloid Proteins: An Endeavour to Probe Amyloid Aggregation. J. Phys. Chem. B 2020, 124, 11133–11144. [Google Scholar] [CrossRef]
- Ghosh, D.; Batuta, S.; Begum, N.A.; Mandal, D. Unusually slow intramolecular proton transfer dynamics of 4′-N,N-dimethylamino-3-hydroxyflavone in high n-alcohols: Involvement of solvent relaxation. Photochem. Photobiol. Sci. 2016, 15, 266–277. [Google Scholar] [CrossRef]
- Das, K.; Sappati, S.; Bisht, G.S.; Hazra, P. Proton-Coupled Electron Transfer in the Aqueous Nanochannels of Lyotropic Liquid Crystals: Interplay of H-Bonding and Polarity Effects. J. Phys. Chem. Lett. 2021, 12, 2651–2659. [Google Scholar] [CrossRef]
- Furukawa, K.; Hino, K.; Yamamoto, N.; Awasthi, K.; Nakabayashi, T.; Ohta, N.; Sekiya, H. External Electric Field Effects on Excited-State Intramolecular Proton Transfer in 4′-N,N-Dimethylamino-3-hydroxyflavone in Poly(methyl methacrylate) Films. J. Phys. Chem. A 2015, 119, 9599–9608. [Google Scholar] [CrossRef]
- Ushakou, D.V.; Tomin, V.I. Spectroscopic methods for the study of energetic characteristics of the normal and photoproduct forms of 3-hydroxyflavones. Spectrochim. Acta Part A 2018, 204, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.W.; Han, K. Unraveling the Detailed Mechanism of Excited-State Proton Transfer. Acc. Chem. Res. 2018, 51, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Jacquemin, D.; Wathelet, V.; Perpete, E.A.; Adamo, C. Extensive TD-DFT Benchmark: Singlet-Excited States of Organic Molecules. J. Chem. Theory Comput. 2009, 5, 2420–2435. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Klamt, A.; Moya, C.; Palomar, J. A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J. Chem. Theory Comput. 2015, 11, 4220–4225. [Google Scholar] [CrossRef] [Green Version]
- Roch, L.M.; Baldridge, K.K. General optimization procedure towards the design of a new family of minimal parameter spin-component-scaled double-hybrid density functional theory. Phys. Chem. Chem. Phys. 2017, 19, 26191–26200. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Son, H.J.; Choe, J.I. mPW1PW91 study for conformational isomers of methylene bridge-monosubstituted tetramethoxycalix 4 arenes. J. Ind. Eng. Chem. 2014, 20, 3276–3282. [Google Scholar] [CrossRef]
- Ramalingam, S.; Periandy, S.; Mohan, S. Vibrational spectroscopy (FTIR and FTRaman) investigation using ab initio (HF) and DFT (B3LYP and B3PW91) analysis on the structure of 2-amino pyridine. Spectrochim. Acta Part A 2010, 77, 73–81. [Google Scholar] [CrossRef]
- Babu, P.D.S.; Periandy, S.; Mohan, S.; Ramalingam, S.; Jayaprakash, B.G. Molecular structure and vibrational investigation of benzenesulfonic acid methyl ester using DFT (LSDA, B3LYP, B3PW91 and MPW1PW91) theory calculations. Spectrochim. Acta Part A 2011, 78, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Khajehzadeh, M.; Moghadam, M. Molecular structure, FT IR, NMR, UV, NBO and HOMO-LUMO of 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran- 5-carbonitrile by DFT/B3LYP and PBEPBE methods with LanL2DZ and 6-311++G(d,2p) basis sets. Spectrochim. Acta Part A 2017, 180, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.J.; Wang, L.L.; Cao, Y.J.; Yu, X.R.; Li, Y.Z.; Sun, C.F.; Cui, J.G. Is it possible to switch ESIPT-channel of hydroxyanthraquinones with the strategy of modifying electronic groups? J. Mol. Liq. 2022, 347, 118343. [Google Scholar] [CrossRef]
- Sun, C.F.; Li, H.; Yin, H.; Li, Y.Z.; Shi, Y. Effects of the cyano substitution at different positions on the ESIPT properties of alizarin: A DFT/TD-DFT investigation. J. Mol. Liq. 2018, 269, 650–656. [Google Scholar] [CrossRef]
- Sun, C.F.; Zhao, H.F.; Liu, X.C.; Yin, H.; Shi, Y. Tunable ESIPT reaction and antioxidant activities of 3-hydroxyflavone and its derivatives by altering atomic electronegativity. Org. Chem. Front. 2018, 5, 3435–3442. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Bond Order Analysis Based on the Laplacian of Electron Density in Fuzzy Overlap Space. J. Phys. Chem. A 2013, 117, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Duran, M.; Sola, M.; Silvi, B. Theoretical evaluation of electron delocalization in aromatic molecules by means of atoms in molecules (AIM) and electron localization function (ELF) topological approaches. Chem. Rev. 2005, 105, 3911–3947. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W.T. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Chen, Q. Interaction Region Indicator: A Simple Real Space Function Clearly Revealing Both Chemical Bonds and Weak Interactions. Chemistry-Methods 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Bao, J.L.; Truhlar, D.G. Variational transition state theory: Theoretical framework and recent developments. Chem. Soc. Rev. 2017, 46, 7548–7596. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Harabuchi, Y.; Ono, Y.; Taketsugu, T.; Morokuma, K. Intrinsic Reaction Coordinate: Calculation, Bifurcation, and Automated Search. Int. J. Quantum Chem. 2015, 115, 258–269. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Su, X.; Zhou, Q.; Li, Y.; Cao, B.F.; Li, B.; Zhang, X.; Yin, H.; Shi, Y. Revised the excited-state intramolecular proton transfer direction of the BTHMB molecule: A theoretical study. Spectrochim. Acta Part A 2021, 249, 119327. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.J.; Cao, Y.J.; Sun, C.F.; Li, Y.Z. Unveiling the influence of atomic electronegativity on the double ESIPT processes of uralenol: A theoretical study. Spectrochim. Acta Part A 2022, 268, 120660. [Google Scholar] [CrossRef]
- Matito, E.; Poater, J.; Sola, M.; Duran, M.; Salvador, P. Comparison of the AIM delocalization index and the Mayer and Fuzzy atom bond orders. J. Phys. Chem. A 2005, 109, 9904–9910. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.C.; Contreras-Garcia, J.; Henon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Narahara, K. Atoms-in-Molecules Dual Parameter Analysis of Weak to Strong Interactions: Behaviors of Electronic Energy Densities versus Laplacian of Electron Densities at Bond Critical Points. J. Phys. Chem. A 2008, 112, 13593–13599. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.F.; Su, X.; Zhou, Q.; Shi, Y. Regular tuning of the ESIPT reaction of 3-hydroxychromone-based derivatives by substitution of functional groups. Org. Chem. Front. 2019, 6, 3093–3100. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Yang, Y.F.; Lan, R.F.; Li, Y.Q. Effect of Different Substituted Groups on Excited-State Intramolecular Proton Transfer of 1-(Acylamino)-anthraquinons. J. Phys. Chem. C 2017, 121, 14779–14786. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4031. [Google Scholar] [CrossRef]
- Peng, X.J.; Song, F.L.; Lu, E.; Wang, Y.N.; Zhou, W.; Fan, J.L.; Gao, Y.L. Heptamethine cyanine dyes with a large stokes shift and strong fluorescence: A paradigm for excited-state intramolecular charge transfer. J. Am. Chem. Soc. 2005, 127, 4170–4171. [Google Scholar] [CrossRef] [PubMed]



| PBEPBE | B3PW91 | Cam-B3LYP | B3LYP | M062x | mPW1PW91 | ωB97XD | Exp a | |
|---|---|---|---|---|---|---|---|---|
| λflu 1 | 532.94 | 456.48 | 396.43 | 458.03 | 396.64 | 441.74 | 388.21 | 510 |
| λflu 2 | 586.30 | 545.90 | 520.40 | 547.40 | 513.09 | 537.39 | 518.42 | 570 |
| State | O1-H1 | H1-O2 | ∠(O1-H1⋯O2) | |
|---|---|---|---|---|
| DMA3HF-enol | S0 | 0.977 | 2.025 | 117.886 |
| S1 | 0.988 | 1.909 | 122.179 | |
| DMA3HF-keto | S0 | 1.936 | 0.988 | 120.425 |
| S1 | 2.009 | 0.981 | 117.761 | |
| DMA3HF-CN-enol | S0 | 0.977 | 2.044 | 117.187 |
| S1 | 0.986 | 1.933 | 121.196 | |
| DMA3HF-CN-keto | S0 | 1.960 | 0.987 | 119.440 |
| S1 | 2.018 | 0.981 | 117.213 | |
| DMA3HF-NH2-enol | S0 | 0.978 | 2.019 | 118.153 |
| S1 | 0.988 | 1.906 | 122.390 | |
| DMA3HF-NH2-keto | S0 | 1.934 | 0.988 | 120.556 |
| S1 | 2.004 | 0.981 | 118.011 |
| DMA3HF | DMA3HF-CN | DMA3HF-NH2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| State/Δ | S0 | S1 | Δ | S0 | S1 | Δ | S0 | S1 | Δ |
| O1 | −0.6917 | −0.6580 | −0.0337 | −0.6860 | −0.6540 | −0.0320 | −0.6947 | −0.6639 | −0.0308 |
| O2 | −0.6948 | −0.7646 | +0.0698 | −0.6819 | −0.7413 | +0.0594 | −0.7024 | −0.7741 | +0.0717 |
| State | FBO (O1-H1) | FBO (H1⋯O2) | |
|---|---|---|---|
| DMA3HF-enol | S0 | 0.75626 | 0.06129 |
| S1 | 0.72953 | 0.07995 | |
| DMA3HF-keto | S0 | 0.08263 | 0.72416 |
| S1 | 0.07044 | 0.74092 | |
| DMA3HF-CN-enol | S0 | 0.75760 | 0.05773 |
| S1 | 0.73284 | 0.07482 | |
| DMA3HF-CN-keto | S0 | 0.07747 | 0.72767 |
| S1 | 0.06834 | 0.73979 | |
| DMA3HF-NH2-enol | S0 | 0.75591 | 0.06242 |
| S1 | 0.72957 | 0.08095 | |
| DMA3HF-NH2-keto | S0 | 0.08309 | 0.72530 |
| S1 | 0.07136 | 0.74132 |
| ρ(r) α | ∇2ρ(r) β | V(r) γ | G(r) δ | H(r) ε | ELF ζ | EHB η | |
|---|---|---|---|---|---|---|---|
| DMA3HF-enol-S0 | 0.0253 | 0.1026 | −0.0204 | 0.0230 | 0.0027 | 0.0689 | −4.9016 |
| DMA3HF-enol-S1 | 0.0320 | 0.1209 | −0.0270 | 0.0286 | 0.0016 | 0.0950 | −6.3963 |
| DMA3HF-keto-S0 | 0.0310 | 0.1133 | −0.0254 | 0.0268 | 0.0015 | 0.0964 | −6.1732 |
| DMA3HF-keto-S1 | 0.0267 | 0.1009 | −0.0212 | 0.0232 | 0.0020 | 0.0805 | −5.2139 |
| DMA3HF-CN-enol-S0 | 0.0239 | 0.1241 | −0.0222 | 0.0266 | 0.0044 | 0.0439 | −4.5893 |
| DMA3HF-CN-enol-S1 | 0.0304 | 0.1168 | −0.0253 | 0.0272 | 0.0020 | 0.0887 | −6.0393 |
| DMA3HF-CN-keto-S0 | 0.0294 | 0.1095 | −0.0238 | 0.0256 | 0.0018 | 0.0899 | −5.8163 |
| DMA3HF-CN-keto-S1 | 0.0262 | 0.1000 | −0.0207 | 0.0229 | 0.0022 | 0.0781 | −5.1024 |
| DMA3HF-NH2-enol-S0 | 0.0257 | 0.1035 | −0.0207 | 0.0233 | 0.0026 | 0.0704 | −4.9909 |
| DMA3HF-NH2-enol-S1 | 0.0323 | 0.1215 | −0.0272 | 0.0288 | 0.0016 | 0.0959 | −6.4632 |
| DMA3HF-NH2-keto-S0 | 0.0311 | 0.1137 | −0.0255 | 0.0270 | 0.0015 | 0.0969 | −6.1955 |
| DMA3HF-NH2-keto-S1 | 0.0270 | 0.1017 | −0.0214 | 0.0234 | 0.0020 | 0.0817 | −5.2809 |
| State | λabs (nm) | Contribution MO a | Strength f | |
|---|---|---|---|---|
| DMA3HF | S1 | 506.63 | (68.325%) H→L | 0.5655 |
| S2 | 369.48 | (56.740%) H→L + 1 (34.285%) H→L + 2 | 0.1037 | |
| S3 | 366.37 | (67.241%) H-1→L | 0.0431 | |
| S4 | 344.55 | (70.680%) H-2→L | 0.0000 | |
| S5 | 344.03 | (55.193%) H→L + 2 | 0.2958 | |
| S6 | 329.47 | (45.859%) H-3→L (46.421%) H→L + 3 | 0.0086 | |
| DMA3HF-CN | S1 | 546.80 | (67.535%) H→L | 0.5329 |
| S2 | 504.39 | (69.415%) H→L + 1 | 0.0359 | |
| S3 | 383.18 | (65.797%) H-1→L | 0.0241 | |
| S4 | 359.31 | (63.652%) H→L + 2 | 0.5336 | |
| S5 | 355.10 | (70.573%) H-2→L | 0.0000 | |
| S6 | 343.13 | (66.814%) H-3→L | 0.0040 | |
| DMA3HF-NH2 | S1 | 504.00 | (68.324%) H→L | 0.5836 |
| S2 | 445.18 | (67.881%) H-1→L | 0.0474 | |
| S3 | 353.16 | (47.579%) H-2→L (46.050%) H→L + 1 | 0.3820 | |
| S4 | 343.13 | (69.751%) H-3→L | 0.0051 | |
| S5 | 339.41 | (45.346%) H-2→L (45.640%) H→L + 1 | 0.1166 | |
| S6 | 336.45 | (58.989%) H→L + 2 (31.026%) H→L + 3 | 0.0147 |
| State | Eflu (eV) | λflu (nm) | Contribution MO a | Strength f | |
|---|---|---|---|---|---|
| DMA3HF-enol | S1 | 2.3264 | 532.94 | H→L (68.670%) | 0.6049 |
| DMA3HF-keto | S1 | 2.1147 | 586.30 | H→L (71.559%) | 0.7774 |
| DMA3HF-CN-enol | S1 | 2.1655 | 572.55 | H→L (68.886%) | 0.5805 |
| DMA3HF-CN-keto | S1 | 2.0561 | 603.01 | H→L (70.403%) | 0.7765 |
| DMA3HF-NH2-enol | S1 | 2.3439 | 528.97 | H→L (68.663%) | 0.6291 |
| DMA3HF-NH2-keto | S1 | 2.1173 | 585.59 | H→L (71.017%) | 0.7374 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Shang, C.; Cao, Y.; Cui, J.; Sun, C. A DFT/TD-DFT Study on the ESIPT-Type Flavonoid Derivatives with High Emission Intensity. Materials 2022, 15, 2896. https://doi.org/10.3390/ma15082896
Yu X, Shang C, Cao Y, Cui J, Sun C. A DFT/TD-DFT Study on the ESIPT-Type Flavonoid Derivatives with High Emission Intensity. Materials. 2022; 15(8):2896. https://doi.org/10.3390/ma15082896
Chicago/Turabian StyleYu, Xiangrui, Changjiao Shang, Yunjian Cao, Jingang Cui, and Chaofan Sun. 2022. "A DFT/TD-DFT Study on the ESIPT-Type Flavonoid Derivatives with High Emission Intensity" Materials 15, no. 8: 2896. https://doi.org/10.3390/ma15082896







