Nonlinear Elasticity Assessment with Optical Coherence Elastography for High-Selectivity Differentiation of Breast Cancer Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. OCE Imaging and Assessment of Tissue Nonlinearity
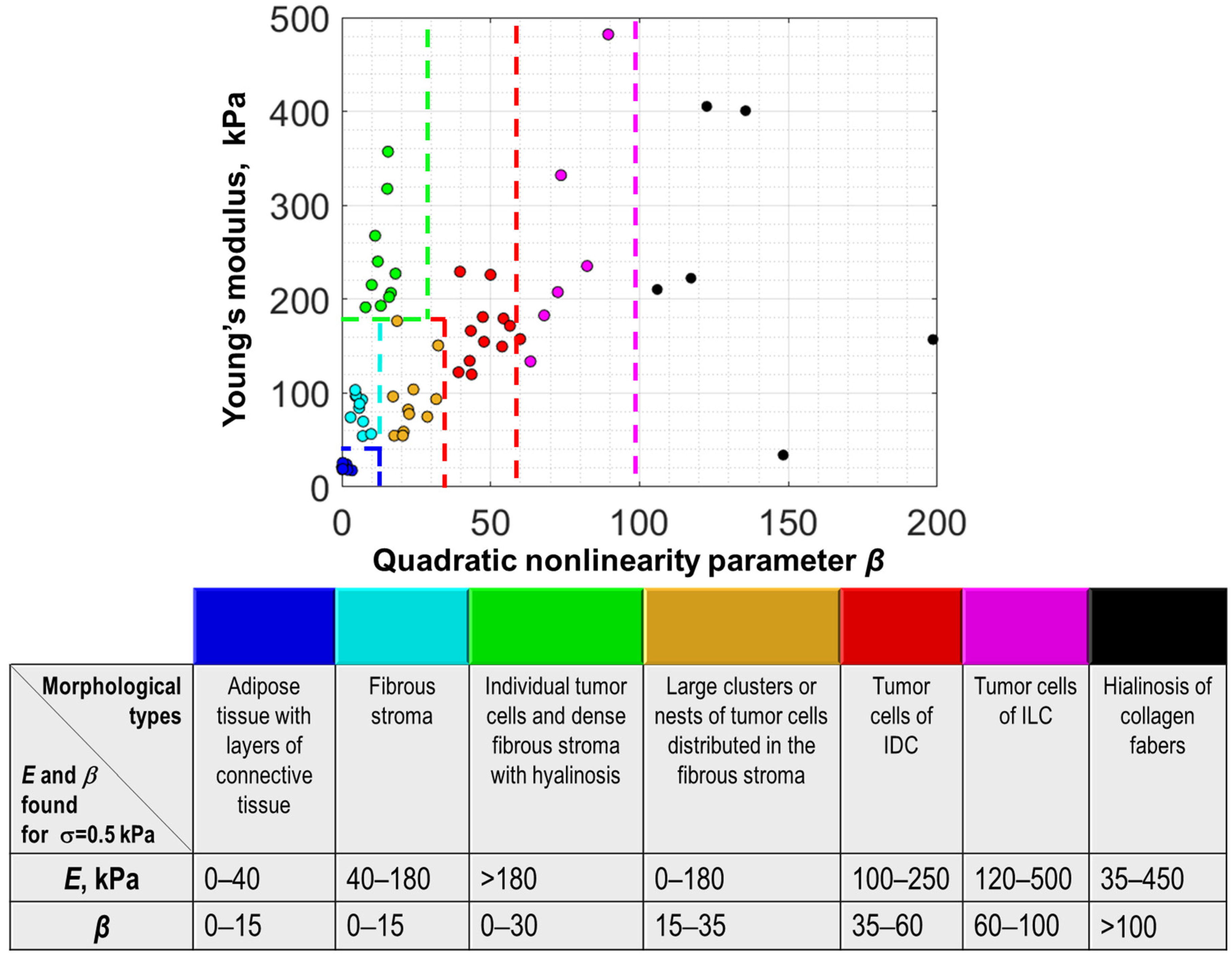
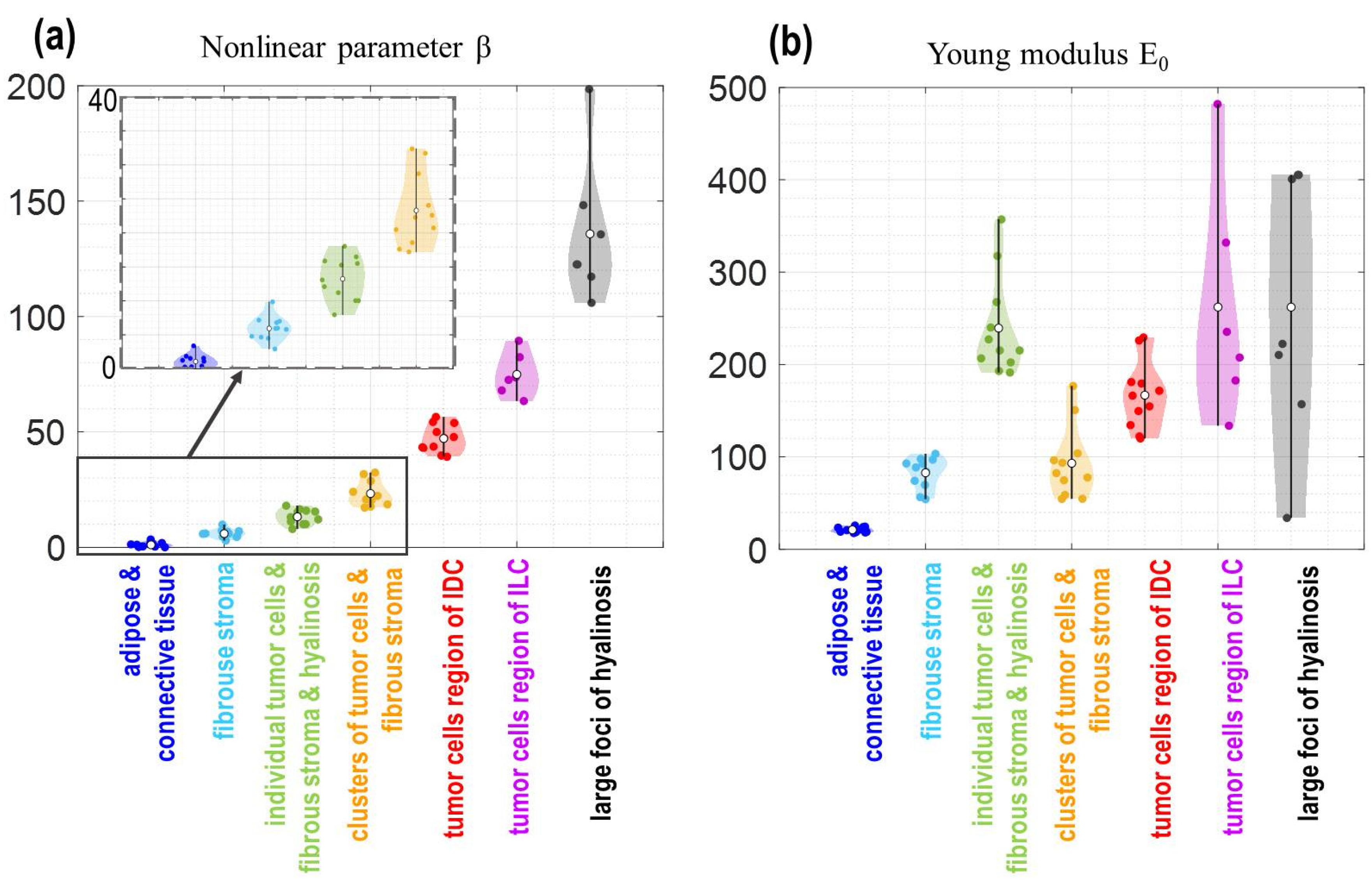
2.2. Principle of Segmentation of OCE-Images Using Linear and Nonlinear Elastic Parameters
2.3. Patient Selection and Data Collection
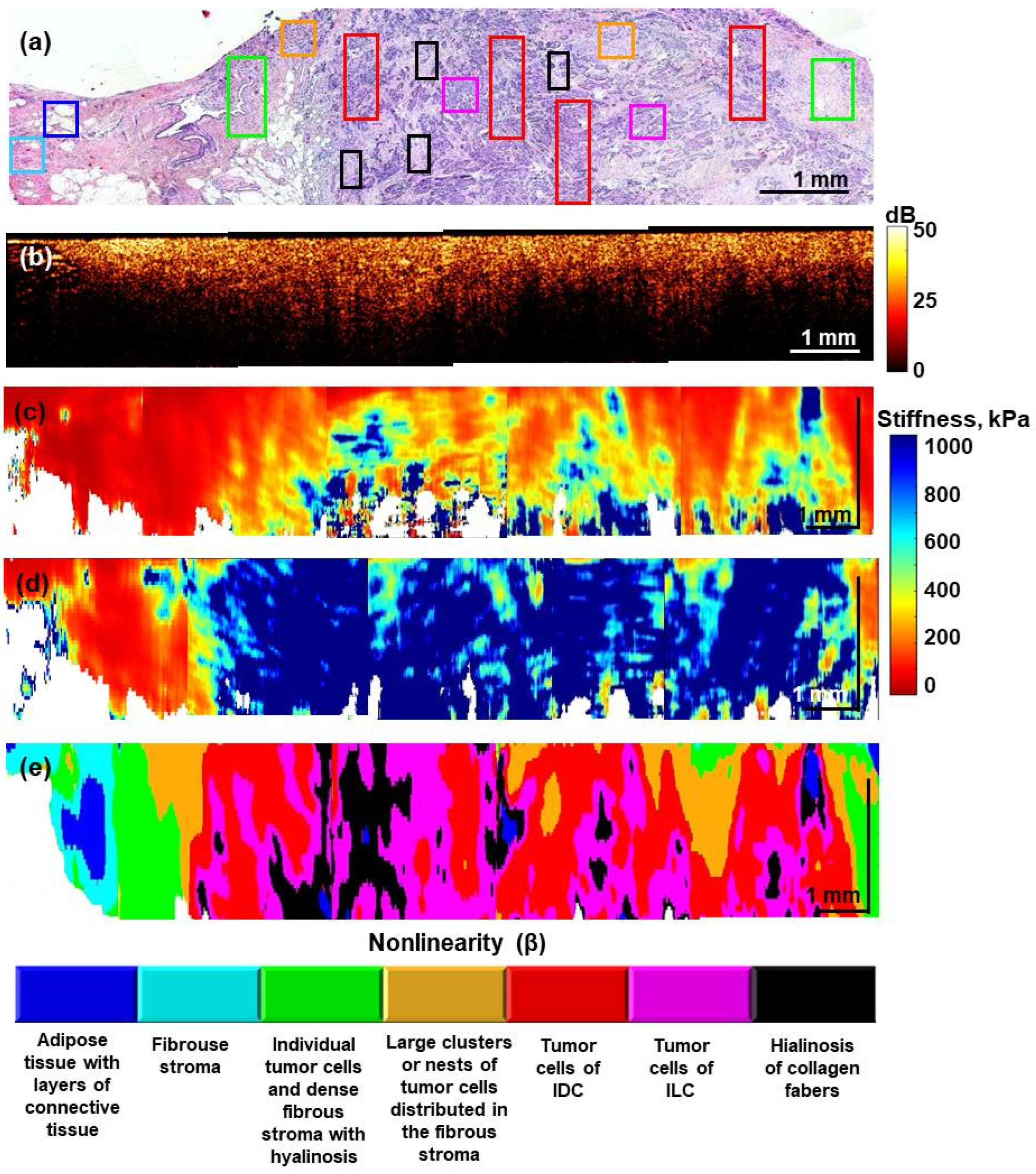
2.4. Histopathology and Its Comparison with OCE-Based Segmentation
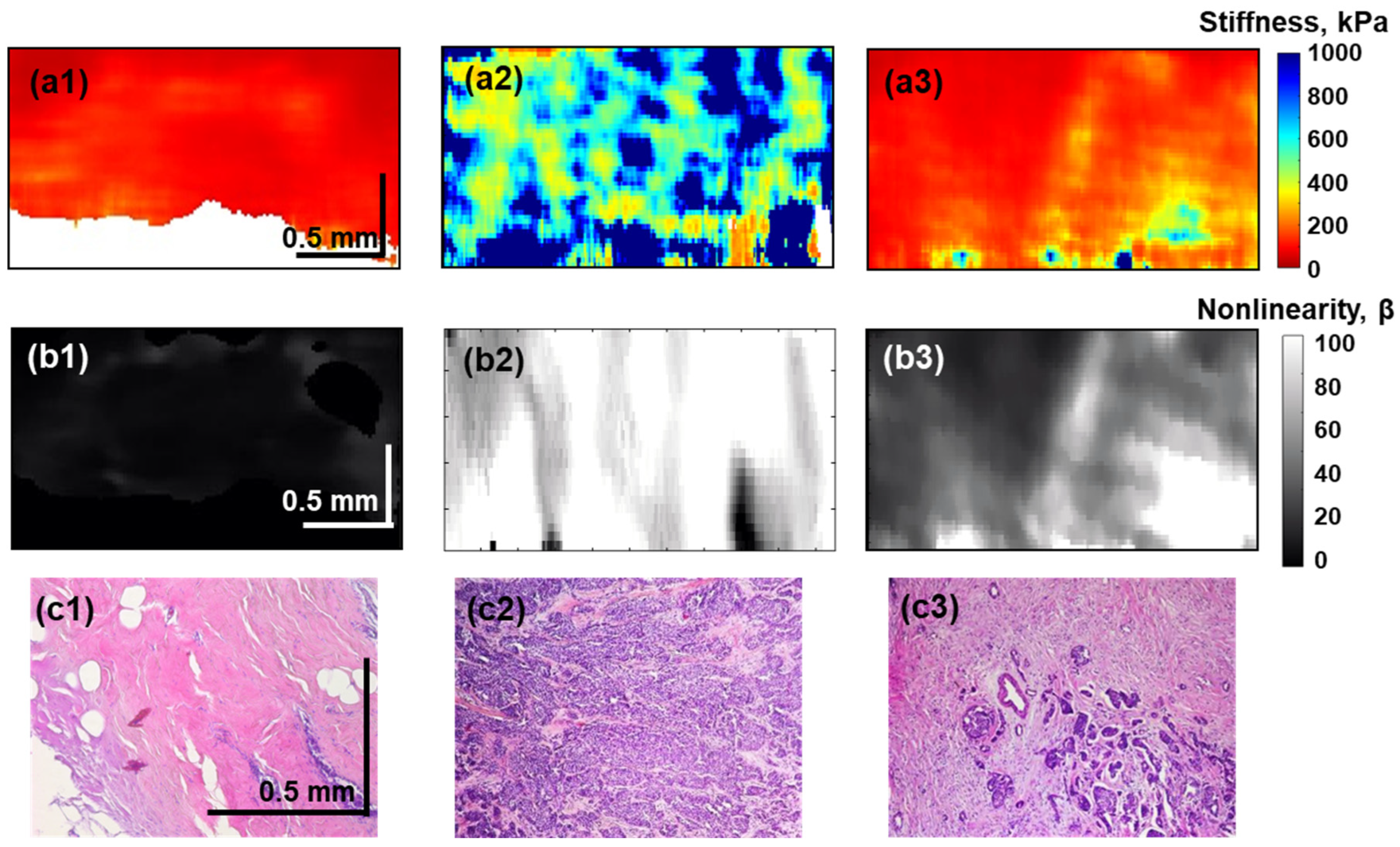
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, K.M.; Chin, L.; McLaughlin, R.A.; Latham, B.; Saunders, C.M.; Sampson, D.D.; Kennedy, B.F. Quantitative Micro-Elastography: Imaging of Tissue Elasticity Using Compression Optical Coherence Elastography. Sci. Rep. 2015, 5, 15538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Hepburn, M.S.; Mowla, A.; Kennedy, B.F. Strain and Elasticity Imaging in Compression Optical Coherence Elastography: The Two-decade Perspective and Recent Advances. J. Biophotonics 2021, 14, e202000257. [Google Scholar] [CrossRef] [PubMed]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A Quantitative Method for Imaging the Elasticity of Biological Tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Ueno, E.; Tohno, E.; Kamma, H.; Takahashi, H.; Shiina, T.; Yamakawa, M.; Matsumura, T. Breast Disease: Clinical Application of US Elastography for Diagnosis. Radiology 2006, 239, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Faruk, T.; Islam, M.K.; Arefin, S.; Haq, M.Z. The Journey of Elastography: Background, Current Status, and Future Possibilities in Breast Cancer Diagnosis. Clin. Breast Cancer 2015, 15, 313–324. [Google Scholar] [CrossRef]
- Seo, M.; Ahn, H.S.; Park, S.H.; Lee, J.B.; Choi, B.I.; Sohn, Y.-M.; Shin, S.Y. Comparison and Combination of Strain and Shear Wave Elastography of Breast Masses for Differentiation of Benign and Malignant Lesions by Quantitative Assessment: Preliminary Study: Strain and Shear Wave Elastography of Breast Masses. J. Ultrasound. Med. 2018, 37, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, J.M. OCT Elastography: Imaging Microscopic Deformation and Strain of Tissue. Opt. Express 1998, 3, 199. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.K.; Ma, Z.; Kirkpatrick, S.J. Tissue Doppler Optical Coherence Elastography for Real Time Strain Rate and Strain Mapping of Soft Tissue. Appl. Phys. Lett. 2006, 89, 144103. [Google Scholar] [CrossRef]
- Wang, R.K.; Kirkpatrick, S.; Hinds, M. Phase-Sensitive Optical Coherence Elastography for Mapping Tissue Microstrains in Real Time. Appl. Phys. Lett. 2007, 90, 164105. [Google Scholar] [CrossRef]
- Kennedy, B.F.; Koh, S.H.; McLaughlin, R.A.; Kennedy, K.M.; Munro, P.R.T.; Sampson, D.D. Strain Estimation in Phase-Sensitive Optical Coherence Elastography. Biomed. Opt. Express 2012, 3, 1865. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Gelikonov, G.V.; Sovetsky, A.A.; Vitkin, A. Optimized Phase Gradient Measurements and Phase-Amplitude Interplay in Optical Coherence Elastography. J. Biomed. Opt. 2016, 21, 116005. [Google Scholar] [CrossRef] [PubMed]
- Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Gelikonov, G.V.; Moiseev, A.A.; Zaitsev, V.Y. Vector Method for Strain Estimation in Phase-Sensitive Optical Coherence Elastography. Laser Phys. Lett. 2018, 15, 065603. [Google Scholar] [CrossRef]
- Li, J.; Pijewska, E.; Fang, Q.; Szkulmowski, M.; Kennedy, B.F. Analysis of Strain Estimation Methods in Phase-Sensitive Compression Optical Coherence Elastography. Biomed. Opt. Express 2022, 13, 2224. [Google Scholar] [CrossRef]
- Krouskop, T.A.; Wheeler, T.M.; Kallel, F.; Garra, B.S.; Hall, T. Elastic Moduli of Breast and Prostate Tissues under Compression. Ultrason. Imaging 1998, 20, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Gubarkova, E.V.; Sovetsky, A.A.; Sirotkina, M.A.; Gelikonov, G.V.; Zagaynova, E.V.; Gladkova, N.D.; Vitkin, A. Practical Obstacles and Their Mitigation Strategies in Compressional Optical Coherence Elastography of Biological Tissues. J. Innov. Opt. Health Sci. 2017, 10, 1742006. [Google Scholar] [CrossRef] [Green Version]
- Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Shabanov, D.V.; Zaitsev, V.Y. Manually-Operated Compressional Optical Coherence Elastography with Effective Aperiodic Averaging: Demonstrations for Corneal and Cartilaginous Tissues. Laser Phys. Lett. 2018, 15, 085602. [Google Scholar] [CrossRef]
- Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Gubarkova, E.V.; Plekhanov, A.A.; Sirotkina, M.A.; Gladkova, N.D.; Zaitsev, V.Y. Full-Optical Method of Local Stress Standardization to Exclude Nonlinearity-Related Ambiguity of Elasticity Estimation in Compressional Optical Coherence Elastography. Laser Phys. Lett. 2020, 17, 065601. [Google Scholar] [CrossRef]
- Qiu, Y.; Zaki, F.R.; Chandra, N.; Chester, S.A.; Liu, X. Nonlinear Characterization of Elasticity Using Quantitative Optical Coherence Elastography. Biomed. Opt. Express 2016, 7, 4702. [Google Scholar] [CrossRef] [Green Version]
- Oberai, A.A.; Gokhale, N.H.; Goenezen, S.; Barbone, P.E.; Hall, T.J.; Sommer, A.M.; Jiang, J. Linear and Nonlinear Elasticity Imaging of Soft Tissue in Vivo: Demonstration of Feasibility. Phys. Med. Biol. 2009, 54, 1191–1207. [Google Scholar] [CrossRef] [Green Version]
- Latorre-Ossa, H.; Gennisson, J.; De Brosses, E.; Tanter, M. Quantitative Imaging of Nonlinear Shear Modulus by Combining Static Elastography and Shear Wave Elastography. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2012, 59, 833–839. [Google Scholar] [CrossRef]
- Sayed, A.; Layne, G.; Abraham, J.; Mukdadi, O. Nonlinear Characterization of Breast Cancer Using Multi-Compression 3D Ultrasound Elastography in Vivo. Ultrasonics 2013, 53, 979–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goenezen, S.; Dord, J.-F.; Sink, Z.; Barbone, P.E.; Jiang, J.; Hall, T.J.; Oberai, A.A. Linear and Nonlinear Elastic Modulus Imaging: An Application to Breast Cancer Diagnosis. IEEE Trans. Med. Imaging 2012, 31, 1628–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, W.M.; Kennedy, K.M.; Fang, Q.; Chin, L.; Curatolo, A.; Watts, L.; Zilkens, R.; Chin, S.L.; Dessauvagie, B.F.; Latham, B.; et al. Wide-Field Quantitative Micro-Elastography of Human Breast Tissue. Biomed. Opt. Express 2018, 9, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, L.; Latham, B.; Saunders, C.M.; Sampson, D.D.; Kennedy, B.F. Simplifying the Assessment of Human Breast Cancer by Mapping a Micro-scale Heterogeneity Index in Optical Coherence Elastography. J. Biophotonics 2017, 10, 690–700. [Google Scholar] [CrossRef]
- Gubarkova, E.V.; Sovetsky, A.A.; Zaitsev, V.Y.; Matveyev, A.L.; Vorontsov, D.A.; Sirotkina, M.A.; Matveev, L.A.; Plekhanov, A.A.; Pavlova, N.P.; Kuznetsov, S.S.; et al. OCT-Elastography-Based Optical Biopsy for Breast Cancer Delineation and Express Assessment of Morphological/Molecular Subtypes. Biomed. Opt. Express 2019, 10, 2244. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Manapuram, R.K.; Menodiado, F.M.; Ingram, D.R.; Twa, M.D.; Lazar, A.J.; Lev, D.C.; Pollock, R.E.; Larin, K.V. Noncontact Measurement of Elasticity for the Detection of Soft-Tissue Tumors Using Phase-Sensitive Optical Coherence Tomography Combined with a Focused Air-Puff System. Opt. Lett. 2012, 37, 5184. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, K.M.; Zilkens, R.; Allen, W.M.; Foo, K.Y.; Fang, Q.; Chin, L.; Sanderson, R.W.; Anstie, J.; Wijesinghe, P.; Curatolo, A.; et al. Diagnostic Accuracy of Quantitative Micro-Elastography for Margin Assessment in Breast-Conserving Surgery. Cancer Res. 2020, 80, 1773–1783. [Google Scholar] [CrossRef] [Green Version]
- Allen, W.M.; Foo, K.Y.; Zilkens, R.; Kennedy, K.M.; Fang, Q.; Chin, L.; Dessauvagie, B.F.; Latham, B.; Saunders, C.M.; Kennedy, B.F. Clinical Feasibility of Optical Coherence Micro-Elastography for Imaging Tumor Margins in Breast-Conserving Surgery. Biomed. Opt. Express 2018, 9, 6331. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rakha, E.A.; Reis-Filho, J.S.; Baehner, F.; Dabbs, D.J.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Breast Cancer Prognostic Classification in the Molecular Era: The Role of Histological Grade. Breast Cancer Res. 2010, 12, 207. [Google Scholar] [CrossRef] [Green Version]
- Plekhanov, A.A.; Sirotkina, M.A.; Sovetsky, A.A.; Gubarkova, E.V.; Kuznetsov, S.S.; Matveyev, A.L.; Matveev, L.A.; Zagaynova, E.V.; Gladkova, N.D.; Zaitsev, V.Y. Histological Validation of in Vivo Assessment of Cancer Tissue Inhomogeneity and Automated Morphological Segmentation Enabled by Optical Coherence Elastography. Sci. Rep. 2020, 10, 11781. [Google Scholar] [CrossRef]
- Sirotkina, M.A.; Moiseev, A.A.; Matveev, L.A.; Zaitsev, V.Y.; Elagin, V.V.; Kuznetsov, S.S.; Gelikonov, G.V.; Ksenofontov, S.Y.; Zagaynova, E.V.; Feldchtein, F.I.; et al. Accurate early prediction of tumour response to PDT using optical coherence angiography. Sci. Rep. 2019, 9, 6492. [Google Scholar] [CrossRef] [PubMed]
- Gubarkova, E.V.; Feldchtein, F.I.; Zagaynova, E.V.; Gamayunov, S.V.; Sirotkina, M.A.; Sedova, E.S.; Kuznetsov, S.S.; Moiseev, A.A.; Matveev, L.A.; Zaitsev, V.Y.; et al. Optical Coherence Angiography for Pre-Treatment Assessment and Treatment Monitoring Following Photodynamic Therapy: A Basal Cell Carcinoma Patient Study. Sci. Rep. 2019, 9, 18670. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.Y.; Ksenofontov, S.Y.; Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Zykov, A.A.; Gelikonov, G.V. Real-Time Strain and Elasticity Imaging in Phase-Sensitive Optical Coherence Elastography Using a Computationally Efficient Realization of the Vector Method. Photonics 2021, 8, 527. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues, 2nd ed.; Springer: New York, NY, USA, 1993; ISBN 9780387979472. [Google Scholar]
- Ramião, N.G.; Martins, P.S.; Rynkevic, R.; Fernandes, A.A.; Barroso, M.; Santos, D.C. Biomechanical Properties of Breast Tissue, a State-of-the-Art Review. Biomech. Model Mechanobiol. 2016, 15, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Sirotkina, M.A.; Gubarkova, E.V.; Plekhanov, A.A.; Sovetsky, A.A.; Elagin, V.V.; Matveyev, A.L.; Matveev, L.A.; Kuznetsov, S.S.; Zagaynova, E.V.; Gladkova, N.D.; et al. In Vivo Assessment of Functional and Morphological Alterations in Tumors under Treatment Using OCT-Angiography Combined with OCT-Elastography. Biomed. Opt. Express 2020, 11, 1365. [Google Scholar] [CrossRef]
- Gubarkova, E.V.; Kiseleva, E.B.; Sirotkina, M.A.; Vorontsov, D.A.; Achkasova, K.A.; Kuznetsov, S.S.; Yashin, K.S.; Matveyev, A.L.; Sovetsky, A.A.; Matveev, L.A.; et al. Diagnostic Accuracy of Cross-Polarization OCT and OCT-Elastography for Differentiation of Breast Cancer Subtypes: Comparative Study. Diagnostics 2020, 10, 994. [Google Scholar] [CrossRef]
- Zaitsev, V.Y. Nonlinear Acoustics in Studies of Structural Features of Materials. MRS Bulletin 2019, 44, 350–360. [Google Scholar] [CrossRef]
- Gubarkova, E.V.; Sovetsky, A.A.; Vorontsov, D.A.; Buday, P.A.; Sirotkina, M.A.; Plekhanov, A.A.; Kuznetsov, S.S.; Matveyev, A.L.; Matveev, L.A.; Gamayunov, S.V.; et al. Compression optical coherence elastography versus strain ultrasound elastography for breast cancer detection and differentiation: Pilot study. Biomed. Opt. Express 2022, 13, 2859–2881. [Google Scholar] [CrossRef]
- Rippy, J.R.; Singh, M.; Aglyamov, S.R.; Larin, K.V. Ultrasound Shear Wave Elastography and Transient Optical Coherence Elastography: Side-by-Side Comparison of Repeatability and Accuracy. IEEE Open J. Eng. Med. Biol. 2021, 2, 179–186. [Google Scholar] [CrossRef]
- Ormachea, J.; Parker, K.J. Elastography Imaging: The 30 Year Perspective. Phys. Med. Biol. 2020, 65, 24TR06. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.; Ophir, J.; Krouskop, T.A. Nonlinear Stress-Strain Relationships in Tissue and Their Effect on the Contrast-to-Noise Ratio in Elastograms. Ultrasound Med. Biol. 2000, 26, 839–851. [Google Scholar] [CrossRef]
- Samani, A.; Plewes, D. A Method to Measure the Hyperelastic Parameters of Ex Vivo Breast Tissue Samples. Phys. Med. Biol. 2004, 49, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, J.J.; Samani, A. Measurement of the Hyperelastic Properties of 44 Pathological Ex Vivo Breast Tissue Samples. Phys. Med. Biol. 2009, 54, 2557–2569. [Google Scholar] [CrossRef]
- Catheline, S.; Gennisson, J.-L.; Fink, M. Measurement of Elastic Nonlinearity of Soft Solid with Transient Elastography. J. Acoust. Soc. Am. 2003, 114, 3087–3091. [Google Scholar] [CrossRef]
- Erkamp, R.Q.; Emelianov, S.Y.; Skovoroda, A.R.; O’Donnell, M. Nonlinear Elasticity Imaging:Theory and Phantom Study. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2004, 51, 532–539. [Google Scholar] [CrossRef]
- Gennisson, J.-L.; Rénier, M.; Catheline, S.; Barrière, C.; Bercoff, J.; Tanter, M.; Fink, M. Acoustoelasticity in Soft Solids: Assessment of the Nonlinear Shear Modulus with the Acoustic Radiation Force. J. Acoust. Soc. Am. 2007, 122, 3211–3219. [Google Scholar] [CrossRef]
- Mehrabian, H.; Campbell, G.; Samani, A. A Constrained Reconstruction Technique of Hyperelasticity Parameters for Breast Cancer Assessment. Phys. Med. Biol. 2010, 55, 7489–7508. [Google Scholar] [CrossRef]
- Koo, T.K.; Cohen, J.H.; Zheng, Y. A Mechano-Acoustic Indentor System for In Vivo Measurement of Nonlinear Elastic Properties of Soft Tissue. J. Manip. Physiol. Ther. 2011, 34, 584–593. [Google Scholar] [CrossRef]
- Guzina, B.B.; Dontsov, E.V.; Urban, M.W.; Fatemi, M. The ‘Sixth Sense’ of Ultrasound: Probing Nonlinear Elasticity with Acoustic Radiation Force. Phys. Med. Biol. 2015, 60, 3775–3794. [Google Scholar] [CrossRef]
- Bernal, M.; Chammings, F.; Couade, M.; Bercoff, J.; Tanter, M.; Gennisson, J.-L. In Vivo Quantification of the Nonlinear Shear Modulus in Breast Lesions: Feasibility Study. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2016, 63, 101–109. [Google Scholar] [CrossRef]
- Nazari, N.; Barbone, P. Shear Wave Speed in Pressurized Soft Tissue. J. Mech. Phys. Solids 2018, 119, 60–72. [Google Scholar] [CrossRef]
- Napoli, M.E.; Freitas, C.; Goswami, S.; McAleavey, S.; Doyley, M.; Howard, T.M. Hybrid Force/Velocity Control “With Compliance Estimation via Strain Elastography for Robot Assisted Ultrasound Screening. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; IEEE: Enschede, The Netherlands, 2018; pp. 1266–1273. [Google Scholar]
- Rosen, D.P.; Jiang, J. A Comparison of Hyperelastic Constitutive Models Applicable to Shear Wave Elastography (SWE) Data in Tissue-Mimicking Materials. Phys. Med. Biol. 2019, 64, 055014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osapoetra, L.O.; Watson, D.M.; McAleavey, S.A. Intraocular Pressure–Dependent Corneal Elasticity Measurement Using High-Frequency Ultrasound. Ultrason. Imaging 2019, 41, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Ahmed, R.; Doyley, M.M.; McAleavey, S.A. Nonlinear Shear Modulus Estimation with Bi-Axial Motion Registered Local Strain. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2019, 66, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bayer, M.; Jiang, J.; Hall, T.J. Large-Strain 3-D in Vivo Breast Ultrasound Strain Elastography Using a Multi-Compression Strategy and a Whole-Breast Scanning System. Ultrasound Med. Biol. 2019, 45, 3145–3159. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Gelikonov, G.V.; Baum, O.I.; Omelchenko, A.I.; Shabanov, D.V.; Sovetsky, A.A.; Yuzhakov, A.V.; Fedorov, A.A.; et al. Revealing structural modifications in thermomechanical reshaping of collagenous tissues using optical coherence elastography. J. Biophotonics 2019, 12, e201800250. [Google Scholar] [CrossRef]
- Matveev, L.A.; Sovetsky, A.A.; Matveyev, A.L.; Baum, O.; Omelchenko, A.; Yuzhakov, A.; Sobol, E.; Zaitsev, V.Y. Optical Coherence Elastography for Characterization of Natural Interstitial Gaps and Laser-Irradiation-Produced Porosity in Corneal and Cartilaginous Samples. Proc. SPIE Biomed. Spectrosc. Microsc. Imaging 2020, 11359, 113590G. [Google Scholar] [CrossRef]
- Zhang, D.; Li, C.; Huang, Z. Relaxation Time Constant Based Optical Coherence Elastography. J. Biophotonics 2020, 13, e201960233. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Matveev, L.A.; Matveyev, A.L.; Sovetsky, A.A.; Shabanov, D.V.; Ksenofontov, S.Y.; Gelikonov, G.V.; Baum, O.I.; Omelchenko, A.I.; Yuzhakov, A.V.; et al. Optimization of Phase-Resolved Optical Coherence Elastography for Highly-Sensitive Monitoring of Slow-Rate Strains. Laser Phys. Lett. 2019, 16, 065601. [Google Scholar] [CrossRef]
- Alexandrovskaya, Y.M.; Baum, O.I.; Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Sobol, E.N.; Zaitsev, V.Y. Observation of Internal Stress Relaxation in Laser-Reshaped Cartilaginous Implants Using OCT-Based Strain Mapping. Laser Phys. Lett. 2020, 17, 085603. [Google Scholar] [CrossRef]
- Baum, O.I.; Zaitsev, V.Y.; Yuzhakov, A.V.; Sviridov, A.P.; Novikova, M.L.; Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Sobol, E.N. Interplay of Temperature, Thermal-stresses and Strains in Laser-assisted Modification of Collagenous Tissues: Speckle-contrast and OCT-based Studies. J. Biophotonics 2020, 13, e201900199. [Google Scholar] [CrossRef] [PubMed]
- Alexandrovskaya, Y.; Baum, O.; Sovetsky, A.; Matveyev, A.; Matveev, L.; Sobol, E.; Zaitsev, V. Optical Coherence Elastography as a Tool for Studying Deformations in Biomaterials: Spatially-Resolved Osmotic Strain Dynamics in Cartilaginous Samples. Materials 2022, 15, 904. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubarkova, E.V.; Sovetsky, A.A.; Matveev, L.A.; Matveyev, A.L.; Vorontsov, D.A.; Plekhanov, A.A.; Kuznetsov, S.S.; Gamayunov, S.V.; Vorontsov, A.Y.; Sirotkina, M.A.; et al. Nonlinear Elasticity Assessment with Optical Coherence Elastography for High-Selectivity Differentiation of Breast Cancer Tissues. Materials 2022, 15, 3308. https://doi.org/10.3390/ma15093308
Gubarkova EV, Sovetsky AA, Matveev LA, Matveyev AL, Vorontsov DA, Plekhanov AA, Kuznetsov SS, Gamayunov SV, Vorontsov AY, Sirotkina MA, et al. Nonlinear Elasticity Assessment with Optical Coherence Elastography for High-Selectivity Differentiation of Breast Cancer Tissues. Materials. 2022; 15(9):3308. https://doi.org/10.3390/ma15093308
Chicago/Turabian StyleGubarkova, Ekaterina V., Aleksander A. Sovetsky, Lev A. Matveev, Aleksander L. Matveyev, Dmitry A. Vorontsov, Anton A. Plekhanov, Sergey S. Kuznetsov, Sergey V. Gamayunov, Alexey Y. Vorontsov, Marina A. Sirotkina, and et al. 2022. "Nonlinear Elasticity Assessment with Optical Coherence Elastography for High-Selectivity Differentiation of Breast Cancer Tissues" Materials 15, no. 9: 3308. https://doi.org/10.3390/ma15093308







