Synthesis, Structural, and Mechanical Behavior of β-Ca3(PO4)2–ZrO2 Composites Induced by Elevated Thermal Treatments
Abstract
:1. Introduction
2. Materials and Methods
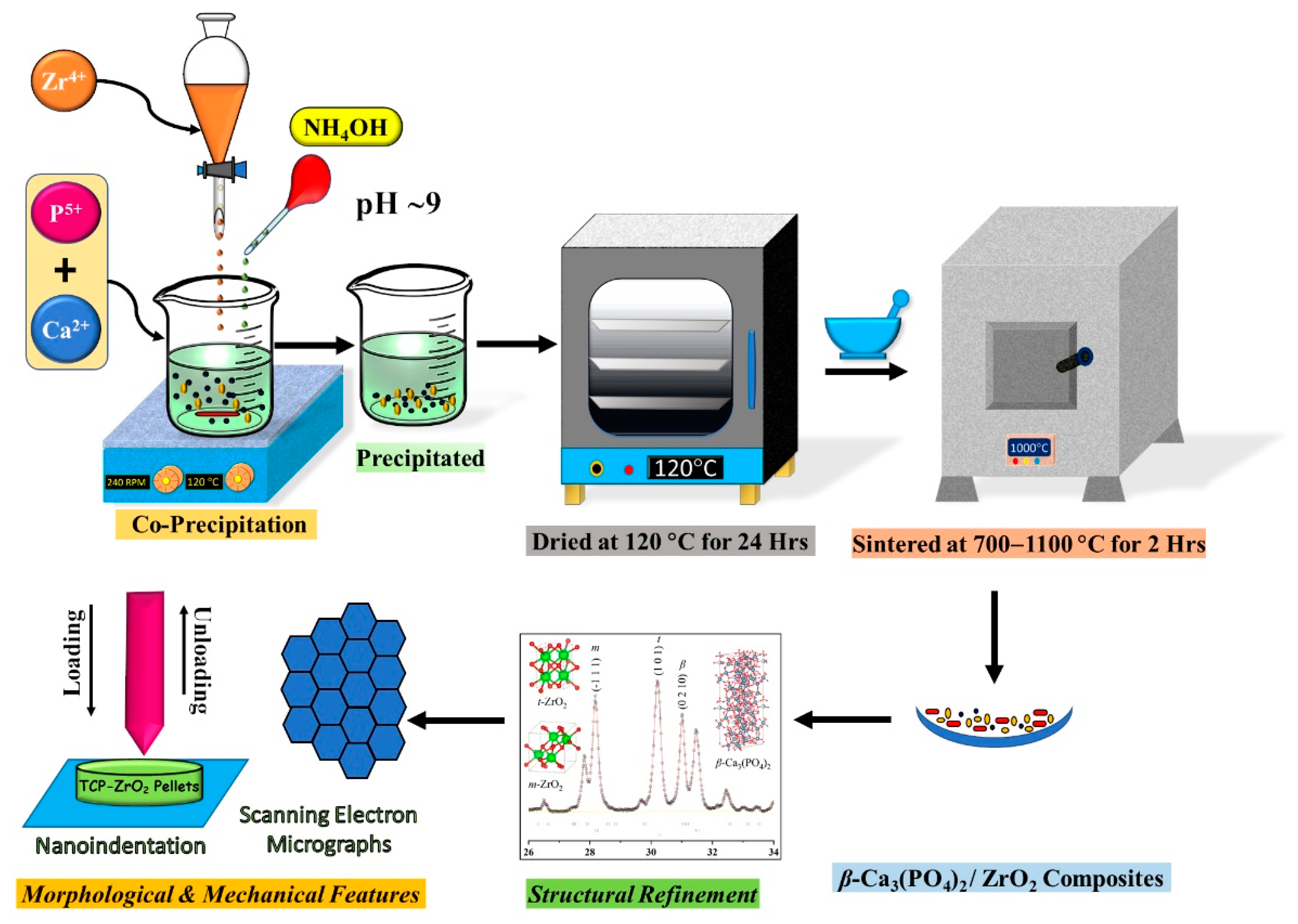
2.1. Powder Synthesis
2.2. Physiochemical Characterization
3. Results
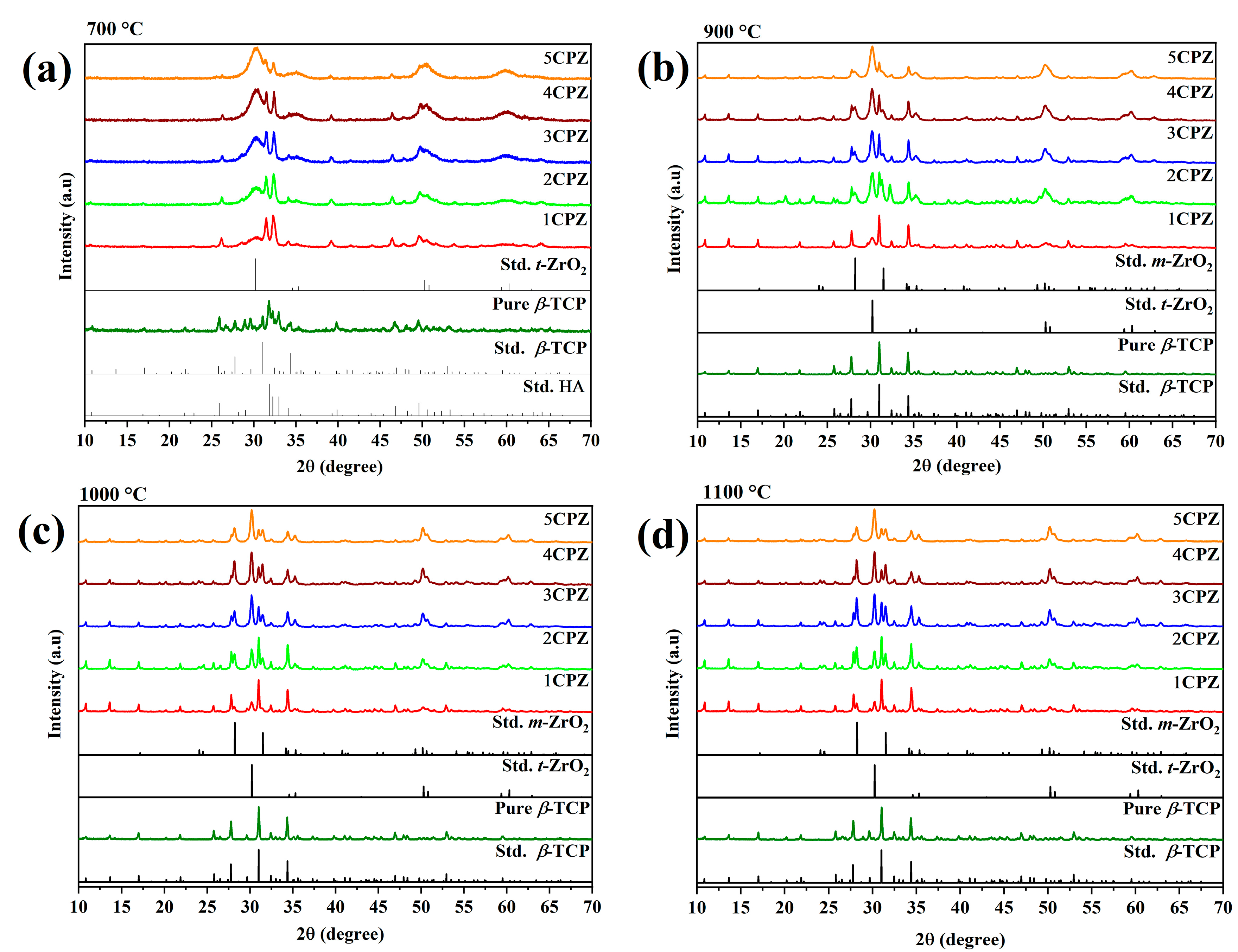
3.1. X-Ray Diffraction Analysis
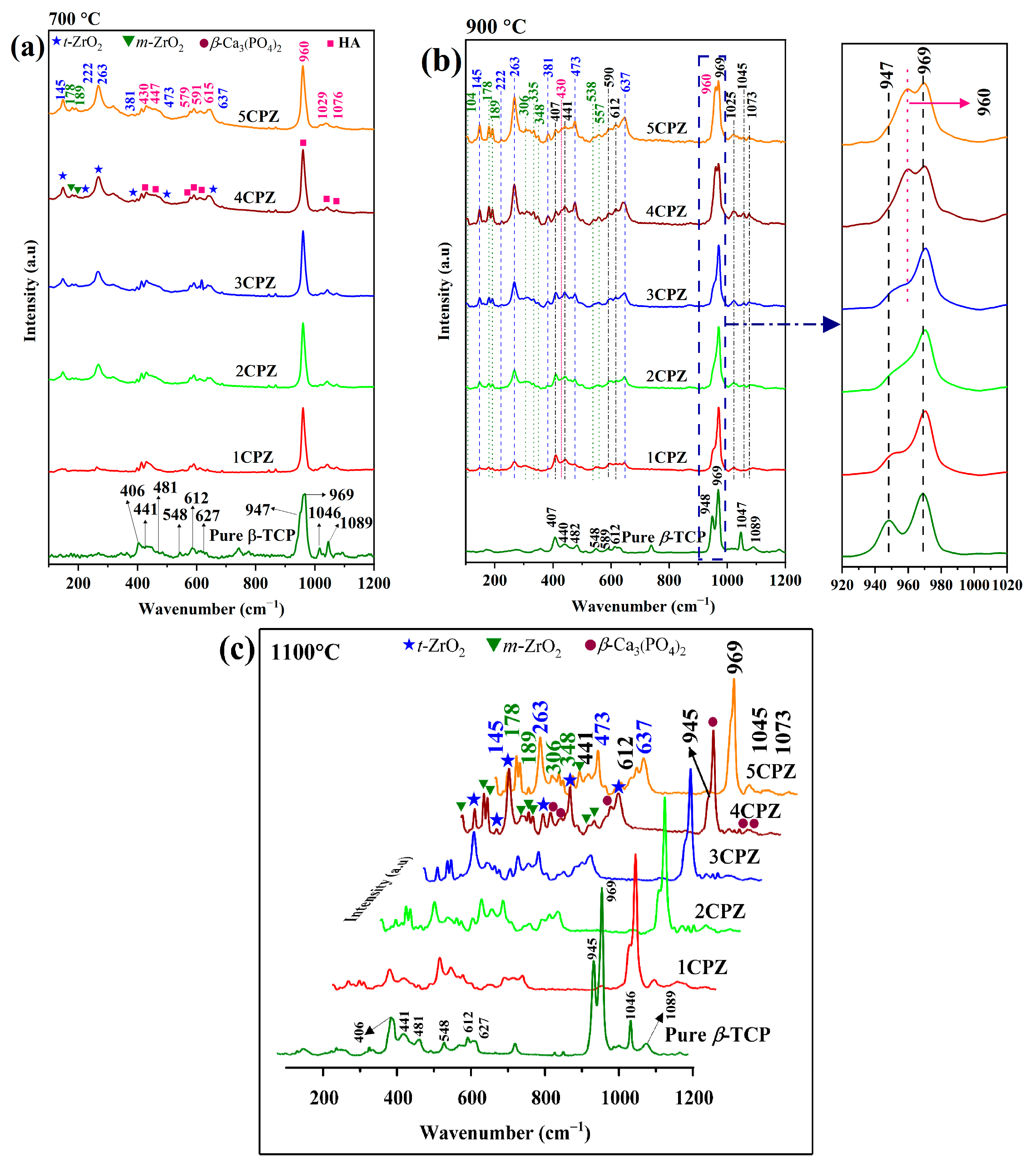
3.2. Raman Spectroscopy
3.3. Fourier-Transform Infrared Spectroscopy
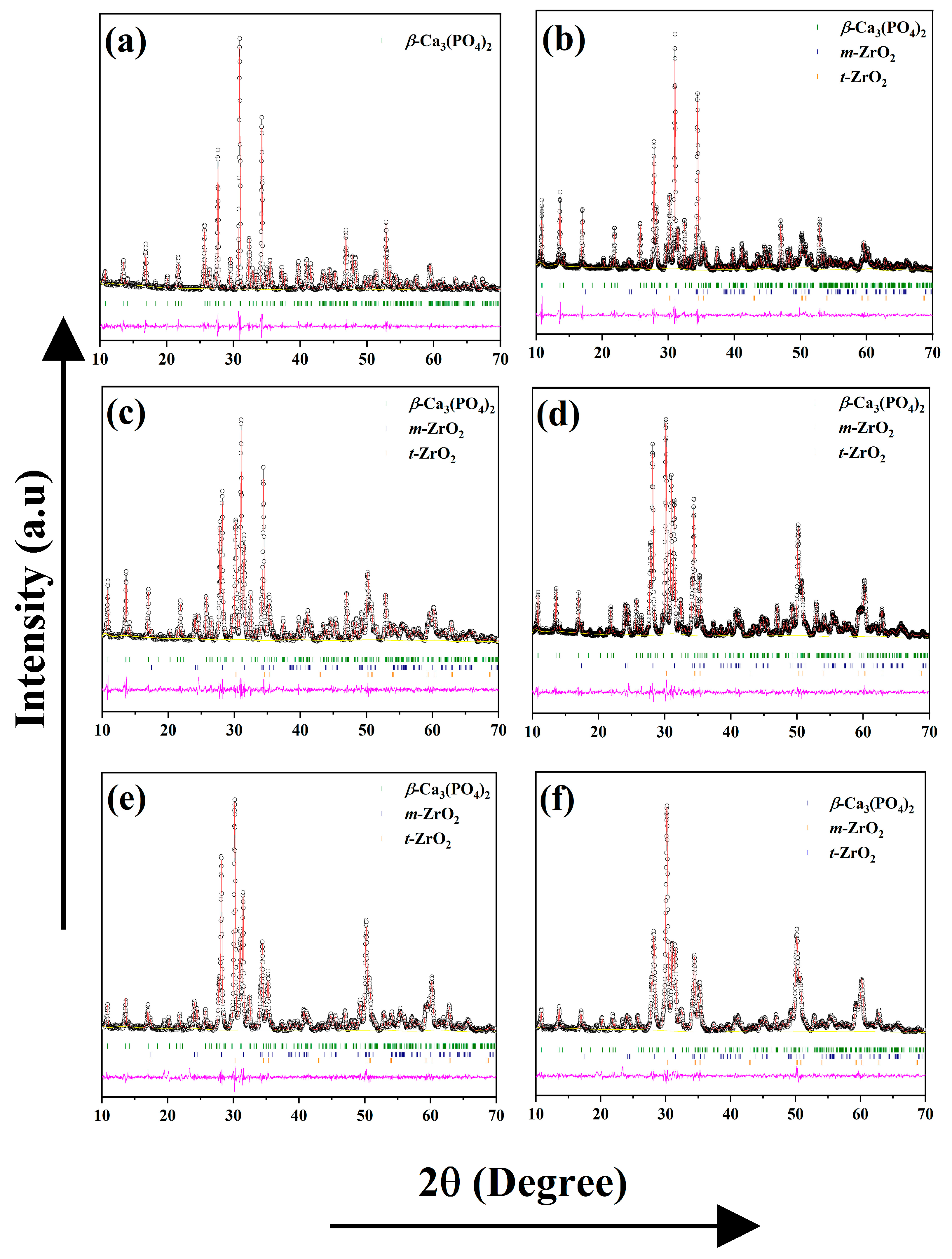
3.4. Rietveld Refinement
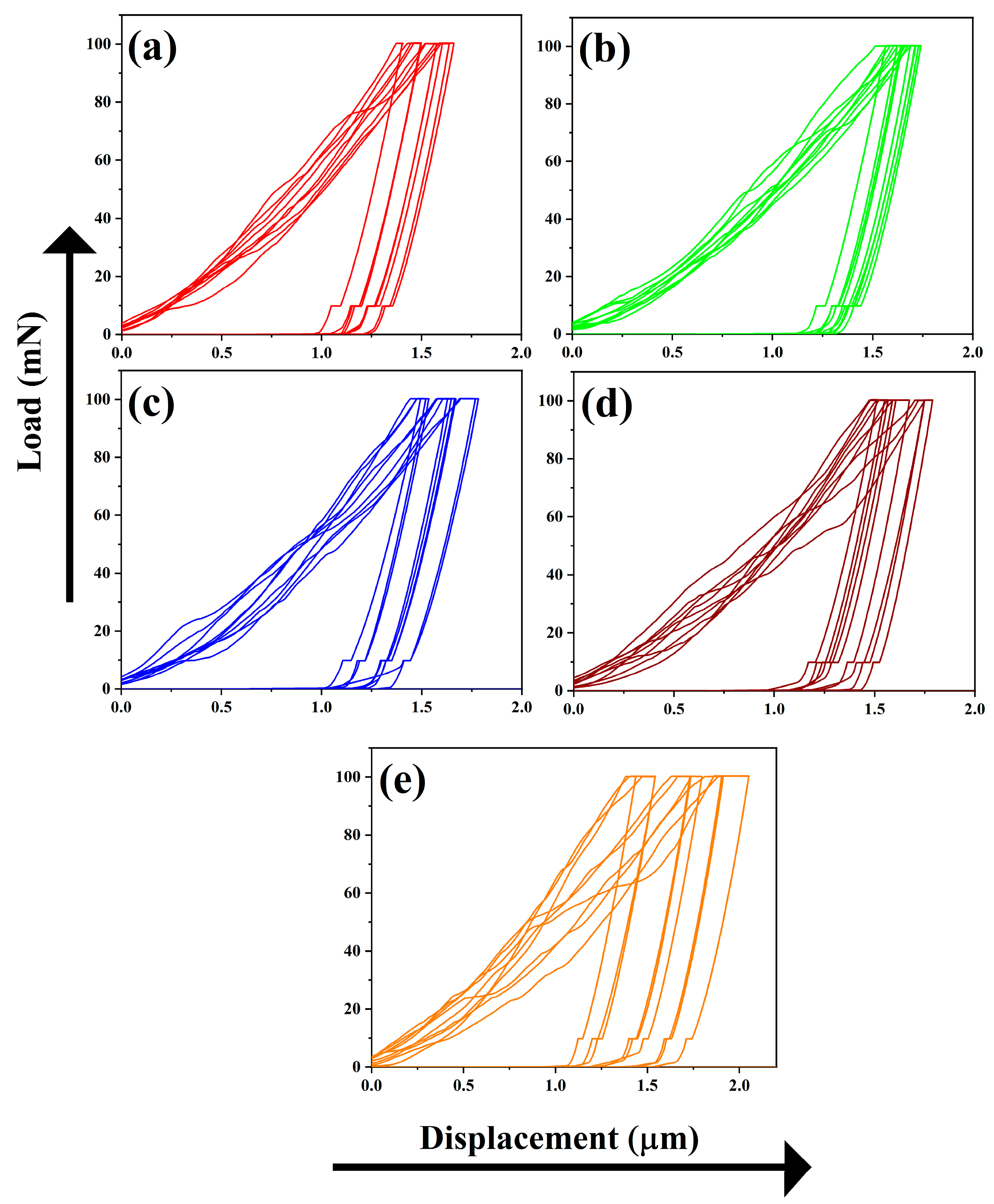
3.5. Morphological and Mechanical Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature List
| β-TCP (β-Ca3(PO4)2) | Beta-tricalcium phosphate |
| TCP | Tricalcium phosphate |
| ZrO2 | Zirconia |
| t-ZrO2 | Tetragonal zirconia |
| m-ZrO2 | Monoclinic zirconia |
| HA | Hydroxyapatite |
| CaO | Calcium oxide |
| CaZrO3 | Calcium zirconate |
| Ca4(PO4)2O | Tetracalcium phosphate |
| α-Ca3(PO4)2 | Alpha-tricalcium phosphate |
| Ca2+ | Calcium |
| P+5 | Phosphorus |
| Zr4+ | Zirconium |
| O2− | Oxygen |
| Y3+ | Yttrium |
| Gd3+ | Gadolinium |
| Dy3+ | Dysprosium |
| TiO2 | Titanium dioxide |
| Al2O3 | Aluminum oxide |
| CDA | Calcium deficient apatite |
| CIF | Crystallographic Information File |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared |
| GPa | Gigapascal |
References
- Wagner, W.R.; Sakiyama-Elbert., S.E.; Zhang, G.; Yaszemski, M.J. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Alvarez Echazu, M.I.; Perna, O.; Olivetti, C.E.; Antezana, P.E.; Municoy, S.; Tuttolomondo, M.V.; Galdoporpora, J.M.; Alvarez, G.S.; Olmedo, D.G.; Desimone, M.F. Recent Advances in Synthetic and Natural Biomaterials-Based Therapy for Bone Defects. Macromol. Biosci. 2022, E2100383. [Google Scholar] [CrossRef] [PubMed]
- Roohani, I.; Yeo, G.C.; Mithieux, S.M.; Weiss, A.S. Emerging concepts in bone repair and the premise of soft materials. Curr. Opin. Biotechnol. 2021, 74, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Trojani, M.C.; Santucci-Darmanin, S.; Breuil, V.; Carle, G.F.; Pierrefite-Carle, V. Autophagy and bone diseases. Jt. Bone Spine 2021, 89, 105301. [Google Scholar] [CrossRef] [PubMed]
- Silvio, L.D. Cellular Response to Biomaterials; Woodhead Publishing: Sawston, UK, 2009. [Google Scholar]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Chawla, K. Biomaterials for Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Tanzi, M.; Farè, S.; Candiani, G. Foundations of Biomaterials Engineering; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lee, H.; Yoo, J.M.; Ponnusamy, N.K.; Nam, S.Y. 3D-printed hydroxyapatite/gelatin bone scaffolds reinforced with graphene oxide: Optimized fabrication and mechanical characterization. Ceram. Int. 2022, 48, 10155–10163. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Ducheyne, P. Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1. [Google Scholar]
- Cao, Y.; Shi, T.; Jiao, C.; Liang, H.; Chen, R.; Tian, Z.; Zou, A.; Yang, Y.; Wei, Z.; Wang, C.; et al. Fabrication and properties of zirconia/hydroxyapatite composite scaffold based on digital light processing. Ceram. Int. 2020, 46, 2300–2308. [Google Scholar] [CrossRef]
- Wong, J.Y.; Bronzino, J.D.; Wong, J.Y.; Bronzino, J.D. Biomaterials; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering an Introduction, 10th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Alao, A.-R.; Yin, L. Loading rate effect on the mechanical behavior of zirconia in nanoindentation. Mater. Sci. Eng. A 2014, 619, 247–255. [Google Scholar] [CrossRef]
- Cao, Y.; Li, C.; Xia, Y.; Ren, K.; Zhu, S. Study on the fabrication and mechanical properties of HAp-3YSZ composites by flash sintering. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Shadianlou, F.; Foorginejad, A.; Yaghoubinezhad, Y. Fabrication of zirconia/reduced graphene oxide/hydroxyapatite scaffold by rapid prototyping method and its mechanical and biocompatibility properties. Ceram. Int. 2022, 48, 7031–7044. [Google Scholar] [CrossRef]
- Brzezińska-Miecznik, J.; Haberko, K.; Sitarz, M.; Bućko, M.M.; Macherzyńska, B.; Lach, R. Natural and synthetic hydroxyapatite/zirconia composites: A comparative study. Ceram. Int. 2016, 42, 11126–11135. [Google Scholar] [CrossRef]
- Kumar, V.A.; Rama Murty Raju, P.; Ramanaiah, N.; Siriyala, R. Effect of ZrO2 content on the mechanical properties and microstructure of HAp/ZrO2 nanocomposites. Ceram. Int. 2018, 44, 10345–10351. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Tranquillo, E.; Tuffi, R.; Dell’Era, A.; Ciprioti, S.V. Morphological and thermal characterization of zirconia/hydroxyapatite composites prepared via sol-gel for biomedical applications. Ceram. Int. 2019, 45, 2835–2845. [Google Scholar] [CrossRef]
- Caroff, F.; Oh, K.S.; Famery, R.; Boch, P. Sintering of TCP-TiO2 biocomposites: Influence of secondary phases. Biomaterials 1998, 19, 1451–1454. [Google Scholar] [CrossRef]
- Castkova, K.; Hadraba, H.; Matousek, A.; Roupcova, P.; Chlup, Z.; Novotna, L.; Cihlar, J. Synthesis of Ca,Y-zirconia/hydroxyapatite nanoparticles and composites. J. Eur. Ceram. Soc. 2016, 36, 2903–2912. [Google Scholar] [CrossRef]
- Nandha Kumar, P.; Kannan, S. Sequential elucidation of the beta-Ca3(PO4)2/TiO2 composite development from the solution precursors. Dalton Trans. 2017, 46, 3229–3239. [Google Scholar] [CrossRef] [PubMed]
- Nandha Kumar, P.; Ferreira, J.M.F.; Kannan, S. Phase transition mechanisms involved in the formation of structurally stable β -Ca3(PO4)2 -α-Al2O3 composites. J. Eur. Ceram. Soc. 2017, 37, 2953–2963. [Google Scholar] [CrossRef]
- Nandha Kumar, P.; Ferreira, J.M.; Kannan, S. Formation Mechanisms in beta-Ca3(PO4)2-ZnO Composites: Structural Repercussions of Composition and Heat Treatments. Inorg. Chem. 2017, 56, 1289–1299. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. American Mineralogist 2003, 88, 247–250. [Google Scholar]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P. Rietveld refinement of the crystallographic structure of human dental enamel apatites. Am. Mineral. 1999, 84, 1406–1414. [Google Scholar] [CrossRef]
- Howard, C.J.; Hill, R.J.; Reichert, B.E. Structures of ZrO2 polymorphs at room temperature by high-resolution neutron powder diffraction. Acta Crystallogr. Sect. B Struct. Sci. 1988, 44, 116–120. [Google Scholar] [CrossRef]
- Smith, D.K.; Newkirk, W. The crystal structure of baddeleyite (monoclinic ZrO2) and its relation to the polymorphism of ZrO2. Acta Crystallogr. 1965, 18, 983–991. [Google Scholar] [CrossRef]
- de Aza, P.N.; Santos, C.; Pazo, A.; de Aza, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 1. Raman Spectrum of β-Tricalcium Phosphate. Chem. Mater. 1997, 9, 912–915. [Google Scholar] [CrossRef]
- de Aza, P.N.; Guitián, F.; Santos, C.; de Aza, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 2. Comparison between Hydroxyapatite and β-Tricalcium Phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar] [CrossRef]
- Ishigame, M.; Sakurai, T. Temperature Dependence of the Raman Spectra of ZrO2. J. Am. Ceram. Soc. 1977, 60, 367–369. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]
- Aktug, S.L.; Kutbay, I.; Usta, M. Characterization and formation of bioactive hydroxyapatite coating on commercially pure zirconium by micro arc oxidation. J. Alloys Compd. 2017, 695, 998–1004. [Google Scholar] [CrossRef]
- Gibson, I.R.; Rehman, I.; Best, S.M.; Bonfield, W. Characterization of the transformation from calcium-deficient apatite to beta-tricalcium phosphate. J. Mater. Sci. Mater. Med. 2000, 11, 533–539. [Google Scholar] [CrossRef]
- Mortier, A.; Lemaitre, J.; Rodrique, L.; Rouxhet, P.G. Synthesis and thermal behavior of well-crystallized calcium-deficient phosphate apatite. J. Solid State Chem. 1989, 78, 215–219. [Google Scholar] [CrossRef]
- Yoshida, K.; Hyuga, H.; Kondo, N.; Kita, H.; Sasaki, M.; Mitamura, M.; Hashimoto, K.; Toda, Y. Substitution Model of Monovalent (Li, Na, and K), Divalent (Mg), and Trivalent (Al) Metal Ions for beta-Tricalcium Phosphate. J. Am. Ceram. Soc. 2006, 89, 688–690. [Google Scholar] [CrossRef]
- Meenambal, R.; Nandha Kumar, P.; Poojar, P.; Geethanath, S.; Kannan, S. Simultaneous substitutions of Gd3+ and Dy3+ in β-Ca3(PO4)2 as a potential multifunctional bio-probe. Mater. Des. 2017, 120, 336–344. [Google Scholar] [CrossRef]
- Nandha Kumar, P.; Mishra, S.K.; Kannan, S. Structural Perceptions and Mechanical Evaluation of beta-Ca3(PO4)2/c-CeO2 Composites with Preferential Occupancy of Ce3+ and Ce4+. Inorg. Chem. 2017, 56, 3600–3611. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.N.; Kannan, S.; Ferreira, J.M.F. Combined Occupancy of Gadolinium at the Lattice Sites of β-Ca3(PO4)2 and t-ZrO2 Crystal Structures. Eur. J. Inorg. Chem. 2020, 2020, 1163–1171. [Google Scholar] [CrossRef]
- Nandha Kumar, P.; Subramanian, S.; Vijayalakshmi, U.; Kannan, S. Simultaneous accommodation of Dy3+ at the lattice site of β-Ca3(PO4)2 and t-ZrO2 mixtures: Structural stability, mechanical, optical, and magnetic features. J. Am. Ceram. Soc. 2020, 103, 3528–3540. [Google Scholar] [CrossRef]
- Heimann, R.B.; Vu, T.A. Effect of CaO on thermal decomposition during sintering of composite hydroxyapatite–zirconia mixtures for monolithic bioceramic implants. J. Mater. Sci. Lett. 1997, 16, 437–439. [Google Scholar] [CrossRef]
- Kannan, S.; Rocha, J.H.G.; Ventura, J.M.G.; Lemos, A.F.; Ferreira, J.M.F. Effect of Ca/P ratio of precursors on the formation of different calcium apatitic ceramics—An X-ray diffraction study. Scr. Mater. 2005, 53, 1259–1262. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Dobelin, N. beta-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Gomes, S.; Nedelec, J.-M.; Jallot, E.; Sheptyakov, D.; Renaudin, G. Unexpected Mechanism of Zn2+ Insertion in Calcium Phosphate Bioceramics. Chem. Mater. 2011, 23, 3072–3085. [Google Scholar] [CrossRef]
- Emam, W.I.; Mabied, A.F.; Hashem, H.M.; Selim, M.M.; El-Shabiny, A.M.; Ahmed Farag, I.S. Controlling polymorphic structures and investigating electric properties of Ca-doped zirconia using solid state ceramic method. J. Solid State Chem. 2015, 228, 153–159. [Google Scholar] [CrossRef]
- Hengsberger, S.; Kulik, A.; Zysset, P. Nanoindentation discriminates the elastic properties of individual human bone lamellae under dry and physiological conditions. Bone 2002, 30, 178–184. [Google Scholar] [CrossRef]

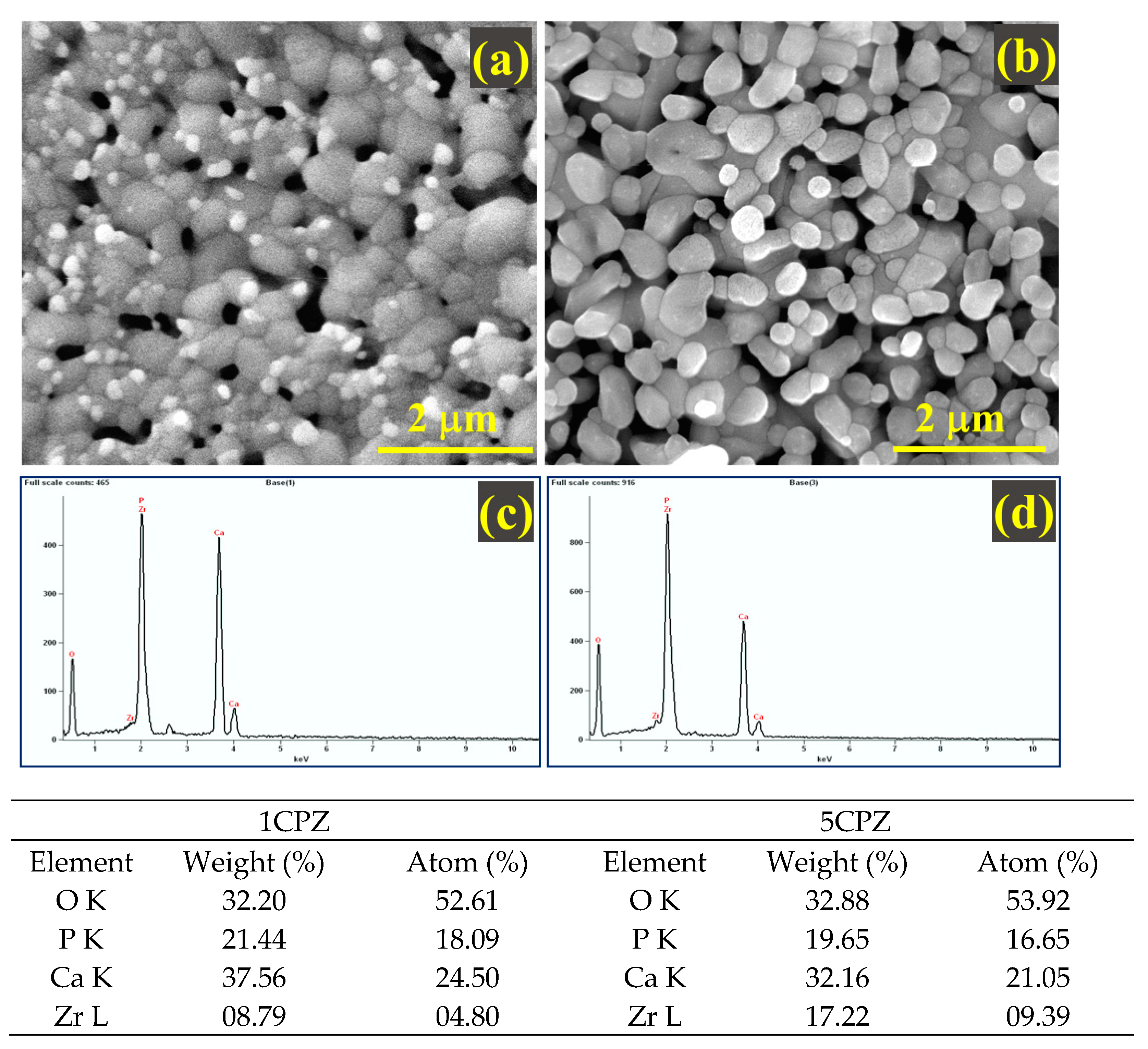
| Precursor Concentration in a Molar Ratio (mol L−1) | |||||
|---|---|---|---|---|---|
| Sample Code | Ca(NO3)2∙4H2O | NH4H2PO4 | ZrOCl2∙8H2O | Ca/P | (Ca + Zr)/P |
| Pure TCP | 1.0 M | 0.6667 | - | 1.50 | 1.500 |
| 1CPZ | 1.0 M | 0.6667 | 0.2 | 1.50 | 1.800 |
| 2CPZ | 1.0 M | 0.6667 | 0.4 | 1.50 | 2.100 |
| 3CPZ | 1.0 M | 0.6667 | 0.6 | 1.50 | 2.400 |
| 4CPZ | 1.0 M | 0.6667 | 0.8 | 1.50 | 2.700 |
| 5CPZ | 1.0 M | 0.6667 | 1.0 | 1.50 | 3.000 |
| Sample Code | Mineralogical Composition (Wt%) | Refinement Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca10(PO4)6(OH)2 | β-Ca3(PO4)2 | m-ZrO2 | t-ZrO2 | Rwp | Rp | χ2 | RBragg | ||
| 900 °C | 1CPZ | 12.67 (2) | 73.95 (5) | 05.30 (1) | 08.08 (2) | 10.95 | 08.51 | 02.12 | 05.13 |
| 2CPZ | 14.58 (3) | 57.86 (2) | 11.28 (2) | 16.28 (2) | 10.58 | 07.89 | 01.51 | 04.64 | |
| 3CPZ | 20.16 (4) | 44.16 (2) | 15.77 (2) | 19.91 (4) | 12.08 | 08.81 | 02.17 | 04.90 | |
| 4CPZ | 25.87 (6) | 29.96 (6) | 20.22 (2) | 23.95 (3) | 12.78 | 09.18 | 02.53 | 05.41 | |
| 5CPZ | 31.47 (8) | 18.50 (1) | 24.32 (8) | 28.03 (9) | 11.03 | 08.43 | 01.91 | 05.28 | |
| 1000 °C | 1CPZ | - | 83.78 (1) | 06.47 (1) | 09.75 (6) | 11.88 | 09.12 | 02.47 | 06.01 |
| 2CPZ | - | 69.31 (5) | 12.25 (3) | 18.44 (2) | 10.91 | 08.45 | 02.38 | 06.16 | |
| 3CPZ | 06.33 (2) | 55.16 (2) | 18.38 (1) | 20.13 (2) | 10.11 | 07.83 | 02.20 | 03.64 | |
| 4CPZ | 11.27 (3) | 39.46 (3) | 22.40 (2) | 26.87 (2) | 10.80 | 08.18 | 02.68 | 05.16 | |
| 5CPZ | 14.43 (6) | 27.98 (2) | 26.01 (1) | 31.58 (2) | 09.93 | 07.82 | 2.319 | 05.48 | |
| 1100 °C | Pure TCP | - | 100 | - | - | 07.15 | 08.44 | 1.114 | 04.29 |
| 1CPZ | - | 79.30 (3) | 07.74 (2) | 12.96 (2) | 11.72 | 09.06 | 02.40 | 06.09 | |
| 2CPZ | - | 66.03 (3) | 13.68 (2) | 20.29 (1) | 10.54 | 08.26 | 2.236 | 05.20 | |
| 3CPZ | - | 59.51 (2) | 15.97 (5) | 24.51 (2) | 08.68 | 06.76 | 1.642 | 05.03 | |
| 4CPZ | - | 42.49 (2) | 19.92 (6) | 25.03 (1) | 09.84 | 07.53 | 02.18 | 05.71 | |
| 5CPZ | - | 37.58 (6) | 26.81 (4) | 35.61 (5) | 08.77 | 06.70 | 1.855 | 03.80 | |
| Sample Code | Refined Lattice Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca10(PO4)6(OH)2 | β-Ca3(PO4)2 | m-ZrO2 | t-ZrO2 | ||||||
| a = b axis | c-axis | a = b axis | c-axis | a-axis | b-axis | c-axis | a = b axis | c-axis | |
| 900 °C | |||||||||
| 1CPZ | 9.5532 (3) | 6.8128 (3) | 10.4112 (5) | 37.4178 (2) | 5.1186 (2) | 5.1972 (4) | 5.2963 (4) | 3.5946 (5) | 5.1914 (1) |
| 2CPZ | 9.5893 (2) | 6.8037(3) | 10.4086 (8) | 37.3946 (3) | 5.1460 (2) | 5.2041 (2) | 5.3156 (2) | 3.5934 (4) | 5.1891 (8) |
| 3CPZ | 9.5924 (8) | 6.8199 (1) | 10.4101 (1) | 37.3925 (4) | 5.1504 (2) | 5.2047 (1) | 5.3159 (2) | 3.5924 (5) | 5.1909 (1) |
| 4CPZ | 9.5636 (5) | 6.7571 (6) | 10.4015 (2) | 37.3617 (7) | 5.1496 (2) | 5.1927 (1) | 5.3159 (1) | 3.5900 (5) | 5.1851 (1) |
| 5CPZ | 9.5465 (5) | 6.7788 (5) | 10.4016 (2) | 37.3638 (7) | 5.1504 (1) | 5.1988 (1) | 5.3139 (2) | 3.5940 (4) | 5.1817 (9) |
| 1100 °C | |||||||||
| Pure TCP | - | - | 10.4384 (3) | 37.4010 (1) | - | - | - | - | - |
| 1CPZ | - | - | 10.4203 (4) | 37.3877 (1) | 5.1481 (8) | 5.2092 (9) | 5.3124 (8) | 3.5948 (3) | 5.1868 (8) |
| 2CPZ | - | - | 10.4118 (4) | 37.3669 (2) | 5.1471 (4) | 5.2090 (5) | 5.3133 (5) | 3.5936 (2) | 5.1889 (6) |
| 3CPZ | - | - | 10.4078 (6) | 37.3630 (2) | 5.1476 (4) | 5.2083 (4) | 5.3135 (4) | 3.5940 (2) | 5.1864 (4) |
| 4CPZ | - | - | 10.4063 (7) | 37.3600 (3) | 5.1452 (4) | 5.2075 (5) | 5.3118(5) | 3.5938(2) | 5.1828 (4) |
| 5CPZ | - | - | 10.4023 (9) | 37.3582 (4) | 5.1465 (7) | 5.2048 (9) | 5.3137 (8) | 3.5935 (3) | 5.1827 (5) |
| Sample Code | Occupancy Factor | Nanoindentation Data | |
|---|---|---|---|
| Ca2+ (5) Sites | Young’s Modulus (GPa) | Hardness (GPa) | |
| 1CPZ | 0.85 (2) | 48.76 ± 02.51 | 01.79 ± 0.18 |
| 2CPZ | 0.72 (9) | 45.97 ± 01.38 | 01.59 ± 0.11 |
| 3CPZ | 0.84 (2) | 42.36 ± 02.08 | 01.51 ± 0.18 |
| 4CPZ | 0.81 (5) | 41.30 ± 04.09 | 01.36 ± 0.34 |
| 5CPZ | 0.91 (3) | 38.30 ± 02.75 | 01.30 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponnusamy, N.K.; Lee, H.; Yoo, J.M.; Nam, S.Y. Synthesis, Structural, and Mechanical Behavior of β-Ca3(PO4)2–ZrO2 Composites Induced by Elevated Thermal Treatments. Materials 2022, 15, 2924. https://doi.org/10.3390/ma15082924
Ponnusamy NK, Lee H, Yoo JM, Nam SY. Synthesis, Structural, and Mechanical Behavior of β-Ca3(PO4)2–ZrO2 Composites Induced by Elevated Thermal Treatments. Materials. 2022; 15(8):2924. https://doi.org/10.3390/ma15082924
Chicago/Turabian StylePonnusamy, Nandha Kumar, Hoyeol Lee, Jin Myoung Yoo, and Seung Yun Nam. 2022. "Synthesis, Structural, and Mechanical Behavior of β-Ca3(PO4)2–ZrO2 Composites Induced by Elevated Thermal Treatments" Materials 15, no. 8: 2924. https://doi.org/10.3390/ma15082924






