Si/SiO2/Al2O3 Supported Growth of CNT Forest for the Production of La/ZnO/CNT Photocatalyst for Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of CNT Forest
2.2. Characterization of Samples
2.3. Production of La/ZnO/CNTs Photocatalyst
2.4. Hydrogen Evolution Experiments
3. Results and Discussion
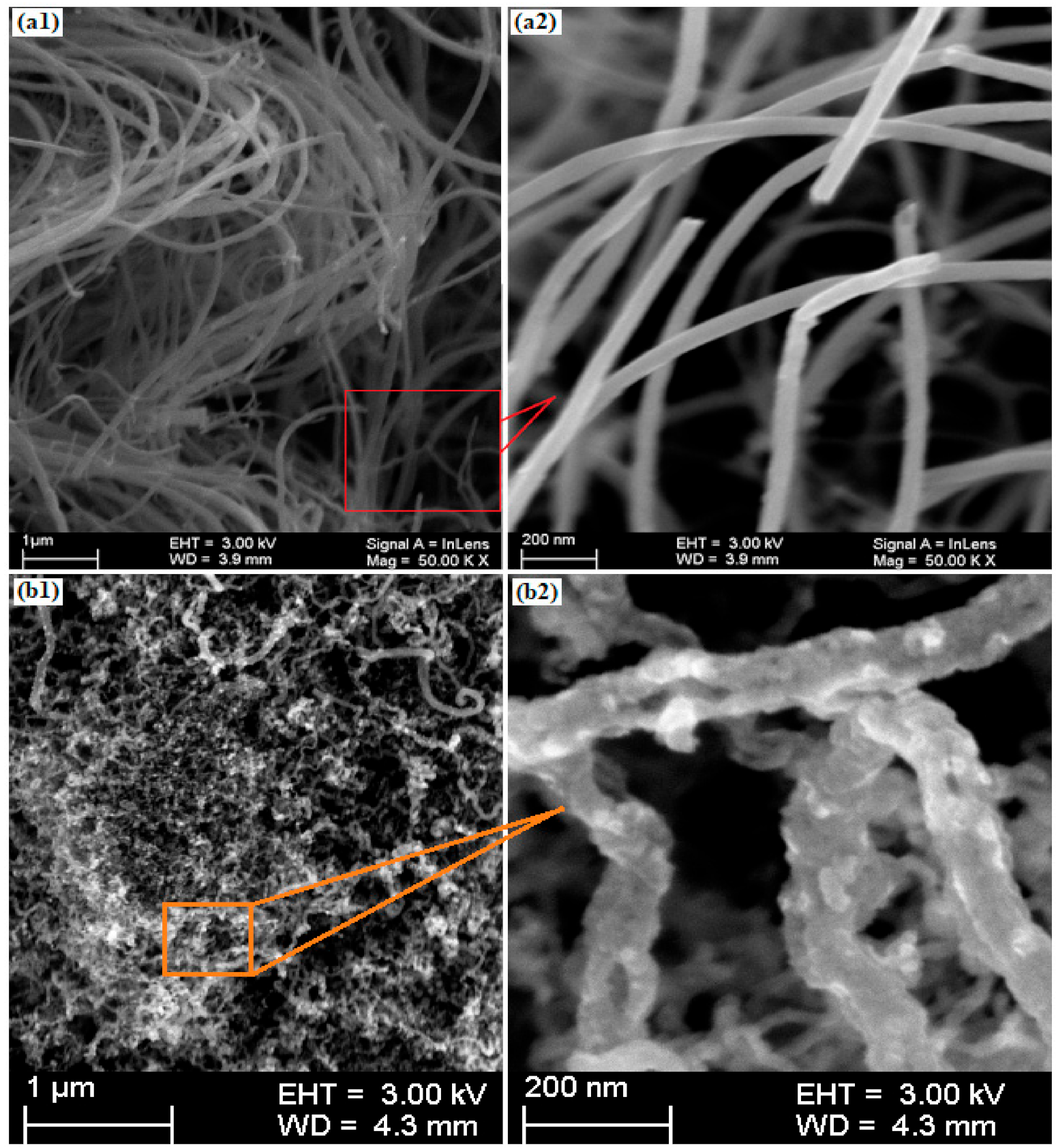
3.1. Structural Formation and Surface Morphology
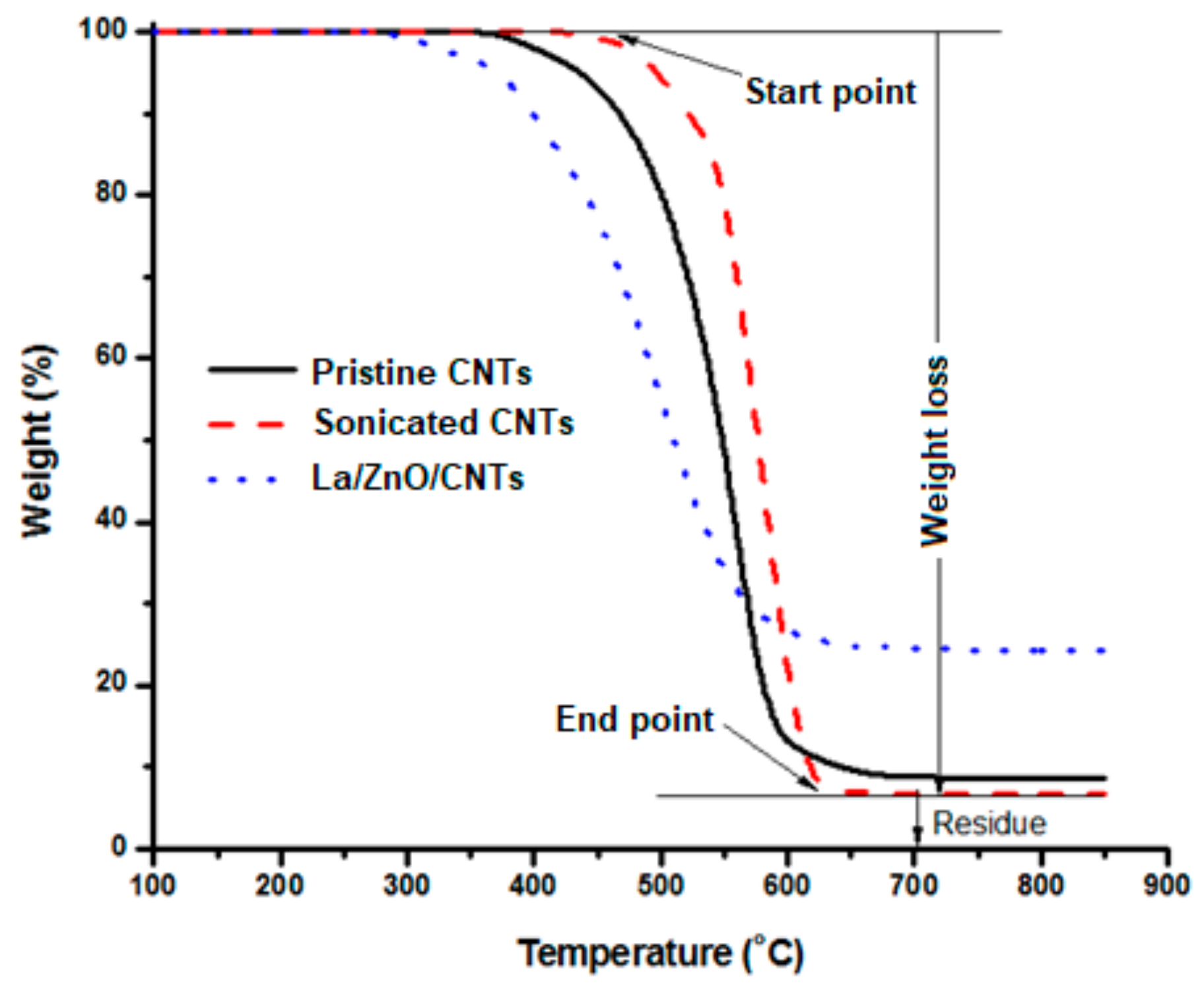
3.2. Structural Purity and Weight Loss Analysis
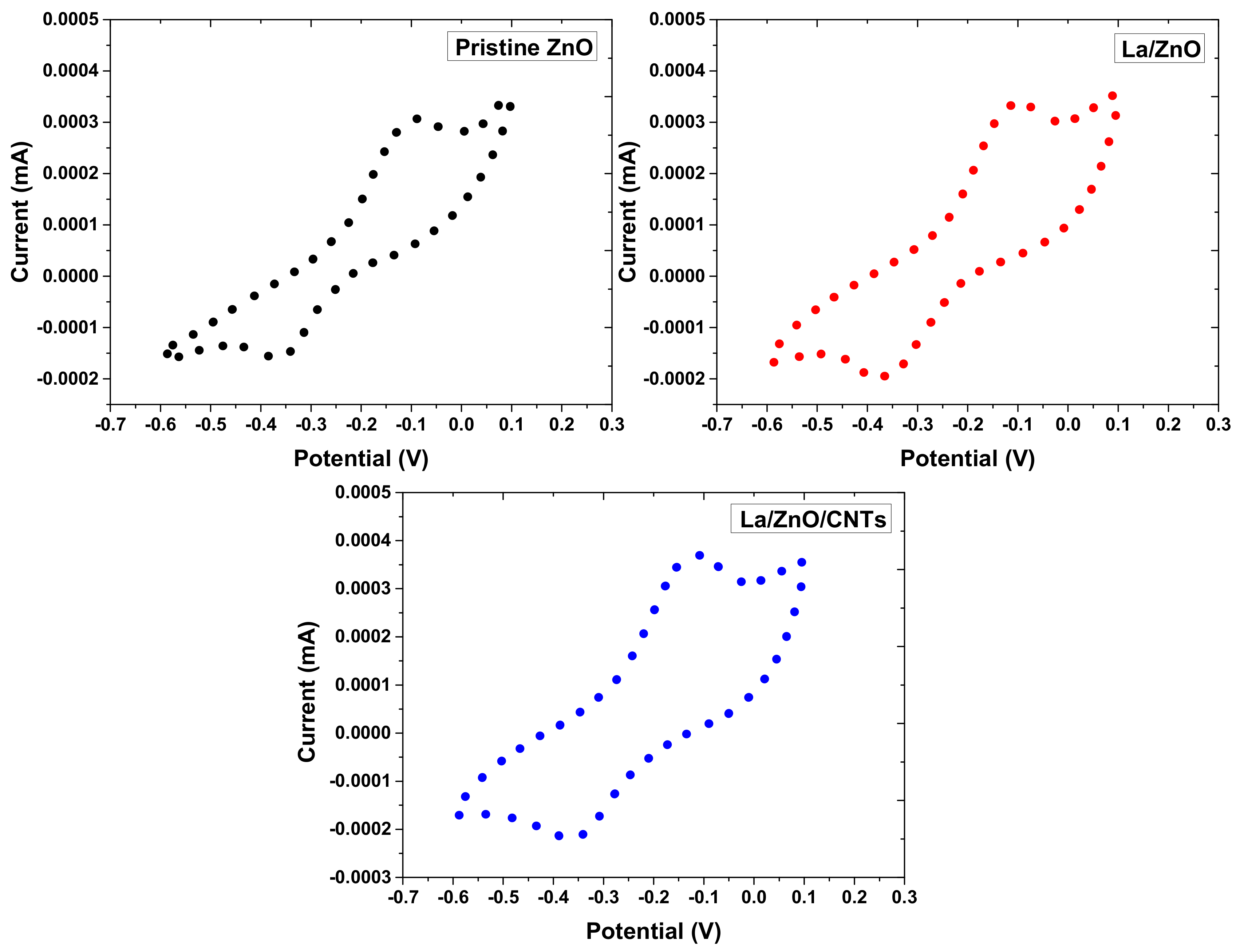
3.3. Cyclic Voltammetry of Photocatalyst
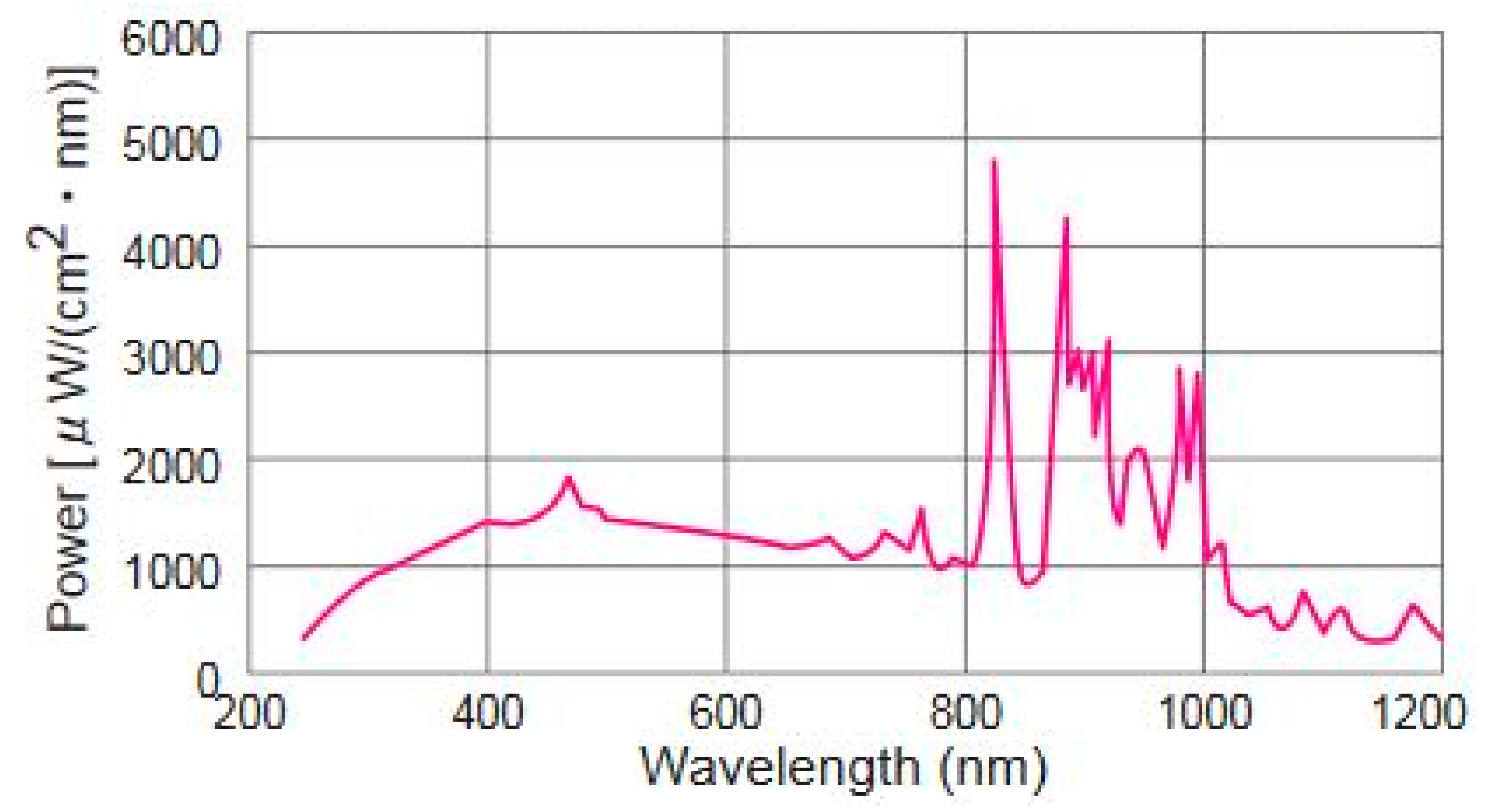
3.4. Hydrogen Evolution
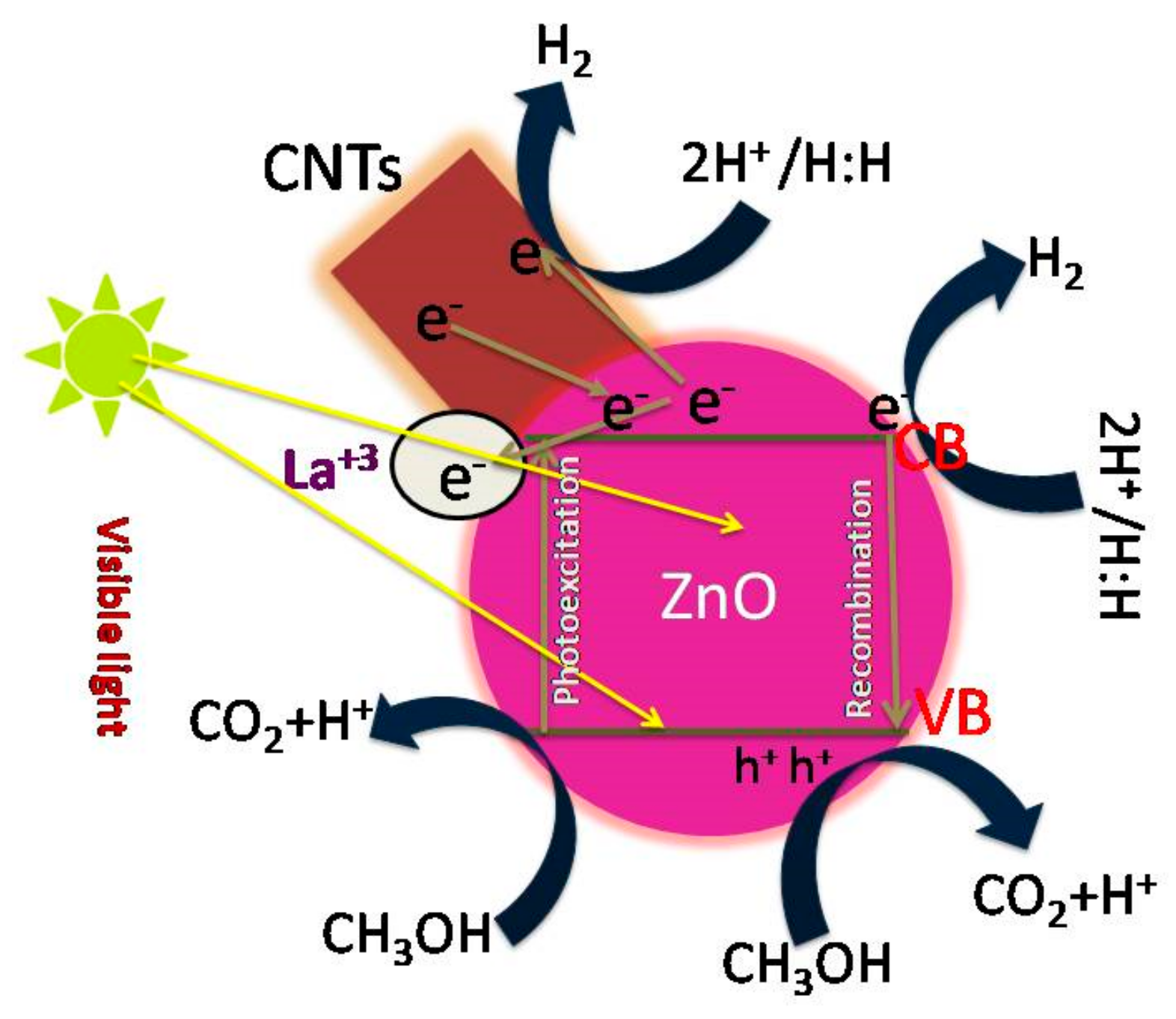
3.5. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spoială, A.; Ilie, C.I.; Trușcă, R.D.; Oprea, O.C.; Surdu, V.A.; Vasile, B.Ș.; Ficai, A.; Ficai, D.; Andronescu, E.; Dițu, L.M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef] [PubMed]
- Machín, A.; Cotto, M.; Duconge, J.; Arango, J.C.; Morant, C.; Pinilla, S.; Soto-Vázquez, L.; Resto, E.; Márquez, F. Hydrogen production via water splitting using different Au@ZnO catalysts under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 385–394. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.Y.; Ahmed, E.; Ahmad, M.; Akhtar, M.S.; Ullah, S.; Farooq, M.U.; Iqbal, S.; Assiri, M.A.; et al. Microwave-assisted one-pot hydrothermal synthesis of V and La co-doped ZnO/CNTs nanocomposite for boosted photocatalytic hydrogen production. Int. J. Hydrogen Energy 2022, 47, 15505–15515. [Google Scholar] [CrossRef]
- Shuai, H.; Wang, J.; Wang, X.; Du, G. Black Talc-Based TiO2/ZnO Composite for Enhanced UV-Vis Photocatalysis Performance. Materials 2021, 14, 6474. [Google Scholar] [CrossRef]
- Feng, Y.J.; Wang, Y.; Wang, K.W.; Ma, J.P.; Duan, Y.Y.; Liu, J.; Lu, X.; Zhang, B.; Wang, G.Y.; Zhou, X.Y. Ultra-fine Cu clusters decorated hydrangea-like titanium dioxide for photocatalytic hydrogen production. Rare Metals 2022, 41, 385–395. [Google Scholar] [CrossRef]
- Liao, Y.W.; Yang, J.; Wang, G.H.; Wang, J.; Wang, K.; Yan, S.D. Hierarchical porous NiO as a noble-metal-free cocatalyst for enhanced photocatalytic H2 production of nitrogen-deficient g-C3N4. Rare Metals 2022, 41, 396–405. [Google Scholar] [CrossRef]
- Oliveira, I.E.; Silva, R.M.; Girão, A.V.; Faria, J.L.; Silva, C.G.; Silva, R.F. Facile Preparation of ZnO/CNTs Nanocomposites via ALD for Photocatalysis Applications. Eur. J. Inorg. Chem. 2020, 2020, 1743–1750. [Google Scholar] [CrossRef]
- Gadisa, B.T.; Appiah-Ntiamoah, R.; Kim, H. In-situ derived hierarchical ZnO/Zn-C nanofiber with high photocatalytic activity and recyclability under solar light. Appl. Surf. Sci. 2019, 491, 350–359. [Google Scholar] [CrossRef]
- Toloman, D.; Popa, A.; Stan, M.; Stefan, M.; Vlad, G.; Ulinici, S.; Baisan, G.; Silipas, T.D.; Macavei, S.; Leostean, C.; et al. Visible-light-driven photocatalytic degradation of different organic pollutants using Cu doped ZnO-MWCNT nanocomposites. J. Alloys Compd. 2021, 866, 159010. [Google Scholar] [CrossRef]
- Elias, M.; Uddin, M.N.; Saha, J.K.; Hossain, M.A.; Sarker, D.R.; Akter, S.; Siddiquey, I.A.; Uddin, J. A Highly Efficient and Stable Photocatalyst; N-Doped ZnO/CNT Composite Thin Film Synthesized via Simple Sol-Gel Drop Coating Method. Molecules 2021, 26, 1470. [Google Scholar] [CrossRef]
- Teo, K.B.K.; Singh, C.; Chhowalla, M.; Milne, W.I. Catalytic synthesis of carbon nanotubes and nanofibers. Encycl. Nanosci. Nanotechnol. 2004, 1, 665–686. [Google Scholar]
- Lee, Y.T.; Kim, N.S.; Bae, S.Y.; Park, J.; Yu, S.C.; Ryu, H.; Lee, H.J. Growth of Vertically Aligned Nitrogen-Doped Carbon Nanotubes: Control of the Nitrogen Content over the Temperature Range 900−1100 °C. J. Phys. Chem. B 2003, 107, 12958–12963. [Google Scholar] [CrossRef]
- Shukrullah, S.; Naz, M.Y.; Mohamed, N.M.; Ibrahim, K.A.; Ghaffar, A.; AbdEl-Salam, N.M. Production of bundled CNTs by floating a compound catalyst in an atmospheric pressure horizontal CVD reactor. Results Phys. 2019, 12, 1163–1171. [Google Scholar] [CrossRef]
- Skudin, V.; Andreeva, T.; Myachina, M.; Gavrilova, N. CVD-Synthesis of N-CNT Using Propane and Ammonia. Materials 2022, 15, 2241. [Google Scholar] [CrossRef] [PubMed]
- Baddour, C.E.; Briens, C. Carbon Nanotube Synthesis: A Review. Int. J. Chem. React. Eng. 2005, 3, 1–22. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Miki-Yoshida, M.; Rendón, L.; Santiesteban, J.G. Catalytic growth of carbon microtubules with fullerene structure. Appl. Phys. Lett. 1993, 62, 202–204. [Google Scholar] [CrossRef]
- Pérez-Mendoza, M.; Vallés, C.; Maser, W.K.; Martínez, M.T.; Benito, A.M. Influence of molybdenum on the chemical vapour deposition production of carbon nanotubes. Nanotechnology 2005, 16, S224–S229. [Google Scholar] [CrossRef]
- Danafar, F.; Fakhru’l-Razi, A.; Salleh, M.A.M.; Biak, D.R.A. Fluidized bed catalytic chemical vapor deposition synthesis of carbon nanotubes—A review. Chem. Eng. J. 2009, 155, 37–48. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, W.; Zhang, Q.; Ning, G.; Luo, G.; Wang, Y.; Wang, D.; Wei, F. Synthesis of high-quality, double-walled carbon nanotubes in a fluidized bed reactor. Chem. Eng. Technol. 2009, 32, 73–79. [Google Scholar] [CrossRef]
- Lanzani, G.; Susi, T.; Ayala, P.; Jiang, T.; Nasibulin, A.G.; Bligaard, T.; Laasonen, K.; Kauppinen, E.I. Mechanism study of floating catalyst CVD synthesis of SWCNTs. Phys. Status Solidi B Basic Solid State Phys. 2010, 247, 2708–2712. [Google Scholar] [CrossRef]
- Hayashi, Y.; Iijima, T.; Miyake, M.; Satoh, M.; Rupesinghe, N.L.; Teo, K.B.K.; Tanmemura, M. Growth evolution of rapid grown aligned carbon nanotube forests without water vapor on Fe/Al2O3/SiO2/Si substrate. Diam. Relat. Mater. 2011, 20, 859–862. [Google Scholar] [CrossRef]
- Aguiar, M.R.; Verissimo, C.; Ramos, A.C.S.; Moshkalev, S.A.; Swart, J.W. Synthesis of Carbon Nanotubes and Nanofibers by Thermal CVD on SiO2 and Al2O3 Support Layers. J. Nanosci. Nanotechnol. 2009, 9, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.D.; Avramchuck, A.V.; Grapov, D.; Tan, C.W.; Tay, B.K.; Aditya, S.; Labunov, V. Enhanced Carbon Nanotubes Growth Using Nickel/Ferrocene-Hybridized Catalyst. ACS Omega 2017, 2, 6063–6071. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Kumar, A.; Sharma, G.; Vo, D.V.N.; Naushad, M. ZnO-based heterostructures as photocatalysts for hydrogen generation and depollution: A review. Environ. Chem. Lett. 2022, 20, 1047–1081. [Google Scholar] [CrossRef]
- Chang, C.J.; Huang, K.L.; Chen, J.K.; Chu, K.W.; Hsu, M.H. Improved photocatalytic hydrogen production of ZnO/ZnS based photocatalysts by Ce doping. J. Taiwan Inst. Chem. Eng. 2015, 55, 82–89. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, S.; Dong, Y.; Fu, Y.; Sun, H.; Yin, S.; Guo, X.; Qin, W. Hydrogen production from methanol aqueous solution by ZnO/Zn(OH)2 macrostructure photocatalysts. RSC Adv. 2018, 8, 11395–11402. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Do, J.Y.; Lee, J.H.; Ra, C.S.; Kim, S.K.; Kang, M. Optical properties of Cu-incorporated ZnO (CuxZnyO) nanoparticles and their photocatalytic hydrogen production performances. Mater. Chem. Phys. 2018, 205, 206–209. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G. Photocatalytic Hydrogen Production from Glycerol Aqueous Solution Using Cu-Doped ZnO under Visible Light Irradiation. Appl. Sci. 2019, 9, 2741. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irfan, M.; Shukrullah, S.; Naz, M.Y.; Ahmad, I.; Shoukat, B.; Legutko, S.; Petrů, J.; Rahman, S.; Alsaiari, M.A. Si/SiO2/Al2O3 Supported Growth of CNT Forest for the Production of La/ZnO/CNT Photocatalyst for Hydrogen Production. Materials 2022, 15, 3226. https://doi.org/10.3390/ma15093226
Irfan M, Shukrullah S, Naz MY, Ahmad I, Shoukat B, Legutko S, Petrů J, Rahman S, Alsaiari MA. Si/SiO2/Al2O3 Supported Growth of CNT Forest for the Production of La/ZnO/CNT Photocatalyst for Hydrogen Production. Materials. 2022; 15(9):3226. https://doi.org/10.3390/ma15093226
Chicago/Turabian StyleIrfan, Muhammad, Shazia Shukrullah, Muhammad Yasin Naz, Irshad Ahmad, Bilal Shoukat, Stanislaw Legutko, Jana Petrů, Saifur Rahman, and Mabkhoot A. Alsaiari. 2022. "Si/SiO2/Al2O3 Supported Growth of CNT Forest for the Production of La/ZnO/CNT Photocatalyst for Hydrogen Production" Materials 15, no. 9: 3226. https://doi.org/10.3390/ma15093226







