Biosynthesis and Response of Zinc Oxide Nanoparticles against Periimplantitis Triggering Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
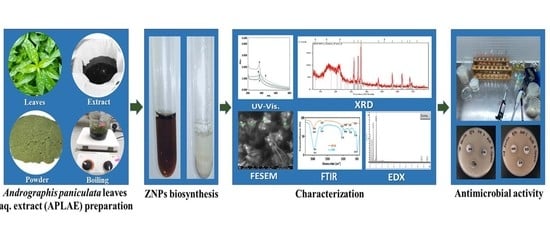
2.2. APLAE Preparation
2.3. ZnPs Biosynthesis
2.4. Optimization of ZnPs Biosynthesis
2.5. Stability of ZnPs
2.6. Antimicrobial Activity of ZnPs
2.7. Characterization of ZnPs
3. Results
3.1. Biosynthesis of ZnPs
3.2. Optimization of ZnPs Biosynthesis Parameters
3.2.1. Optimization of Zinc Acetate Concentration
3.2.2. Optimization of APLAE Volume
3.2.3. Optimization of pH
3.2.4. Optimization of Temperature
3.3. Stability of ZnPs
3.4. Characterization of ZnPs
3.4.1. Fourier Transformed Infrared (FTIR) Analysis
3.4.2. Field Emission Scanning Electron Microscopy (FESEM)
3.4.3. X-ray Diffraction (XRD) Analysis
3.4.4. Energy Dispersive X-ray Diffraction (EDX) Analysis
3.5. Antimicrobial Activity of ZnPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pokrowiecki, R.; Mielczarek, A.; Zaręba, T.; Tyski, S. Oral microbiome and peri-implant diseases: Where are we now? Ther. Clin. Risk Manag. 2017, 13, 1529–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardila Medina, C.M.; Villa-Correa, Y.A. Gram-negative enteric rods associated to early implant failure and peri-implantitis: Case report and systematic literature review. Int. J. Odontostomat. 2015, 9, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steves, G.M.; Esteves, J.; Resende, M.; Mendes, L.; Azevedo, A.S. Antimicrobial and Antibiofilm Coating of Dental Implants—Past and New Perspectives. Antibiotics 2022, 11, 235. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Salinas, J.; Sánchez-Siles, M.; Pato-Mourelo, J.; Cotrina-Veizaga, B.D.; Ortega, N. Synergistic antimicrobial effect of photodynamic therapy and chitosan on the titanium-adherent biofilms of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa: An in vitro study. J. Periodontol. 2021. [Google Scholar] [CrossRef]
- Hang, C.Z.; Fuloria, N.K.; Hong, O.J.; Kim, C.B.; Ting, B.Y.S.; Ru, C.S.; Ko, M.Y.; Fuloria, S. Biosynthesis of DLLAE blended silver nanoparticles and their response against periodontitis triggering bacteria. Int. J. Res. Pharm. Sci. 2020, 11, 1849–1856. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Fuloria, N.K.; Fuloria, S.; YikChia, K.; Karupiah, S.; Sathasivam, K. Response of green synthesized drug blended silver nanoparticles against periodontal disease triggering pathogenic microbiota. J. Appl. Biol. Biotechnol. 2019, 7, 4–6. [Google Scholar] [CrossRef]
- Sayed, M.E.; Mugri, M.H.; Almasri, M.A.; Al-Ahmari, M.M.; Bhandi, S.; Madapusi, T.B.; Varadarajan, S.; Raj, A.T.; Reda, R.; Testarelli, L.; et al. Role of Stem Cells in Augmenting Dental Implant Osseointegration: A Systematic Review. Coatings 2021, 11, 1035. [Google Scholar] [CrossRef]
- Teragundi, A.; Nanjundeswaraswamy, D. Literature review on synthesis of ZnO nano particles using natural and synthetic methods. Int. J. Res. Sci. Innov. 2018, 5, 67–71. [Google Scholar]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.-M. Green approach for synthesis of zinc oxide nanoparticles from andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess. Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Hanumith, S.; Abirami, S.; Velrajan, M. Biosynthesis of zinc oxide nanoparticles potentiates anticancer and antimicrobial activity. IJTSRD 2018, 2, 1797–1802. [Google Scholar]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Vargas-Reus, M.A.; Memarzadeh, K.; Huang, J.; Ren, G.G.; Allaker, R.P. antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int. J. Antimicrob. Agents 2012, 40, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, E.H.; Memarzadeh, K.; Allaker, R.P.; Huang, J.; Pratten, J.; Spratt, D. Anti-Biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jiménez, R.A.; Panecatl-Bernal, Y.; Carrillo-López, J.; Méndez-Rojas, M.Á.; Romero-López, A.; Pacio-Castillo, M.; Vivaldo, I.; Morales-Sánchez, A.; Arce, R.D.; Caram, J.; et al. Influence of ethanolic plant extracts on morphology and size distribution of sol-gel prepared TiO2 nanoparticles. Chem. Sel. 2021, 6, 3958–3968. [Google Scholar]
- Hessien, M.; Da’na, E.; Taha, A. Phytoextract assisted hydrothermal synthesis of ZnO–NiO nanocomposites using neem leaves extract. Ceram. Int 2021, 47, 811–816. [Google Scholar] [CrossRef]
- Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic ZnO nanoparticles synthesized using a novel plant extract: Application to enhance physiological and biochemical traits in maize. Nanomaterials 2021, 11, 1270. [Google Scholar] [CrossRef]
- Ahmed, S.; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Cecilia, S.; Divyarani, S.; Lakshya, K. Preparation of silver nano particles using aqueous solution of Ocimum sanctum and Piper betle and evaluation of its antimicrobial activity against Enterococcus Faecalis. Int. J. Pharm. Clin. Res. 2016, 8, 1118–1120. [Google Scholar]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int 2012, 22, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol 2004, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour. Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Hussein, M.Z.B.; Taufiq-Yap, Y.H. Synthesis and characterization of ZnO nanostructures using palm olein as biotemplate. Chem. Cent. J. 2013, 7, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinni, S.V.; Gopinath, S.C.B.; Anbu, P.; Fuloria, N.K.; Fuloria, S.; Mariappan, P.; Krusnamurthy, K.; Veeranjaneya Reddy, L.; Ramachawolran, G.; Sreeramanan, S.; et al. Characterization and antibacterial response of silver nanoparticles biosynthesized using an ethanolic extract of Coccinia indica leaves. Crystals 2021, 11, 97. [Google Scholar] [CrossRef]
- Anzlovar, A.; Crnjak Orel, Z.; Kogej, K. Polyol-mediated synthesis of zinc oxide nanorods and nanocomposites with poly (methyl methacrylate). J. Nanomater. 2012, 2012, 760872. [Google Scholar] [CrossRef] [Green Version]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2014, 5, 4993–5003. [Google Scholar] [CrossRef]
- Priyatharesini, P.I.; Ganesamoorthy, R.; Sudha, R. Synthesis of zinc oxide nanoparticle using Cocos Nucifera male flower extract and analysis their antimicrobial activity. Res. J. Pharm. Technol. 2020, 13, 2151–2154. [Google Scholar] [CrossRef]
- Hossain, M.S.; Urbi, Z.; Sule, A.; Hafizur Rahman, K.M. Andrographis Paniculata (Burm. f.) Wall. Ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014, 2014, 274905. [Google Scholar] [CrossRef] [Green Version]
- Fakhari, S.; Jamzad, M.; Fard, H.K. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, F.M.; Ghasemi, N. Influence of temperature and concentration on biosynthesis and characterization of zinc oxide nanoparticles using cherry extract. J. Nanostruct. Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Fatehah, M.O.; Aziz, H.A.; Stoll, S. Stability of ZnO nanoparticles in solution. Influence of pH, dissolution, aggregation and disaggregation effects. J. Colloid Sci. Biotechnol. 2014, 3, 75–84. [Google Scholar] [CrossRef]
- Chikkanna, M.M.; Neelagund, S.E.; Rajashekarappa, K.K. Green synthesis of zinc oxide nanoparticles (ZnO NPs) and their biological activity. SN Appl. Sci. 2018, 1, 117. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.H.; Shah, M.; Hashmi, S.S.; Nazir, M.; Naz, S.; Ahmad, W.; Khan, I.U.; Hano, C. Green bio-assisted synthesis, characterization and biological evaluation of biocompatible ZnO NPs Synthesized from different tissues of milk thistle (Silybum marianum). Nanomaterials 2019, 9, 1171. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, V.; Gusain, D.; Sharma, Y.C. Synthesis, characterization and application of zinc oxide nanoparticles(n-ZnO). Ceram. Int. 2013, 39, 9803–9808. [Google Scholar] [CrossRef]
- Malahubban, M.; Alimon, A.R.; Sazili, A.Q.; Fakurazi, S.; Zakry, F.A. Phytochemical analysis of Andrographis Paniculata and Orthosiphon stamineus leaf extracts for their antibacterial and antioxidant potential. Trop. Biomed. 2013, 30, 467–480. [Google Scholar]
- Banoee, M.; Seif, S.; Nazari, Z.E.; Jafari-Fesharaki, P.; Shahverdi, H.R.; Moballegh, A.; Moghaddam, K.M.; Shahverdi, A.R. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize zinc oxide nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, A.; Kumar, N.; Kumar, R.; Salar, R.K. Antimicrobial activity of zinc oxide nanoparticles synthesized from Aloe Vera peel extract. SN Appl. Sci. 2019, 1, 136. [Google Scholar] [CrossRef] [Green Version]
- Soltanian, S.; Sheikhbahaei, M.; Mirtadzadini, M.; Khandani, B.K. Evaluation of anticancer, antioxidant and antibacterial properties of methanol extract of three Acantholimon Boiss. species. Avicenna J. Phytomed. 2020, 10, 641–652. [Google Scholar] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO Nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, K.S.; Ur Rahman, A.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Yan, M.; Majd, M.H. Evaluation of Induced Apoptosis by Biosynthesized Zinc Oxide Nanoparticles in MCF-7 Breast Cancer Cells Using Bak1 and Bclx Expression. Dokl. Biochem. Biophys. 2021, 500, 360–367. [Google Scholar] [CrossRef]
- Fan, P.; Yang, C.; Wang, L.; Wang, Q.; Zhang, Y.; Zhou, J.; Weng, J.; Feng, B. ZnO nanoparticles stimulate oxidative stress to induce apoptosis of B16F10 melanoma cells: In vitro and in vivo studies. Biomed. Phys. Eng. Express 2021, 20, 7. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, M.H.M. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [Green Version]
- Ifeanyichukwu, U.L.; Fayemi, O.E.; Ateba, C.N. Green synthesis of zinc oxide nanoparticles from pomegranate (Punica granatum) extracts and characterization of their antibacterial activity. Molecules 2020, 25, 4521. [Google Scholar] [CrossRef]





| 2θ | hkl | FWHM (β) | D (nm) |
|---|---|---|---|
| 31.70 | 100 | 0.9890 | 87.18 |
| 34.33 | 002 | 0.9700 | 88.89 |
| 36.17 | 101 | 0.9971 | 86.17 |
| 47.45 | 102 | 0.9163 | 98.14 |
| 56.52 | 110 | 0.9515 | 98.97 |
| 62.78 | 103 | 0.9859 | 98.56 |
| 69.02 | 201 | 0.9850 | 98.65 |
| Microorganism | Zone of Inhibition in mm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZnPs | APLAE | Ciprofloxacin | |||||||||||
| Concentration (mg/mL) | 1.0 | 2.0 | 4.0 | 6.0 | 8.0 | 10.0 | 1.0 | 2.0 | 4.0 | 6.0 | 8.0 | 10.0 | 10 µg/mL |
| S. aureus | 16 ± 1 | 18 ± 1 | 18.5 ± 0.5 | 19 ± 2 | 19 ± 3 | 25 ± 1 | N/A | N/A | N/A | N/A | N/A | N/A | 21 ± 0.58 |
| E. coli | 8 ± 1 | 10 ± 0.57 | 15 ± 0.57 | 19 ± 1 | 20.5 ± 1.5 | 22 ± 0.57 | N/A | N/A | N/A | N/A | 15.5 ± 1.5 | 16 ± 0.57 | 20 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, B.Y.S.; Fuloria, N.K.; Subrimanyan, V.; Bajaj, S.; Chinni, S.V.; Reddy, L.V.; Sathasivam, K.V.; Karupiah, S.; Malviya, R.; Meenakshi, D.U.; et al. Biosynthesis and Response of Zinc Oxide Nanoparticles against Periimplantitis Triggering Pathogens. Materials 2022, 15, 3170. https://doi.org/10.3390/ma15093170
Ting BYS, Fuloria NK, Subrimanyan V, Bajaj S, Chinni SV, Reddy LV, Sathasivam KV, Karupiah S, Malviya R, Meenakshi DU, et al. Biosynthesis and Response of Zinc Oxide Nanoparticles against Periimplantitis Triggering Pathogens. Materials. 2022; 15(9):3170. https://doi.org/10.3390/ma15093170
Chicago/Turabian StyleTing, Bernice Yii Shu, Neeraj Kumar Fuloria, Vetriselvan Subrimanyan, Sakshi Bajaj, Suresh V. Chinni, Lebaka Veeranjaneya Reddy, Kathiresan V. Sathasivam, Sundram Karupiah, Rishabha Malviya, Dhanalekshmi Unnikrishnan Meenakshi, and et al. 2022. "Biosynthesis and Response of Zinc Oxide Nanoparticles against Periimplantitis Triggering Pathogens" Materials 15, no. 9: 3170. https://doi.org/10.3390/ma15093170